Marsupial oral cavity microbiome
Philip S Bird A D , Wayne SJ Boardman B , Darren J Trott B and Linda L Blackall CA The University of Queensland, School of Veterinary Science, Faculty of Science, Gatton, Qld 4343, Australia
B The University of Adelaide, School of Animal and Veterinary Sciences, Roseworthy, SA 5371, Australia
C Swinburne University of Technology, School of Science, Faculty of Science, Engineering and Technology, Hawthorn, Vic. 3122, Australia
D Corresponding author. Tel: +61 7 5460 1834, Fax: +61 7 5460 1922, Email: phil.bird@uq.edu.au
Microbiology Australia 36(1) 29-31 https://doi.org/10.1071/MA15009
Published: 6 March 2015
The oral microbiome of humans and animals will cause oral disease within their lifetimes and include a large number of endogenous cariogenic, periodontal and other opportunistic pathogens. Studies over many decades have attempted to determine which bacteria are involved in oral diseases. Earlier studies used exclusively culture-based methods. Now culture-independent methods are being used to determine the composition of the microbiome in health and disease. There have been limited numbers of studies of the marsupial microbiome and this report covers some of the research of those studies.
Dental plaque, a natural oral biofilm is composed of many diverse bacterial species, some of which are involved in the aetiology of periodontal diseases (gingivitis and periodontitis)1. Factors such as indigenous bacteria, host immune system, diet, host susceptibility and time, interplay in these diseases2. There have been many studies determining which of the causative agent(s) initiate oral diseases in humans and domesticated animals. Marsupials also have oral diseases3,4 and culture-dependent studies have shown a range of bacteria can be isolated from the marsupial oral cavity5. However culture-based studies, while useful to enable precise characterisation of putative periodontopathogens, generally underestimate microbial community diversity. Culture-independent methods, such as high-throughput DNA sequencing reveal a rich and diverse bacterial community in the oral cavities of humans and companion animals6–8, and the marsupial microbiome should also be more thoroughly studied too.
The first bacteria ever to be seen under a microscope were the plaque bacteria taken from Antonie van Leeuwenhoek’s own teeth and reported in a letter to the Royal Society on 17 September 1683. These first recorded observations of living bacteria showed ‘an unbelievable great company of living animalcules and of enormous number of a variety of shapes and sizes’. Since then many oral prokaryotic species have been described. The Human Oral Microbiome Database (www.homd.org/) has to-date >200 bacterial species described from culture-dependent methods and approximately 1,000 phylotypes detected by 16S rRNA gene sequencing of oral samples from the human oral cavity using culture-independent methods9. A search of the International Journal of Systematic and Evolutionary Microbiology over the past 10 years for ‘oral’ revealed a host of novel bacterial species isolated from the oral cavity of humans and animals, including domesticated and wild (free-ranging and captive) animals. In marsupials, novel microbial species were revealed in the oral cavity of macropods associated with gingivitis and oral necrobacillosis10.
In domesticated and wild animals, using culture-dependent methods, associations with disease and specific bacterial species have been reported5. More recently, researchers have used high-throughput DNA sequencing to study the oral microbiota of healthy cats8 and dogs7. In adult horses 67% of 203 operational taxonomic units (OTUs) were recovered, with the most frequent genera being Prevotella and Porphyromonas (T Chinkangsadarn, GJ Wilson, SW Corley and PS Bird, 2014, unpublished). In each case, the results revealed a rich and diverse bacterial community in much higher numbers than identified using culture- and cloning-based studies. Therefore, in studies of animals with known oral health status, health and disease could be correlated with the oral microbiota detected using these new technologies.
We have shown that oral diseases such as gingivitis and periodontitis can be found in a range of native Australian animals including macropods, koalas (Phascolarctos cinereus), brushtail possums (Trichosurus vulpecular) and bandicoots (Isoodon macrourus), and that black-pigmented, anaerobic bacteria, belonging to the genera Porphyromonas and Prevotella, are part of the microbiota5. Earlier studies using culture dependent methods, showed that in the normal oral microbiota of macropods, Gram-negative anaerobes were poorly represented11. In contrast, Dent and co-workers12 reported that macropod oral cavities had a noticeable predominance of Gram-negative and Gram-positive rods with the facultative anaerobic Gram-negative rods comprising 40% of the cultivable organisms although no Bacteroides spp. were isolated. In a study of 10 species of kangaroos and wallabies, black-pigmented anaerobic bacteria comprised 21% of their normal oral microbiota13. Thus the early culture-based studies have shown variability in the microbes isolated and suggest that this approach has limitations.
