Necrotic enteritis in chickens: an important disease caused by Clostridium perfringens
Robert J MooreHost-Microbe Interactions Laboratory
School of Applied Sciences
Health Innovations Research Institute
RMIT University
Bundoora, Vic. 3083, Australia
Tel: +61 3 9925 7580
Email: rob.moore@rmit.edu.au
Microbiology Australia 36(3) 118-119 https://doi.org/10.1071/MA15041
Published: 6 August 2015
Clostridium perfringens, a spore-forming, Gram-positive, anaerobic bacterium, causes a variety of diseases throughout the animal kingdom. Each disease in each animal species tends to be caused by particular strains of C. perfringens and is defined by the tissue tropism and toxin profile of the bacteria. In chickens toxinotype A strains cause necrotic enteritis; a disease characterised by tissue damage to the proximal regions of the small intestine. In extreme cases the disease can be lethal but is more commonly seen as a sub-clinical disease that causes welfare issues and productivity losses within the poultry industry. The disease is currently well controlled in Australia by good management practices and, for some poultry producers, the use of antibiotics in the feed. However, the disease does cause significant issues in other regions including North America and Europe. In Europe there was a spike of necrotic enteritis disease when antibiotics were withdrawn from animal feeds. It is probable that the disease will become more of an issue in the Australian poultry industry as in-feed antibiotic use is reduced. Therefore, other methods of disease control are under investigation, including the development of vaccines.
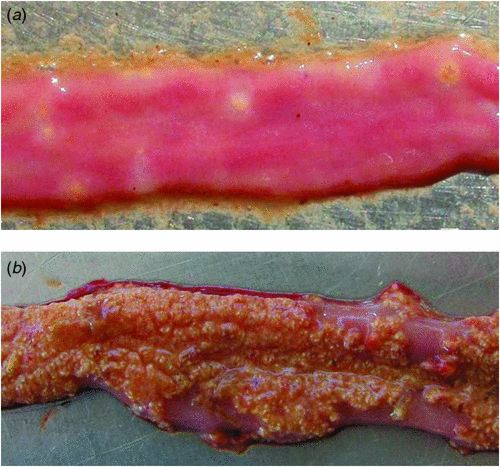
Necrotic enteritis (Figure 1) has been estimated to cost the global poultry industry $2 billion per annum in control measures and productivity losses1. Although C. perfringens is clearly the causative agent of necrotic enteritis a simple infection with the bacterium is not sufficient to induce disease. C. perfringens is a ubiquitous organism commonly found in many environments, in particular within the gastrointestinal tract (GIT) of healthy animals and humans. At low levels it causes no problems in the GIT; it is only in the face of predisposing factors that disease occurs. There are many interacting factors which can predispose birds to the development of necrotic enteritis. They can be broadly classified according to the effects that they have on the birds; they can directly damage the intestinal mucosa (e.g. infection with the Eimeria apicomplexan parasite), alter the gut microbiota (e.g. high protein levels in feed2), or compromise the immune system (e.g. some viral infections). It is only following the development of a better understanding of these predisposing factors, and the application of some of them, that it has been possible to reliably induce disease experimentally. The difficulty of consistently reproducing disease held back research for a number of years but has now been largely overcome.
|
The generally held conceptual model for disease development hypothesised that the predisposing factors induced an expansion of the C. perfringens population in the gut resulting in higher toxin levels, gut damage, and frank disease. However, recent studies have indicated that the C. perfringens strains that are commonly present in low numbers within the healthy gut are generally distinct from the strains that can go on to cause disease3,4. The origin of pathogenic strains has not yet been clarified and the mechanisms driving displacement of non-pathogenic strains by pathogenic strains are not clear but may be partly driven by bacteriocin expression by the pathogenic strains5. So, although C. perfringens population expansion is a critical part of disease development, it appears that non-pathogenic native strains are displaced by virulent strains. Many of the toxins that play key roles in disease development are encoded on large conjugative plasmids that have the potential to move from strain to strain6; this may be an important factor in the emergence and epidemiology of disease causing strains.
