Protecting the heart after HAART; understanding the pathogenesis of cardiovascular disease in people living with HIV
Janine M Trevillyan A and Jennifer F Hoy A BA Department of Infectious Diseases
Alfred Hospital and Monash University
Commercial Road
Melbourne, Vic. 3000, Australia
B Department of Infectious Diseases
Alfred Hospital and Monash University
Commercial Road
Melbourne, Vic. 3000, Australia
Tel: +61 3 9076 6900
Fax: +61 3 90762431
Email: jennifer.hoy@monash.edu
Microbiology Australia 35(2) 91-93 https://doi.org/10.1071/MA14027
Published: 28 April 2014
Advances in the management of HIV and antiretroviral therapy (ART) have led to substantial improvements in disease-free survival for patients with HIV. Life-expectancy is approaching that of the general population. Yet these gains have been tempered by increasing rates of non-AIDS-related co-morbidities. In fact the major burden of illness, health care utilisation and premature death in HIV positive patients is now due to diseases of ageing. Cardiovascular disease (CVD) occurs at two times the rate in the general population and is a cause of significant morbidity and mortality. Lifestyle factors such as cigarette smoking and underlying genetics are clearly important. Yet in HIV patients CVD is also promoted by complex interactions between HIV and ART driven coagulation, dyslipidaemia, inflammation and immune dysfunction. Understanding the pathogenesis of CVD in HIV will be of increasing importance as the HIV population ages. This will enable targeted prevention strategies and personalised antiretroviral regimens to be utilised. Some of the recent advances in the field are discussed in this review.
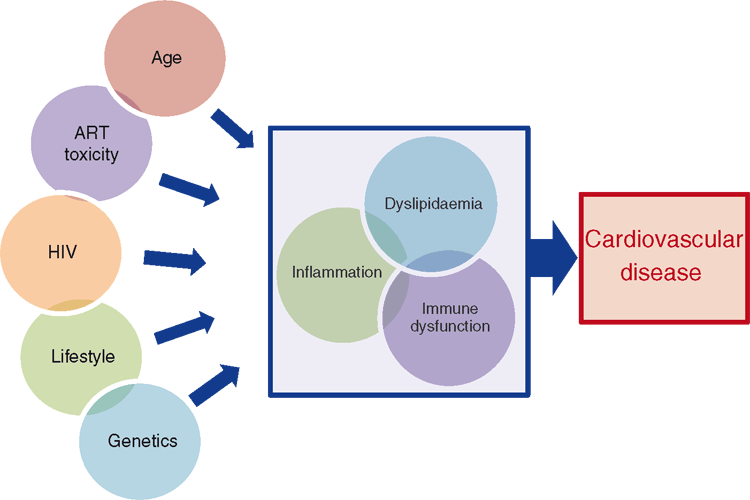
It is important to highlight that the contribution of HIV specific factors (such as immune deregulation and ART) on cardiovascular risk is relatively small when compared with those of traditional risk factors such as age, family history and cigarette smoking (Figure 1). In fact smoking is responsible for over half of the total CVD risk in HIV. This is partly because HIV positive patients are 2–3 times more likely to smoke than age matched controls; in some cohorts up to 59% of patients smoke1. There may also be a synergistic effect between HIV and smoking related endothelial inflammation which could contribute to atherosclerosis. While eliciting behaviour change is difficult, there is growing evidence that improved education and a sustained focus on smoking cessation can lower smoking rates and improve patient outcomes2.
