Bovine theileriosis in Australia: a decade of disease
Cheryl JenkinsElizabeth Macarthur Agricultural Institute
NSW Department of Primary Industries
Menangle, NSW 2568, Australia
Tel: +61 2 4640 6396
Email: cheryl.jenkins@dpi.nsw.gov.au
Microbiology Australia 39(4) 215-219 https://doi.org/10.1071/MA18067
Published: 24 October 2018
Theileriosis refers to the clinical disease caused by organisms from the genus Theileria, tick-borne haemoprotozoans infecting a diverse range of mammalian hosts. In Australia, Theileria spp. have been identified in both domestic and wildlife species but the bovine parasite, Theileria orientalis, has received the most attention due to the emergence and spread of clinical disease over the past 12 years, particularly in cattle herds on the east coast. At an estimated $20 million per annum, the burden to cattle production is significant but despite over a decade of disease, there are still no effective chemotherapeutic treatments or vaccines available in Australia. Recent insights from genome sequencing studies reveal species level diversity within T. orientalis, which may help direct efforts at disease control.
Clinical presentation
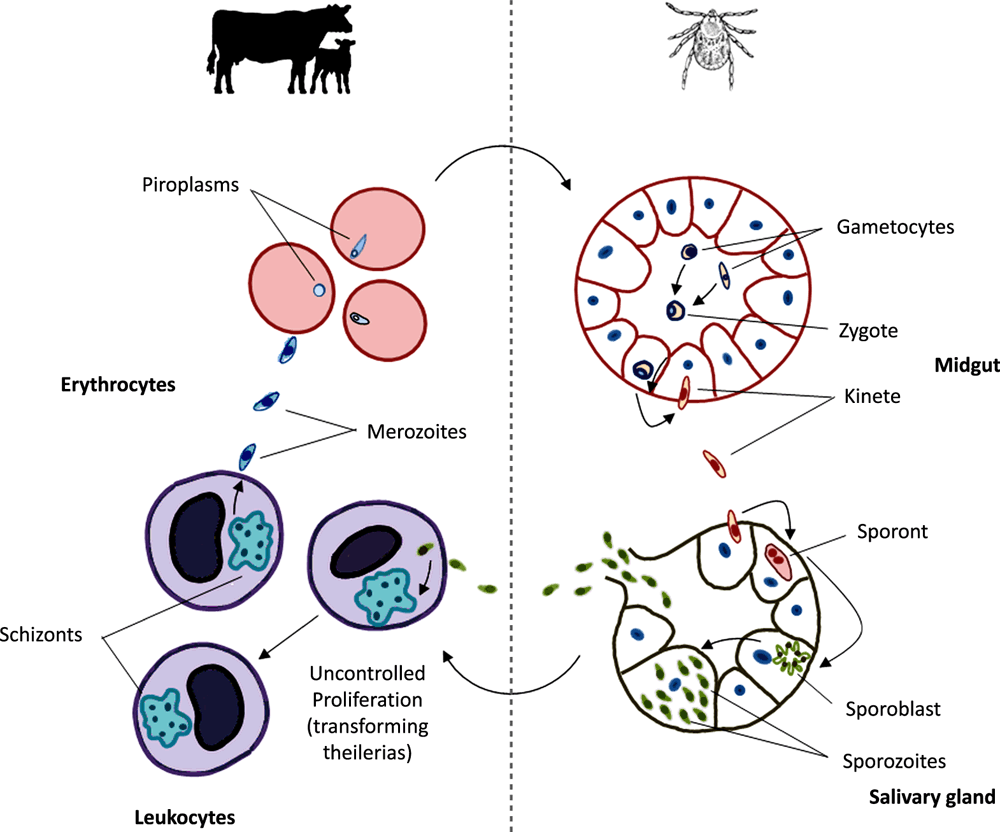
Theileria orientalis is an apicomplexan parasite that requires both a bovine and a tick host in order to complete its lifecycle (Figure 1). In Australia, bovine theileriosis is sometimes referred to as bovine anaemia caused by Theileria orientalis group (BATOG) to distinguish it from the more severe, exotic bovine theilerial diseases East Coast fever and tropical theileriosis (caused by Theileria parva and Theileria annulata respectively). Both T. parva and T. annulata are known as transforming theilerias in that they have their major proliferative stage within bovine leukocytes, inducing a lethal cancer-like state. While T. orientalis causes less severe disease than the transforming theilerias, this organism is nonetheless capable of causing up to 5% mortality in affected cattle herds. The major pathogenic effects of Theileria orientalis are elicited through the destruction of infected erythrocytes and subsequent anaemia. Therefore, the red blood cell phase (piroplasm), rather than the leukocyte phase (schizont) drives pathogenesis in this species. An enlarged spleen is frequently observed upon post-mortem, along with a large ochre-coloured liver and generalised jaundice1 brought about by excessive bilirubin from broken down erythrocytes. Animals frequently present with symptoms related to underlying anaemia including lethargy, ataxia and an increased heart and respiratory rate.