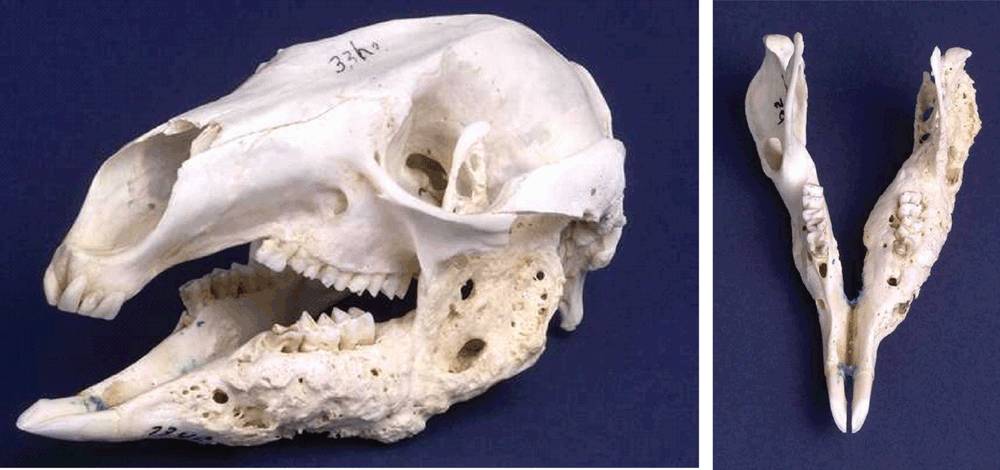
Koalas do present with severe periodontal disease4 and with severe loss of alveolar bone associated with age and conditions such as food impaction3 (Figure 1). In koalas <7 years old with good oral health, there was an absence of black-pigmented bacteria, compared to koalas >7 years of age, where 50% harboured black-pigmented bacteria, the majority of which were identified as Porphyromonas gingivalis-like14. Current work characterising this bacterium, shows the organism to be novel and that it may be associated with periodontal disease in marsupials [Bird et al, 2015, submitted]. Another intriguing question relates to the koala’s diet which consists of Eucalyptus leaves. How have the oral bacteria evolved in the presence of such a toxic diet (e.g. high in essential oils) and how does this affect the koala’s oral microbiome?

|
Oral necrobacillosis or lumpy jaw as it is commonly known, is a leading cause of mortality in captive macropods and has been reported in free-ranging macropods15. The disease progresses from plaque formation, gingivitis and periodontal disease to a necrotising, fatal osteomyelitis,16 (Figure 2) with all macropods susceptible, particularly Eastern grey kangaroos17. Early studies of macropods with lumpy jaw showed that Fusobacterium necrophorum was the most frequent isolate from lesions (81% prevalence) as well as the most abundant organism in mixed cultures. It also was isolated in high abundance from gingival margin samples taken from sites remote from the lumpy jaw lesions in 61% of the animals with disease18. While the principle infective agent in Australia appears to be F. necrophorum, other organisms appear to play a role in this disease. Recent work has shown that F. necrophorum sub-species necrophorum is associated with organisms resembling Porphyromonas gulae in lumpy jaw in macropods in South Australian zoos19. Porphyromonas organisms distinct from both P. gingivalis and P. gulae have been proposed as a novel species14 and were isolated with increasing frequency from the oral cavity of macropods in our studies, which warrants further evaluation into the role of this newly described organism in jaw disease.
A study of the tammar wallaby pouch young suggested factors that protect young animals against potentially pathogenic microbial infections could include the microbiome from the maternal saliva20. The microbiomes of the pouch and saliva from the mother were compared with the gastrointestinal tract (GIT) microbiome of the pouch young using 16S rRNA gene comparative methods. Each study site had a unique microbiome20. The maternal pouch harboured 41 unique Actinobacteria phylotypes, while in the saliva there were 48 unique Proteobacteria phylotypes. The GIT of the pouch young had a complex microbiome of 53 unique phylotypes and of these, only nine were detected at either maternal site. Overall, the majority of bacteria detected were novel species and each study site possessed its own unique microbiome20.
