Movement of resistance genes in hospitals
Sally R Partridge Centre for Infectious Diseases and Microbiology
University of Sydney
Westmead Millennium Institute
Westmead Hospital
Westmead, NSW 2145, Australia
Tel: +61 2 9845 5246
Fax: +61 2 9891 5317
Email: sally.partridge@health.nsw.gov.au
Microbiology Australia 35(1) 60-62 https://doi.org/10.1071/MA14017
Published: 4 February 2014
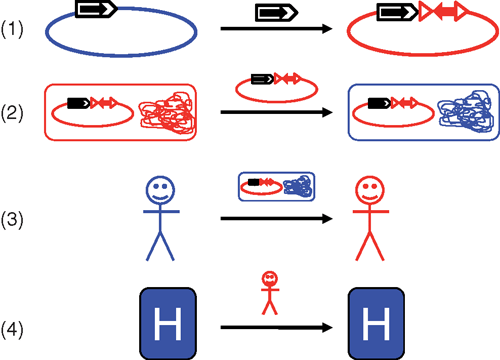
Enterobactericeae resistant to multiple antibiotics are an increasing global health problem that impacts treatment and survival of hospitalised patients. In these organisms much of the antibiotic resistance is due to a wide variety of ‘mobile’ resistance genes that have been captured from the chromosomes of different bacterial species and transferred to plasmids by the actions of various mobile genetic elements. These plasmids can then spread between bacterial cells, including different species. The association of resistance genes with mobile elements, these mobile elements with plasmids and plasmids with particular bacterial strains means that spread of resistance genes can occur at several different levels (Figure 1). Understanding more about the contributions of these different processes and how they interact may enable better prediction and control of the spread of resistance.
|
Several different types of mobile elements are able to capture genes (which may confer antibiotic resistance) from various sources and transfer them to plasmids, enabling spread into other species including pathogenic bacteria. As gene capture events are rare, each particular resistance gene is usually associated with a particular mobile element; these elements have different characteristics but some features are common (Figure 2)1. Insertion sequences (IS) consist of little more than a transposase (tnpA) gene flanked by inverted repeats (IR). These IR are recognised and processed by the encoded transposase protein resulting in copying or transfer of the IS to a new location (transposition). Some unusual IS (ISEcp1, ISCR elements) can capture and move adjacent DNA when present as a single copy, but for most IS two copies flanking a resistance gene are needed to form a composite transposon that moves as a single unit. Transposons (Tn) usually have an additional resolvase (tnpR) gene and can carry resistance genes internally. Tn and most IS create direct repeats (DR) of characteristic length on insertion and these are useful signatures for understanding relationships between different resistance regions and plasmids. Integrons (In) capture resistance genes packaged as gene cassettes by site-specific recombination between the integron attI site and the cassette attC site, catalysed by an IntI integrase encoded in the integron. These mobile genetic elements often provide a promoter for expression of an associated resistance gene and can act as mobile regions of homology, allowing additional movement of resistance genes by recombination. The association of resistance genes with mobile elements provides the first level of movement of these genes, allowing transfer onto and between plasmids.
The ‘backbones’ of these plasmids carries genes for essential plasmid functions such as replication, partitioning and stability, some of which are involved in determining the ‘host range’ of the plasmid, that is, which species (and maybe which strains) it can transfer to and be maintained in. A mobile element/resistance gene combination inserted into this backbone in a location that does not disrupt plasmid integrity can act as a site for further insertions, so that resistance genes and associated mobile genetic elements tend to become clustered in complex multiresistance regions (Figure 2). Conjugative plasmids encode the machinery necessary for their own movement between cells and tend to be large (up to ~200 kb), while mobilisable plasmids, which may be much smaller, lack conjugation genes and can only move between bacterial cells if ‘helped’ by a conjugative plasmid2. This transfer of plasmids carrying resistance genes between bacterial cells creates a second level of movement of resistance and multiresistance.
While the mechanisms by which different mobile elements move and transfer resistance genes between DNA molecules and by which plasmids move between cells have been well studied and the processes demonstrated experimentally, less is known about how these processes operate in the ‘real world’. A few studies illustrate movement of resistance genes between plasmids and plasmids between isolates in a clinical setting. For example, the blaKPC-2 (‘Klebsiella pneumoniae carbapenemase’) gene was identified in two different plasmid types in the same isolate as part of the same transposon Tn44013, implying movement of this transposon. Transfer of a plasmid carrying resistance genes between species in the same patient has also been documented, for example, a plasmid carrying the blaIMP-4 carbapenemase gene plus other resistance genes was identified first in a Serratia isolate and subsequently in an Escherichia coli isolate from the same patient4.
A third level of movement is transfer of bacteria between patients, which could occur via the hospital environment, medical instruments or by carers interacting with multiple patients. Recently, whole genome sequencing has been used to track the spread of bacterial isolates carrying important resistance genes during outbreaks. Single nucleotide polymorphisms (SNP) were used to follow the course of an outbreak of K. pneumoniae ST258 (CC292) isolates carrying the blaKPC-3 gene in the USA5. A similar approach was used in Australia to examine a neonatal outbreak of E. coli, which was identified as ST131 carrying the blaCTX-M-15 gene that confers resistance to third generation cephalosporins6. Generating complete plasmid sequences from this type of data is more difficult than identifying resistance genes and SNPs, due to long repeats (often mobile elements) that complicate assembly, but would be useful in identifying which resistance genes are travelling together and which plasmid type carries each set of genes.