The epidemiology of theileriosis in Australia
Prior to 2006, infections with T. orientalis in Australian cattle were considered benign. The organism had been observed for over 100 years in blood smears but was considered an incidental finding with very few reports of clinical disease. Early serological surveys suggested the parasite was widespread in NSW and QLD but researchers were unable to induce clinical disease experimentally1,2. T. orientalis is currently classified into genotypes based on the sequence of the major piroplasm surface protein (MPSP). Up until 2006, MPSP genotypes identified in Australia were Buffeli and Chitose.
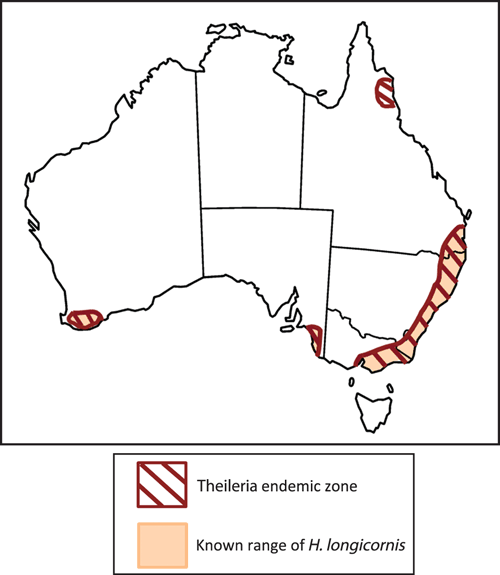
Between 2006 and 2008 theileriosis cases were reported in NSW cattle herds with a history of pregnancy or introduction to new herds. Animals presented with abortion, lethargy, jaundice and anaemia. Attention turned to Theileria as a cause due to the unusually high numbers of parasites observed in blood smears and after alternative causes of anaemia were ruled out. Follow up molecular testing revealed the presence of a new genotype, T. orientalis Ikeda, which was linked to disease in Japan3. We undertook surveillance of a large number of cattle in Australia revealing that this genotype was associated with disease either as the sole agent or in mixed infections with Buffeli and Chitose genotypes4. Reports of BATOG increased substantially, and in the intervening years the disease spread throughout coastal NSW and Victoria, south east Qld and into isolated parts of SA, WA and far north Qld (Figure 2). New disease cases have consistently been associated with T. orientalis Ikeda.
|
Immunity
Since 2015, incursions into new areas of the country ceased, although movement of naïve animals into areas where the disease is enzootic remains a major risk factor for disease. Subclinical infections with T. orientalis Ikeda are common. In areas where the disease is enzootic it is not unusual to find 100% prevalence in the absence of disease implying a level of immunity, although the immune mechanisms are poorly understood. Animals affected by clinical theileriosis usually seroconvert to the MPSP while subclinically infected animals often lack a detectable humoral response5. Calves acquire little protection from the dam via antibodies in colostrum and are highly susceptible to infection6, 7. While calves can sometimes be infected transplacentally, this does not appear to be a major route of transmission. Infection dynamics in calves are consistent with tick transmission and animals routinely become highly parasitaemic between 4–9 weeks of age7. Given the intracellular nature of the parasite, immunity is likely to be cell-mediated although the potential mechanisms behind this are yet to be explored.