As mentioned above, inflammation is a key component in the pathogenesis of atherosclerosis and acute cardiovascular events. Metabolic derangements and inflammation have complex and likely bidirectional relationships. It is known that lipids are more likely to infiltrate the arterial wall in the setting of inflammatory stimuli. There they activate endothelial cells to increase expression of adhesion molecules and recruit further inflammatory cells, in particular T-cells. Ultimately this cycle results in unstable, chronically inflamed atherosclerotic plaques3. HIV positive patients in general have higher baseline levels of inflammatory biomarkers (such as high sensitivity C-reactive protein and interleukin-6) which have been associated with increased rates of cardiovascular events and all-cause mortality4. Immune dysregulation also plays an important role in the progression of HIV related CVD. There is evidence that increased levels of T cell activation predict subclinical carotid artery disease5. Research performed in Melbourne has demonstrated that a change in expression of monocyte surface markers CX3CR1 (a chemokine receptor important in cell signalling) and CD11b (an integrin involved in adhesion to the endothelial wall) are associated with subclinical atherosclerosis6. And while cardiovascular risk does decrease with successful ART it does not return to the levels seen in the general population. This may be partly explained by the finding that chronic inflammation and immune activation associated with HIV infection persists even in the setting of viral suppression7. The pro-atherogenic, and possibly pro-thrombotic, side effects of some antiretroviral agents are clearly also contributing.
The Data collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study provided the first evidence of an increased risk of CVD associated with ART; demonstrating a 26% increased rate of acute myocardial infarction per year of ART use8. The cohort has since gone on to describe that the degree of risk differs according to type of antiretroviral, in particular increased exposure to some protease inhibitors (e.g. indinavir and ritonavir-boosted lopinavir) and current abacavir use have been implicated9. While the relationship between abacavir and CVD has been hotly debated in the literature, a case-control study performed at our institution also identified an association between current abacavir use and symptomatic coronary artery disease10. Our group and others are currently attempting to delineate a pathogenic link between the two, and there is early evidence that increasing platelet activation may be involved11.
A significant portion of the elevated cardiovascular risk in HIV patients can be explained by viral and antiretroviral-associated dyslipidaemia. Australian research has demonstrated that HIV impairs reverse cholesterol transport from macrophages by modifying high density lipoprotein (HDL) cholesterol metabolism and redirecting cholesterol to apoB-containing lipoproteins12. In the presence of HIV macrophages can thus accumulate substantial amounts of lipid and come to resemble foam cells, a classic component of atherosclerotic plaques. Untreated HIV is commonly associated with low total-, HDL and low-density lipoprotein- (LDL) cholesterol along with elevated serum triglycerides. Initiation of ART can lead to an increase in total and LDL cholesterol (at times to well above the normal range) without a corresponding rise in HDL13. The degree of dyslipidaemia is determined in part by the antiretroviral regimen chosen. Lipid profiles consisting of high LDL and low HDL have been shown to be on the causal pathway towards cardiovascular events in the general population.
The optimal management of ART and HIV associated dyslipidaemia is not yet clear with a number of strategies currently being investigated including switching within or between antiretroviral classes to avoid agents most associated with dyslipidaemia, versus adding a lipid modifying agent such as HMG-CoA reductase inhibitors (statins), niacins or fish oils. Statins in particular provide an attractive option in HIV given their dual lipid lowering and anti-inflammatory properties but further work is needed to guide their optimal use.
Conclusion
CVD is a significant cause of morbidity and mortality in HIV positive patients in the modern era. Ongoing work to understand the pathogenesis of CVD must remain a key priority so that recent gains in the health and life-expectancy of patients living with HIV can be maintained.
References
[1] Calvo-Sánchez, M. et al. (2013) Differences between HIV-infected and uninfected adults in the contributions of smoking, diabetes and hypertension to acute coronary syndrome: two parallel case-control studies. HIV Med. 14, 40–48.| Differences between HIV-infected and uninfected adults in the contributions of smoking, diabetes and hypertension to acute coronary syndrome: two parallel case-control studies.Crossref | GoogleScholarGoogle Scholar | 23088307PubMed |
[2] Gritz, E.R. et al. (2013) Long-term outcomes of a cell phone-delivered intervention for smokers living with HIV/AIDS. Clin. Infect. Dis. 57, 608–615.
