ASM NZ Postgraduate Research Travel Award, 2012
Jayne ManningMicrobiology Australia 34(2) 114-115 https://doi.org/10.1071/MA13039
Published: 13 May 2013
Thank you for the great opportunity to attend my first international scientific meeting, the New Zealand Society for Microbiology 2012 conference, and for the chance to visit the laboratory of my collaborator Professor John Tagg, co-discoverer of the probiotic bacterial strain that is the focus of my PhD project.
My time in New Zealand was spent at The University of Otago in Dunedin, where I presented a poster at the 4 day conference and was then based in the laboratories of BLIS Technologies Ltd, located in the Centre of Innovation at the University, for 8 days.
BLIS Technologies Ltd, of which Professor Tagg is a principal scientific consultant, holds the rights to unique collection of bacteriocin-like inhibitory substance (BLIS)-producing bacteria, including Streptococcus salivarius K12, which was originally isolated by Professor Tagg and colleagues at the University’s Department of Microbiology and Immunology. BLIS Technologies Ltd produce K12 in the form of a probiotic throat lozenge aimed to prevent pharyngitis and halitosis (bad breath), available commercially for more than a decade.
Together, The University of Otago and BLIS Technologies Ltd have published numerous studies investigating and characterising BLIS produced by various bacteria. Their findings show strain K12 has the ability to inhibit pathogenic respiratory bacteria such as Streptococcus pyogenes via several megaplasmid-encoded bacteriocins (salivaricins). Fellow commercial probiotic S. salivarius strain M18 also harbours megaplasmid-encoded salivaricins. In our laboratory K12 has been shown to inhibit colonisation of epithelial cells by Streptococcus pneumoniae (the pneumococcus) in vitro. The primary focus of my PhD is to investigate the mechanism of this inhibition. Experiments performed in the BLIS laboratories allowed me to investigate whether these S. salivarius megaplasmids are required for production of BLIS active against pneumococci. I became competent in the technique of deferred antagonism testing, analysing the BLIS activity of 10 Streptococcus salivarius strains against pneumococcal isolates shipped to New Zealand from our laboratory for this purpose.
My results showed S. salivarius strains harbouring known salivaricins all produced BLIS capable of inhibiting the growth of all pneumococcal isolates tested, suggesting salivaricins are required for in vitro BLIS activity against pneumococci.
K12 and M18 megaplasmid-negative strains did not produce BLIS activity against pneumococci (Fig. 1), suggesting these megaplasmids mediate BLIS production in these strains, likely due to their encoded salivaricins.
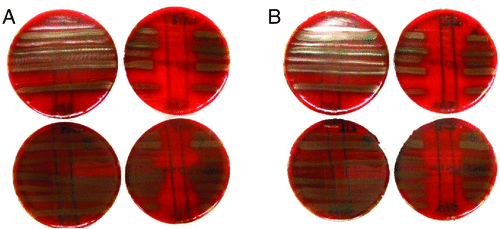
|
Furthermore, K12 strains harbouring only salivaricin A2 (A234) or salivaricin B (NR) were tested to investigate the specificity of the K12 salivaricins. While each salivaricin was capable of producing BLIS activity against all pneumococcal strains tested, salivaricin B showed significantly stronger inhibition (data not shown).
Overall, these results add to already published evidence that S. salivarius bacteriocins are important for the in vitro inhibition of respiratory pathogens, and suggest that for strains K12 and M18 in vitro pneumococcal inhibition is megaplasmid-mediated. I am excited to observe if these megaplasmids are also necessary to inhibit pneumococcal colonisation in the tissue culture pharyngeal cell adhesion model I am currently developing.
In addition to the laboratory-based experience and results I gained in New Zealand, I was able to practice my networking and presentation skills at both the conference and during a company presentation given at BLIS Technologies Ltd. I also had the opportunity to hear about a diverse range of interesting microbiology research while at the conference. Aside from Professor Tagg, I had the opportunity to meet with five scientists specifically interested in my field from several university departments and biotechnology companies including John Hale and Philip Wescombe from BLIS Technologies Ltd, Nick Heng from the Oral Sciences Department, Otago University Dental School and Dan Power from Life Technologies, who generously found time to meet and discuss my project, enlightening me to information outside of the scope of my current literature searches. I also gained valuable insight into the structure of a biotechnology company, extending my knowledge of possible post-doctoral career paths.
I thank Professor Tagg and the team at BLIS Technologies (especially John Hale and Philip Wescombe) for having me in their lab and sharing their wealth of S. salivarius knowledge, and I look forward to continuing our future collaborations. Finally, I thank the ASM once again for this much appreciated opportunity.
Biography
Jayne Manning is completing her PhD in the Pneumococcal Research Group at the Murdoch Childrens Research Institute.