Transmission
A range of tick species have been implicated in transmission of theileriosis overseas, but members of the genus Haemaphysalis are considered the main vectors. Studies conducted in Japan demonstrated transmission of T. orientalis Ikeda with Haemaphysalis longicornis, which was introduced to Australia in the 19th or 20th century. Conversely, transmission work conducted in Australia in the 1980s demonstrated that H. bancrofti and H. humerosa (latterly believed to be H. bremneri) were competent transmitters of T. orientalis, while H. longicornis was not2. The likely explanation for this discrepancy lies in the fact that Japanese studies were conducted with T. orientalis Ikeda stock, while studies in Australia were conducted with T. orientalis Buffeli. To investigate this further we undertook sampling of ticks from cattle and other domestic and wildlife species within the endemic area, identified the tick species with DNA barcoding, and screened the mouthparts by PCR for T. orientalis. A total of 135 ticks were collected representing eight different species; however, only H. longicornis ticks tested positive for T. orientalis, lending further weight to H. longicornis as the likely vector for theileriosis in Australia8. Indeed the extent of disease spread in Australia is almost perfectly defined by the known range of H. longicornis, which prefers the wetter areas of east coast and is rarely found west of the Great Dividing Range (Figure 2). Small pockets of H. longicornis occur in the moist areas of southwest WA and southeast SA where T. orientalis Ikeda outbreaks have also been reported. Thus, while never directly demonstrated, the evidence overwhelmingly points to H. longicornis as the major vector for bovine theileriosis.
In addition to tick transmission, mechanical transmission can be achieved experimentally with as little as 0.1 mL of blood when parasite levels are high and can induce clinically relevant levels of Theileria in the recipient animal6. While merozoites can undergo several rounds of proliferation within erythrocytes, mechanical transfer (via non-tick arthropods or iatrogenic means) is unlikely to support parasite persistence in the long term as it bypasses the sexual stage of the lifecycle that is required to maintain genetic diversity within the population.
Control
There are currently no effective chemotherapeutics or vaccines for the control of bovine theileriosis in Australia. Treatment of vector ticks via acaricides and minimising the movement of cattle from non-endemic to endemic areas are the main methods of disease management. Imidocarb, erythromycin or oxytetracycline are sometimes administered to affected animals, but to little effect9. In New Zealand, blood transfusion is regularly undertaken on animals that are moderately to severely anaemic, but this practice is costly and time consuming. Buparvaquone, a known anti-protozoal is used to treat BATOG in New Zealand and is also used to treat East Coast fever in Africa. When administered in a timely fashion, buparvaquone is effective against T. orientalis, yet this drug is not approved for use in Australia due to its tendency to leave