In conclusion, a number of culture-based studies on the oral microbiota of marsupials were conducted some 20–30 years ago. Now with the deep sequencing culture-independent methods it will be possible to detect novel bacterial species in their oral cavities and these are likely to have unique properties. In addition, new studies will undoubtedly lead to insights into their evolution, diversity and ecological role. We can speculate that co-evolution of the marsupial oral microbiome has occurred with its host and organisms such as the newly identified porphyromonad unique to marsupials may represent an ancestral lineage distinct from P. gulae and P. gingivalis. The oral microbiome of our marsupials has received little attention and therefore definitely warrants more thorough exploration by keen and dedicated microbiologists for novel bacteria and associated oral diseases.
References
[1] Tsang, K.L. et al. (2005) Caries and periodontal disease: two diseases, one biofilm. Microbiol. Aust. 23, 110–112.[2] Marsh, P.D. (2003) Are dental diseases examples of ecological catastrophes? Microbiology 149, 279–294.
| Are dental diseases examples of ecological catastrophes?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhvFSru7o%3D&md5=62161652f1d50a63cc61cdfd55fc0b9cCAS | 12624191PubMed |
[3] Lee, E.F. et al. (2011) Loss of tooth-supporting bone in the koala (Phascolarctos cinereus) with age. Aust. J. Zool. 59, 49–53.
| Loss of tooth-supporting bone in the koala (Phascolarctos cinereus) with age.Crossref | GoogleScholarGoogle Scholar |
[4] Pettett, L.M. et al. (2012) The development of an oral health charting system for koalas (Phascolarctos cinereus). J. Vet. Dent. 29, 232–241.
| 23505786PubMed |
[5] Bird, P.S. et al. (2002) Oral disease in animals: the Australian perspective. Isolation and characterisation of black-pigmented bacteria from the oral cavity of marsupials. Anaerobe 8, 79–87.
| Oral disease in animals: the Australian perspective. Isolation and characterisation of black-pigmented bacteria from the oral cavity of marsupials.Crossref | GoogleScholarGoogle Scholar |
[6] Pérez-Chaparro, P.J. et al. (2014) Newly identified pathogens associated with periodontitis: A systematic review. J. Dent. Res. 93, 846–858.
| Newly identified pathogens associated with periodontitis: A systematic review.Crossref | GoogleScholarGoogle Scholar | 25074492PubMed |
[7] Sturgeon, A. et al. (2014) Characterization of the oral microbiota of healthy cats using next-generation sequencing. Vet. J. 201, 223–229.
| Characterization of the oral microbiota of healthy cats using next-generation sequencing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXltFCgsbY%3D&md5=6c6005f83360b2965ca9b58429316390CAS | 24680670PubMed |
[8] Sturgeon, A. et al. (2013) Metagenomic analysis of the canine oral cavity as revealed by high-throughput pyrosequencing of the 16S rRNA gene. Vet. Microbiol. 162, 891–898.
| Metagenomic analysis of the canine oral cavity as revealed by high-throughput pyrosequencing of the 16S rRNA gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVWit73K&md5=59eadc3f435e630c30232093eec4849cCAS | 23228621PubMed |
[9] Dewhirst, F.E. et al. (2010) The human oral microbiome. J. Bacteriol. 192, 5002–5017.
| The human oral microbiome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFCitbrP&md5=c33667927bc198989c9485de67a54bcfCAS | 20656903PubMed |
[10] Antiabong, J.F. et al. (2013) The oral microbial community of gingivitis and lumpy jaw in captive macropods. Res. Vet. Sci. 95, 996–1005.
| The oral microbial community of gingivitis and lumpy jaw in captive macropods.Crossref | GoogleScholarGoogle Scholar | 24012349PubMed |
[11] Beighton, D. and Miller, W.A. (1977) A microbiological study of normal flora of macropod dental plaque. J. Dent. Res. 56, 995–1000.
| A microbiological study of normal flora of macropod dental plaque.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1c%2Fks1Ciug%3D%3D&md5=dbe398f65c0595e9495b847a3166de25CAS | 270499PubMed |
[12] Dent, V.E. (1979) The bacteriology of dental plaque from a variety of zoo-maintained mammalian species. Arch. Oral Biol. 24, 277–282.