A range of potential therapeutic and prophylactic treatments are being actively developed to address the necrotic enteritis disease burden within the global poultry industry. These include the application of prebiotics7, probiotics8, organic acids9, and plant extracts10 as well as vaccines. Vaccines against other animal clostridial diseases have been available for many years. Such vaccines are produced using toxoids; chemically inactivated culture supernatants containing the disease causing toxins and a range of other minor antigens. They are cheap to produce and very effective. However, satisfactory vaccines to protect poultry from necrotic enteritis have not been commercially available.
The toxins produced by C. perfringens are excellent vaccine antigens because they are the major virulence factors responsible for disease induction and, as secreted proteins, are readily accessible to the host immune system. For decades after the definitive description of necrotic enteritis by Parish11 it was thought that alpha-toxin, a toxin produced by all isolates of C. perfringens, was the main virulence determinant. However, in 2006, we demonstrated that alpha-toxin was not essential for experimental disease induction12 and then went on to discover and characterise the toxin, necrotic enteritis toxin B-like (NetB), that does play an essential role in virulence13,14.
The belief that alpha-toxin was important in disease misdirected vaccine efforts for many years but the recent advances made in our fundamental understanding of the basis of pathogenesis is now enabling the development of effective vaccines15–17. The key to successful vaccine design has been to ensure that there is a sufficient level of the key NetB protein to elicit a strong protective immune response. With efficacious experimental vaccines demonstrated the challenge for the industry now is to be able to formulate and deliver the vaccines in a cost effective and useful way. The broiler (meat) chicken industry presents interesting challenges for vaccine application as vaccines must be provided at very low cost and must be effective in very young birds – for instance the peak risk of developing necrotic enteritis is between 2 to 4 weeks of age. We are continuing to address industry needs by investigating the use of both conventional killed vaccines and live vector delivered vaccines for necrotic enteritis.
Acknowledgements
I thank my two key collaborators, Professor Julian Rood and Dr Anthony Keyburn, who have contributed so much to necrotic enteritis research. The fundamental and applied research to understand disease pathogenesis and apply that learning to vaccine development has been carried out within the Australian Animal Health Laboratory, CSIRO, Geelong, and the Department of Microbiology, Monash University, with the support of the Poultry Cooperative Research Centre, established and supported under the Australian Government’s Cooperative Research Centres Program, and the ARC Centre of Excellence in Structural and Functional Microbial Genomics.
References
[1] Van der Sluis, W. (2000) Clostridial enteritis is an often underestimated problem. World Poult. 16, 42–43.[2] Wu, S.-B. et al. (2014) Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 169, 188–197.
| Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens.Crossref | GoogleScholarGoogle Scholar | 24522272PubMed |
[3] Keyburn, A.L. et al. (2010) Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin. Vet. Res. 41, 21.
| Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin.Crossref | GoogleScholarGoogle Scholar | 19931005PubMed |
[4] Hibberd, M.C. et al. (2011) Multilocus sequence typing subtypes of poultry Clostridium perfringens isolates demonstrates disease niche partitioning. J. Clin. Microbiol. 49, 1556–1567.
| Multilocus sequence typing subtypes of poultry Clostridium perfringens isolates demonstrates disease niche partitioning.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFKrtL%2FJ&md5=6d9483ccac2b8701c28b1434890280b6CAS | 21270221PubMed |
[5] Timbermont, L. et al. (2009) Intra-species growth-inhibition by Clostridium perfringens is a possible virulence trait in necrotic enteritis in broilers. Vet. Microbiol. 137, 388–391.
| Intra-species growth-inhibition by Clostridium perfringens is a possible virulence trait in necrotic enteritis in broilers.Crossref | GoogleScholarGoogle Scholar | 19201552PubMed |
[6] Bannam, T.L. et al. (2011) Necrotic enteritis-derived Clostridium perfringens with three closely related three independently conjugative toxin and antibiotic resistance plasmids. MBio 2, e00190-e11.
| Necrotic enteritis-derived Clostridium perfringens with three closely related three independently conjugative toxin and antibiotic resistance plasmids.Crossref | GoogleScholarGoogle Scholar |
[7] Vidanarachchi, J.K. et al. (2013) Natural plant extracts and prebiotic compounds as alternatives to antibiotics in broiler chicken diets in a necrotic enteritis challenge model. Anim. Prod. Sci. 53, 1247–1259.