residues in meat and milk10 and the need to observe lengthy withholding periods. Vaccination would be the preferred option for disease control but there has been little progress towards a vaccine for this disease worldwide. Despite assertions that a live vaccine based on the benign Buffeli genotype would be a potential way forward11, there is little hard evidence that this genotype provides protection in naturally infected animals. In other Theileria species, immunisation with one variant does not result in heterologous immunity against other variants. Furthermore, high seroprevalence of animals to T. orientalis Buffeli and/or Chitose in NSW prior to 20061 failed to prevent widespread outbreaks of disease caused by T. orientalis Ikeda. Development of subunit vaccines is generally regarded as problematic for apicomplexan parasites due to genetic diversity within parasite populations. Nonetheless some early work in Japan demonstrated partial protection against theileriosis using a subunit vaccine formulation of the Ikeda MPSP antigen with Freund’s adjuvant or liposomes12. Despite these initially promising results, no further vaccine development has been undertaken with this or with other antigens.
Lessons from genome sequencing
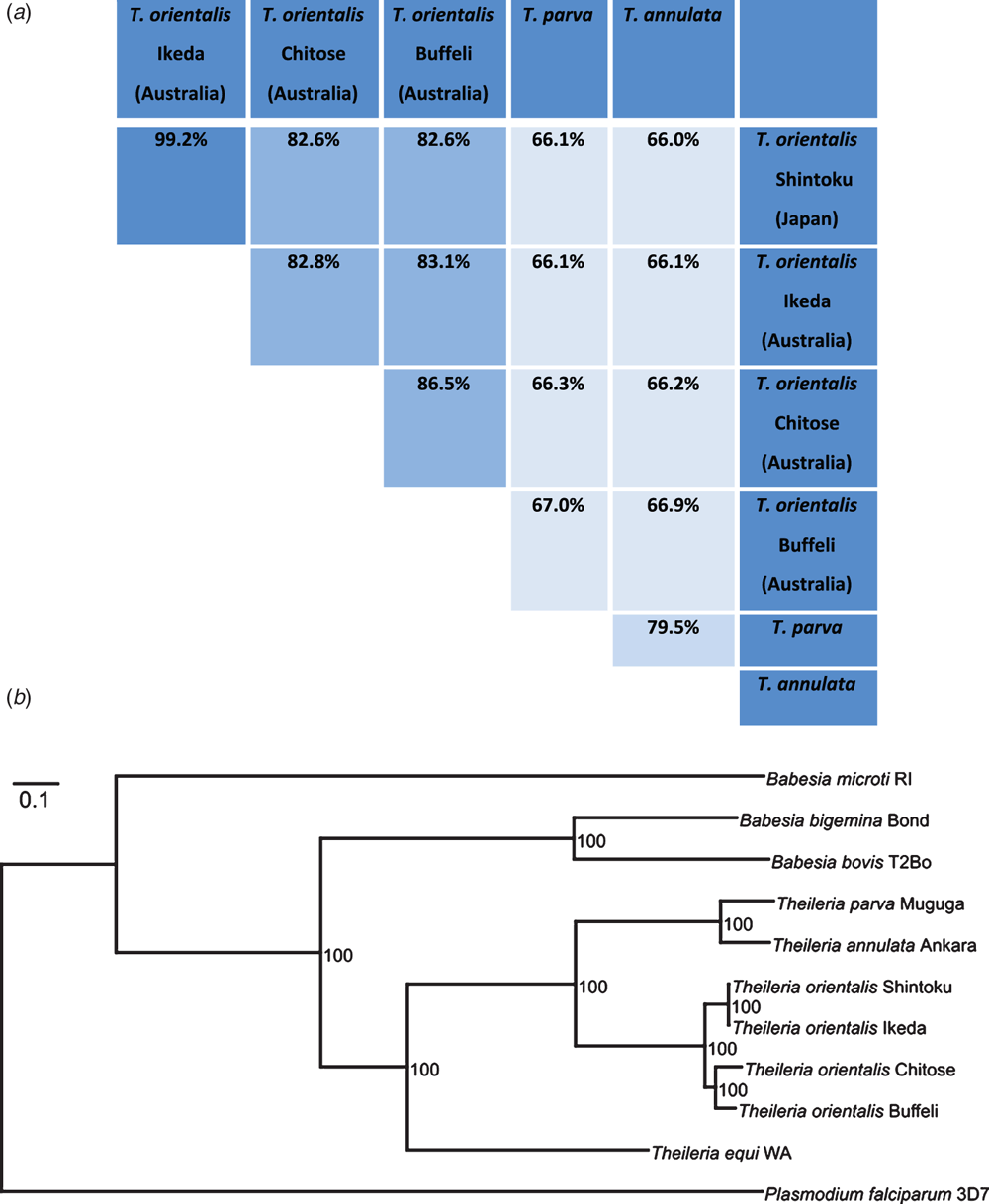
Recent draft genomes of Ikeda, Chitose and Buffeli genotypes of T. orientalis may assist in providing insights into the differential pathogenesis of these subtypes13. Surprisingly, these genomes revealed potential species level diversity within T. orientalis with average nucleotide identities almost as low as observed between T. annulata and T. parva (Figure 3). Phylogenetic analysis of 654 protein-coding genes also showed that T. orientalis Ikeda forms its own lineage relative to T. orientalis Buffeli and Chitose, while the Japanese and Australian strains of T. orientalis Ikeda are remarkably similar13. The origin of T. orientalis Ikeda in Australia has never been elucidated although the importation of a small number of Wagyu breed cattle into Australia from Japan in the late 1990s has been proposed as one potential route of introduction. Genome sequencing of further international isolates of T. orientalis may lend weight to this theory. The origin of introduction is potentially highly relevant to the issue of vaccine development. If T. orientalis Ikeda in Australia arose from only a limited parasite population, then genetic diversity would be expected to be relatively low, making the prospect of developing a long-lasting vaccine for this disease more likely. Further genome-based studies are currently being undertaken to establish the genetic diversity within the Ikeda genotype in Australia.
|
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
Daniel Bogema, Melinda Micallef, Graeme Eamens, Graham Bailey and Shayne Fell from the NSW Department of Primary Industries are gratefully acknowledged for their contributions to this work. I also thank Jade Hammer and David Emery of the University of Sydney for their collaboration on various aspects of the Theileria transmission work. This work was supported by Meat & Livestock Australia and the McGarvie Smith Trust.