| The bacteriology of dental plaque from a variety of zoo-maintained mammalian species.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3c%2FmsVCqug%3D%3D&md5=d9787c863ef6ebc7e4a5cc48063962ecCAS | 116634PubMed |
[13] Samuel, J.L. (1982) The normal flora of the mouths of macropods (Marsupialia: macropodidae). Arch. Oral Biol. 27, 141–146.
| The normal flora of the mouths of macropods (Marsupialia: macropodidae).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL383gsFCqsA%3D%3D&md5=166eb0df3d6d12bea711f62d98e6109bCAS | 7044349PubMed |
[14] Mikkelsen, D. et al. (2008) Phylogenetic analysis of Porphyromonas species isolated from the oral cavity of Australian marsupials. Environ. Microbiol. 10, 2425–2432.
| Phylogenetic analysis of Porphyromonas species isolated from the oral cavity of Australian marsupials.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFSksLjO&md5=1a30db8bef0e7a9d32e82a785982ca40CAS | 18564186PubMed |
[15] Borland, D. et al. (2012) Oral necrobacillosis (‘lumpy jaw’) in a free-ranging population of eastern grey kangaroos (Macropus giganteus) in Victoria. Aust. Mammal. 34, 29–35.
| Oral necrobacillosis (‘lumpy jaw’) in a free-ranging population of eastern grey kangaroos (Macropus giganteus) in Victoria.Crossref | GoogleScholarGoogle Scholar |
[16] Bakal-Weiss, M. et al. (2010) Use of a sustained release chlorhexidine varnish as treatment of oral necrobacillosis in Macropus spp. J. Zoo Wildl. Med. 41, 371–373.
| Use of a sustained release chlorhexidine varnish as treatment of oral necrobacillosis in Macropus spp.Crossref | GoogleScholarGoogle Scholar | 20597238PubMed |
[17] Butler, R. (1981) Epidemiology and management of ‘lumpy jaw’ in macropods. In Fourth International Conference on the Wildlife Diseases Association. Sydney, Australia.
[18] Samuel, J.L. (1983) Jaw disease in macropod marsupials: bacterial flora isolated from lesions and from the mouths of affected animals. Vet. Microbiol. 8, 373–387.
| Jaw disease in macropod marsupials: bacterial flora isolated from lesions and from the mouths of affected animals.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c%2Fks1KhtA%3D%3D&md5=70ff1b22abfa4866af3663f45548fa09CAS | 6636509PubMed |
[19] Antiabong, J.F. et al. (2013) A molecular survey of a captive wallaby population for periodontopathogens and the co-incidence of Fusobacterium necrophorum subspecies necrophorum with periodontal diseases. Vet. Microbiol. 163, 335–343.
| A molecular survey of a captive wallaby population for periodontopathogens and the co-incidence of Fusobacterium necrophorum subspecies necrophorum with periodontal diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXislemsLg%3D&md5=fd667febc0530038a60ad66a79480a29CAS | 23428381PubMed |
[20] Chhour, K.L. et al. (2010) An observational study of the microbiome of the maternal pouch and saliva of the tammar wallaby, Macropus eugenii, and of the gastrointestinal tract of the pouch young. Microbiology 156, 798–808.
| An observational study of the microbiome of the maternal pouch and saliva of the tammar wallaby, Macropus eugenii, and of the gastrointestinal tract of the pouch young.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktFOksb8%3D&md5=7ab3405474734a22356c03856756cbbcCAS | 19833775PubMed |
Biographies
Dr Philip Bird is an Adjunct Associate Professor at the School of Veterinary Science. His research interests are the oral microbiology of human and animals, especially that of native Australian animals.
Dr Wayne Boardman is Senior Lecturer, at the School of Animal and Veterinary Sciences, University of Adelaide. An experienced wildlife veterinarian, he is a diplomate of the European College of Zoological Medicine in Wildlife Population Health.
Dr Darren Trott is Associate Professor of Veterinary Microbiology at The University of Adelaide. His research interests cover comparative aspects of antimicrobial resistance in animals and humans, new drug development and gastrointestinal microbial ecology.
Linda L Blackall is a microbial ecologist who has studied many different complex microbial communities ranging from host associated through to free living in numerous environments. Her research has covered mammalian microbiomes spanning marsupials, humans, ruminants and horses and the methods used allow elucidation of massive microbial complexity and function in these diverse biomes. She is a Professor of Biosciences at Swinburne University of Technology in the Faculty of Science, Engineering and Technology.