| Natural plant extracts and prebiotic compounds as alternatives to antibiotics in broiler chicken diets in a necrotic enteritis challenge model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslWls7bJ&md5=e49dfabae51684b502212d508a5866a5CAS |
[8] Cao, L. et al. (2012) Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1.20291. Poult. Sci. 91, 3065–3071.
| Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1.20291.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvFShs7%2FE&md5=50e4a9517416f67c5b0ef837a5fbde6eCAS | 23155014PubMed |
[9] Geier, M.S. et al. (2010) Comparison of alternatives to in-feed antimicrobials for the prevention of clinical necrotic enteritis. J. Appl. Microbiol. 109, 1329–1338.
| Comparison of alternatives to in-feed antimicrobials for the prevention of clinical necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlejs77I&md5=b03121e24eaadab3c4a4460b3d453589CAS | 20497278PubMed |
[10] Lee, S.H. et al. (2013) Dietary supplementation of young broiler chickens with capsicum and turmeric oleoresins increases resistance to necrotic enteritis. Br. J. Nutr. 110, 840–847.
| Dietary supplementation of young broiler chickens with capsicum and turmeric oleoresins increases resistance to necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht12jtL%2FL&md5=6b832920cdb73f2ee7884e8f69317979CAS | 23566550PubMed |
[11] Parish, W.E. (1961) Necrotic enteritis in the fowl Gallus gallus domesticus. I. Histopathology of the disease and isolation of a strain of Clostridium welchii. J. Comp. Pathol. 71, 377–393.
| Necrotic enteritis in the fowl Gallus gallus domesticus. I. Histopathology of the disease and isolation of a strain of Clostridium welchii.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF38XjsVWnsw%3D%3D&md5=4f7fb3b530877c015608fcac57525b08CAS | 14483884PubMed |
[12] Keyburn, A.L. et al. (2006) The alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 74, 6496–6500.
| The alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Khu73E&md5=715cb440f922a02c31cf9a968cdd5915CAS | 16923791PubMed |
[13] Keyburn, A.L. et al. (2008) NetB, a new toxin is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 4, e26.
| NetB, a new toxin is associated with avian necrotic enteritis caused by Clostridium perfringens.Crossref | GoogleScholarGoogle Scholar | 18266469PubMed |
[14] Yan, X.-X. et al. (2013) Structural and functional analysis of the pore-forming toxin NetB from Clostridium perfringens. MBio 4, e00019-e13.
| Structural and functional analysis of the pore-forming toxin NetB from Clostridium perfringens.Crossref | GoogleScholarGoogle Scholar |
[15] Keyburn, A.L. et al. (2013) Vaccination with recombinant NetB toxin protects broiler chickens from necrotic enteritis. Vet. Res. 44, 54.
| Vaccination with recombinant NetB toxin protects broiler chickens from necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXht1OntbfM&md5=81f20447466ad8e63f0ff190f3fd83ffCAS | 23865568PubMed |
[16] Keyburn, A.L. et al. (2013) Maternal immunization with vaccine containing recombinant NetB toxin partially protects progeny chickens from necrotic enteritis. Vet. Res. 44, 108.
| Maternal immunization with vaccine containing recombinant NetB toxin partially protects progeny chickens from necrotic enteritis.Crossref | GoogleScholarGoogle Scholar | 24219318PubMed |
[17] Fernandes da Costa, S.P. et al. (2013) Protection against avian necrotic entertitis after immunisation with NetB genetic or formaldehyde toxoids. Vaccine 31, 4003–4008.
| Protection against avian necrotic entertitis after immunisation with NetB genetic or formaldehyde toxoids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXptlGktbk%3D&md5=09b95622039a986b18ec5ba672cd802aCAS | 23727000PubMed |
Biography
Rob Moore has recently moved from CSIRO to establish a new laboratory at RMIT University, where he is a Professor of Biotechnology. He is also an Adjunct Associate Professor in the Microbiology Department at Monash University and an Honorary Fellow within the CSIRO Biosecurity Flagship at the Australian Animal Health Laboratory, Geelong. His research interests are in bacterial pathogenesis, vaccine design, the role of gut microbiota in health and productivity, and the identification and development of probiotic strains of bacteria.