A fourth level of movement is transfer of patients carrying resistance genes between different healthcare facilities. A simulation using hospital admission data from England demonstrated that connections between hospitals due to patient transfers can have a profound effect on the epidemic behaviour of high-risk clones, with formation of specialist centres with very large catchment areas potentially greatly increasing the spread of resistance7. There are also many examples of the introduction of bacteria carrying different resistance genes by hospitalisation of patients who have been treated overseas8 and certain bacterial strains and/or plasmids appear to be very successful at spreading globally9. For example, blaKPC genes are found in strains belonging to K. pneumoniae clonal complex (CC) 292 in many countries. Similarly, E. coli multilocus sequence type (ST) 131 is a successful clone often associated with the globally dominant blaCTX-M-15 gene that confers resistance to third-generation cephalosporins. Closely related plasmids have been found in isolates of the same ST from different locations10, suggesting that particular plasmid types may be linked to particular bacterial clones.
Tracking, understanding and trying to limit the spread of antibiotic resistance in hospitals is clearly a complex problem. Currently, identifying and isolating patients carrying problematic resistance genes and other interventions that prevent the spread of bacterial strains between patients seem most achievable. More extensive screening to detect particular combinations of resistance genes and selected plasmid and/or strain markers may allow identification of particularly troublesome plasmids and/or strains and indicate most important level(s) of movement for different resistance genes. This should inform isolation and infection control policies, as well as providing sets of samples that can be examined in more detail to try and understand how the genetic context of a resistance gene really influences its spread.
References
[1] Partridge, S.R. (2011) Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiol. Rev. 35, 820–855.| Analysis of antibiotic resistance regions in Gram-negative bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVyjsr7J&md5=c2b67eb6eba2a77bfd0b80351a1eab5eCAS | 21564142PubMed |
[2] Smillie, C. et al. (2010) Mobility of plasmids. Microbiol. Mol. Biol. Rev. 74, 434–452.
| Mobility of plasmids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVSqurnP&md5=eba040bcddf2362d7f30641bbc2cdac5CAS | 20805406PubMed |
[3] Gootz, T.D. et al. (2009) Genetic organization of transposase regions surrounding bla KPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob. Agents Chemother. 53, 1998–2004.
| Genetic organization of transposase regions surrounding bla KPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlvVGgurw%3D&md5=09583f9a04d1e26a84d94d5775860b6dCAS | 19258268PubMed |
[4] van Hal, S.J. et al. (2009) Immediate appearance of plasmid-mediated resistance to multiple antibiotics upon antibiotic selection: an argument for systematic resistance epidemiology. J. Clin. Microbiol. 47, 2325–2327.
| Immediate appearance of plasmid-mediated resistance to multiple antibiotics upon antibiotic selection: an argument for systematic resistance epidemiology.Crossref | GoogleScholarGoogle Scholar | 19420178PubMed |
[5] Snitkin, E.S. et al. (2012) Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 4, 148ra116.
| Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing.Crossref | GoogleScholarGoogle Scholar | 22914622PubMed |
[6] Sherry, N.L. et al. (2013) Outbreak investigation using high-throughput genome sequencing within a diagnostic microbiology laboratory. J. Clin. Microbiol. 51, 1396–1401.
| Outbreak investigation using high-throughput genome sequencing within a diagnostic microbiology laboratory.Crossref | GoogleScholarGoogle Scholar | 23408689PubMed |
[7] Donker, T. et al. (2014) Dispersal of antibiotic-resistant high-risk clones by hospital networks: changing the patient direction can make all the difference. J. Hosp. Infect. 86, 34–41.
| Dispersal of antibiotic-resistant high-risk clones by hospital networks: changing the patient direction can make all the difference.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2c%2FhvFOisw%3D%3D&md5=75a4a04e77f06ed35ac715678c0ee9bdCAS | 24075292PubMed |
[8] van der Bij, A.K. and Pitout, J.D. (2012) The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae. J. Antimicrob. Chemother. 67, 2090–2100.
| The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1egt7%2FJ&md5=48b32590152883addc21f89220c3c075CAS | 22678728PubMed |
[9] Woodford, N. et al. (2011) Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 35, 736–755.
| Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVyjsr7N&md5=e44166a71d452acdad2d3fd0be37f26cCAS | 21303394PubMed |
[10] Partridge, S.R. et al. (2011) Recombination in IS26 and Tn2 in the evolution of multi-resistance regions carrying bla CTX-M-15 on conjugative IncF plasmids from Escherichia coli. Antimicrob. Agents Chemother. 55, 4971–4978.
| Recombination in IS26 and Tn2 in the evolution of multi-resistance regions carrying bla CTX-M-15 on conjugative IncF plasmids from Escherichia coli.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVSru7rL&md5=541019d79437a7f4e74d3e962bb4777bCAS | 21859935PubMed |
Biography
Dr Sally Partridge is a Senior Research Fellow who has been working on the genetics of mobile antibiotic resistance in Gram-negative bacteria since 1997, first at CSIRO and Macquarie University and from 2005 at the Centre for Infectious Diseases and Microbiology, The University of Sydney, and the Westmead Millennium Institute, Westmead Hospital.