| Long-term outcomes of a cell phone-delivered intervention for smokers living with HIV/AIDS.Crossref | GoogleScholarGoogle Scholar | 23704120PubMed |
[3] Lo, J. and Plutzky, J. (2012) The biology of atherosclerosis: general paradigms and distinct pathogenic mechanisms among HIV-infected patients. J. Infect. Dis. 205, S368–S374.
| The biology of atherosclerosis: general paradigms and distinct pathogenic mechanisms among HIV-infected patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xnt1egsrs%3D&md5=f0c9ef0029d29a5571be0fed0bc74c61CAS | 22577210PubMed |
[4] Kuller, L.H. et al. (2008) Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 5, e203.
| Inflammatory and coagulation biomarkers and mortality in patients with HIV infection.Crossref | GoogleScholarGoogle Scholar | 18942885PubMed |
[5] Kaplan, R.C. et al. (2011) T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J. Infect. Dis. 203, 452–463.
| T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVegur8%3D&md5=427178b2b11260e1653313ec6b721f02CAS | 21220772PubMed |
[6] Westhorpe, C.L. et al. (2013) Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol. Cell Biol. 92, 133–138.
| 24296810PubMed |
[7] Neuhaus, J. et al. (2010) Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J. Infect. Dis. 201, 1788–1795.
| Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXovVKht7Y%3D&md5=20d59d7b39dc66a1ee79b8474fb03fc2CAS | 20446848PubMed |
[8] Friis-Moller, N. et al. (2003) Combination antiretroviral therapy and the risk of myocardial infarction. N. Engl. J. Med. 349, 1993–2003.
| Combination antiretroviral therapy and the risk of myocardial infarction.Crossref | GoogleScholarGoogle Scholar | 14627784PubMed |
[9] Worm, S.W. et al. (2010) Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J. Infect. Dis. 201, 318–330.
| Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFGqtLY%3D&md5=817f7428afd82acba75f62843f0fdaedCAS | 20039804PubMed |
[10] Trevillyan, J.M. et al. (2013) Abacavir exposure and cardiovascular risk factors in HIV-positive patients with coronary heart disease: a retrospective case-control study. Sex. Health 10, 97–101.
| Abacavir exposure and cardiovascular risk factors in HIV-positive patients with coronary heart disease: a retrospective case-control study.Crossref | GoogleScholarGoogle Scholar | 23256968PubMed |
[11] Falcinelli, E. et al. (2013) In vivo platelet activation and platelet hyperreactivity in abacavir-treated HIV-infected patients. Thromb. Haemost. 110, 349–357.
| In vivo platelet activation and platelet hyperreactivity in abacavir-treated HIV-infected patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlCrsrnM&md5=176ce96c5d654623979db8a37a172a38CAS | 23703656PubMed |
[12] Rose, H. et al. (2008) HIV infection and high density lipoprotein metabolism. Atherosclerosis 199, 79–86.
| HIV infection and high density lipoprotein metabolism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXns1Sntro%3D&md5=4c8b2a124dab3e959a2e98501eea98caCAS | 18054941PubMed |
[13] Penzak, S.R. and Chuck, S.K. (2000) Hyperlipidemia associated with HIV protease inhibitor use: pathophysiology, prevalence, risk factors and treatment. Scand. J. Infect. Dis. 32, 111–123.
| Hyperlipidemia associated with HIV protease inhibitor use: pathophysiology, prevalence, risk factors and treatment.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3cvktVKnsQ%3D%3D&md5=d4941f52ed0a59e4d92d37c019d85558CAS | 10826894PubMed |
Biographies
Jennifer Hoy is Professor Director of HIV Medicine at the Alfred Hospital and Monash University. She has over 25 years experience in both research and care in HIV infection. Her main research interest is in non-AIDS morbidity, including the pathogenesis of cardiovascular disease in HIV, dissecting the roles of traditional risk factors, HIV and chronic inflammation as well as the role of antiretroviral therapy.
Dr Janine Trevillyan is an Infectious Diseases Physician at the Alfred Hospital, Melbourne. She is currently completing a PhD with Monash University investigating the pathogenesis and prevention of cardiovascular disease in people living with HIV.