References
[1] Izzo, M.M. et al. (2010) Haemolytic anaemia in cattle in NSW associated with Theileria infections. Aust. Vet. J. 88, 45–51.| Haemolytic anaemia in cattle in NSW associated with Theileria infections.Crossref | GoogleScholarGoogle Scholar |
[2] Stewart, N.P. et al. (1987) Haemaphysalis humerosa, not H. longicornis, is the likely vector of Theileria buffeli in Australia. Aust. Vet. J. 64, 280–282.
| Haemaphysalis humerosa, not H. longicornis, is the likely vector of Theileria buffeli in Australia.Crossref | GoogleScholarGoogle Scholar |
[3] Kamau, J. et al. (2011) Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks. Parasit. Vectors 4, 22.
| Emergence of new types of Theileria orientalis in Australian cattle and possible cause of theileriosis outbreaks.Crossref | GoogleScholarGoogle Scholar |
[4] Eamens, G.J. et al. (2013) Theileria orientalis MPSP types in Australian cattle herds associated with outbreaks of clinical disease and their association with clinical pathology findings. Vet. Parasitol. 191, 209–217.
| Theileria orientalis MPSP types in Australian cattle herds associated with outbreaks of clinical disease and their association with clinical pathology findings.Crossref | GoogleScholarGoogle Scholar |
[5] Jenkins, C. and Bogema, D.R. (2016) Factors associated with seroconversion to the major piroplasm surface protein of the bovine haemoparasite Theileria orientalis. Parasit. Vectors 9, 106.
| Factors associated with seroconversion to the major piroplasm surface protein of the bovine haemoparasite Theileria orientalis.Crossref | GoogleScholarGoogle Scholar |
[6] Hammer, J.F. et al. (2016) Mechanical transfer of Theileria orientalis: possible roles of biting arthropods, colostrum and husbandry practices in disease transmission. Parasit. Vectors 9, 34.
| Mechanical transfer of Theileria orientalis: possible roles of biting arthropods, colostrum and husbandry practices in disease transmission.Crossref | GoogleScholarGoogle Scholar |
[7] Swilks, E. et al. (2017) Transplacental transmission of Theileria orientalis occurs at a low rate in field-affected cattle: infection in utero does not appear to be a major cause of abortion. Parasit. Vectors 10, 227.
| Transplacental transmission of Theileria orientalis occurs at a low rate in field-affected cattle: infection in utero does not appear to be a major cause of abortion.Crossref | GoogleScholarGoogle Scholar |
[8] Hammer, J.F. et al. (2015) Detection of Theileria orientalis genotypes in Haemaphysalis longicornis ticks from southern Australia. Parasit. Vectors 8, 229.
| Detection of Theileria orientalis genotypes in Haemaphysalis longicornis ticks from southern Australia.Crossref | GoogleScholarGoogle Scholar |
[9] Glassop, A. and Kerr, J. (2013) Theileriosis research on the mid-north coast of NSW 2011. Flock and Herd Case Notes. http://flockandherd.net.au/cattle/reader/theileriosis-research-north-coast.html
[10] Bailey, G. (2013) Buparvaquone tissue residue study. Meat & Livestock Australia, North Sydney, Australia. https://www.mla.com.au/Research-and-development/Search-RD-reports/final-report-details/Animal-Health-and-Biosecurity/Buparvaquone-tissue-residue-study/170
[11] de Vos, A.J. (2011) Theileria: assess potential to develop a vaccine for Theileria orientalis infection. Meat & Livestock Australia, North Sydney, Australia. https://www.mla.com.au/CustomControls/PaymentGateway/ViewFile.aspx?AP68dQqEokAFxb8ISxN5cczVSxStHmOaOAIwUA7Vs47gPGuShn/+8qFmJgf2C9A/3EYMKKAfsht7d1Tnt3BqiA==
[12] Onuma, M. et al. (1997) Control of Theileria sergenti infection by vaccination. Trop. Anim. Health Prod. 29, 119S–123S.
| Control of Theileria sergenti infection by vaccination.Crossref | GoogleScholarGoogle Scholar |
[13] Bogema, D.R. et al. (2018) Analysis of Theileria orientalis draft genome sequences reveals potential species-level divergence of the Ikeda, Chitose and Buffeli genotypes. BMC Genomics 19, 298.
| Analysis of Theileria orientalis draft genome sequences reveals potential species-level divergence of the Ikeda, Chitose and Buffeli genotypes.Crossref | GoogleScholarGoogle Scholar |
Biography
Dr Cheryl Jenkins is a Principal Research Scientist at the NSW Department of Primary Industries with an interest in parasitic and bacterial diseases of production animals.