“Gone in the back legs”
Richard Malik A , Derek Spielman B and Jan Šlapeta BA Centre for Veterinary Education
The University of Sydney,
NSW 2006, Australia
B Faculty of Veterinary Science,
The University of Sydney,
NSW 2006, Australia
Microbiology Australia 34(1) 8-12 https://doi.org/10.1071/MA13004
Published: 20 March 2013
Neural angiostrongyliasis and neosporosis are the two most common infectious causes of spinal cord disease in young dogs. The former is caused by migration of Angiostrongylus cantonensis (rat lungworm) larvae following ingestion of mollusc intermediate hosts, while the latter is caused by the apicomplexan protozoan Neospora caninum, acquired transplacentally, during parturition or in the neonatal period. This article gives the reader the perspective of a veterinarian confronted with the diagnosis and treatment of these two potentially life-threatening infections, taking into account differences in epidemiology and pathogenesis, and listing diagnostic tests available and affordable for most owners. The broader implications of these infections for other species, including people and wildlife, are discussed.
Most readers of Microbiology Australia are scientists. In this article, we want you to leave the comfort zone of the laboratory and embrace the world of companion animal medicine. An old proverb says: to understand a man, you’ve got to walk a mile in his shoes, whether they fit or not. So metaphorically, we ask you to “wear” the shoes of a suburban veterinarian asked to look at a young dog who has “gone in the back legs”, as many owners would put it. First we need to give you the rules of engagement, because although a profession, veterinary science is also a small business where owners expect an accurate diagnosis and a successful cost-effective solution. Good outcomes are expected even if the patient is seen at nights or at weekends, when laboratories do not offer a comprehensive service.
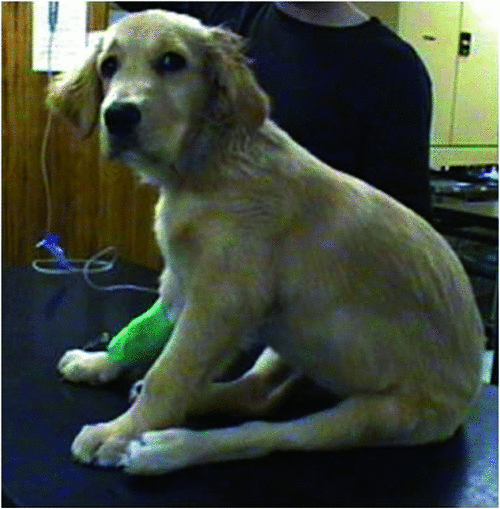
The dog in question is a 12-week-old Golden Retriever called “Honey” normally domiciled in Bellevue Hill (Fig. 1). She has a history of progressive hind limb dysfunction over several days. The onset was sudden, without evidence of trauma. Neurologic testing shows poor proprioceptive ability in the hind limbs, weakness that seems to be lower motor neuron (LMN) in type with poor tail carriage, urinary and faecal incontinence and marked hyperesthesia over the tail base and adjacent spine. This unusual combination of signs is most suggestive of a disease affecting the spinal cord (especially the part subserving the back end of the dog), or the corresponding nerves and/or nerve roots innervating the legs, tail and rear-quarters. Hyperaesthesia suggests meningeal inflammation. Not many diseases produce this combination of signs and common diseases such as tick paralysis (due to the toxin of Ixodes holocyclus) and traumatic vertebral injury do not fit the clinical picture (trust us, we are vets!). Congenital vertebral anomalies are rare in this breed and vertebral or spinal cord neoplasia is extremely rare in young dogs. So, the two most likely possibilities are neural angiostronglyosis (NA)1–5 and neosporosis6–11, two parasitic diseases which seem disproportionately common in eastern Australia compared to the rest of the world.
|
Now if a young child was presented with these signs, the investigation would likely involve a neurological consultation, perhaps an infectious diseases consultation, magnetic resonance (MR) imaging of the spinal cord and brain, electrodiagnostic tests (needle electromyography and nerve conduction studies), collection and analysis of cerebrospinal fluid (CSF), blood cultures and various serological tests12–14. Such a “work up” is possible for canine patients but many owners are unwilling to pay thousands of dollars to cover the cost of investigations. Unfortunately, Medicare doesn’t cover dogs and most people don’t take out pet health insurance. Welcome to the world of your local vet! To better understand the most likely diagnostic possibilities, to help decide which tests are most efficient, let’s consider the epidemiology and pathogenesis of these infections.
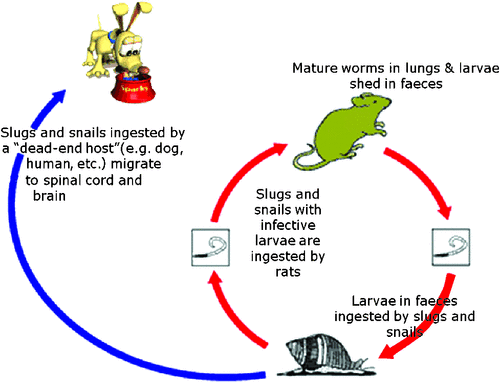
Neural angiostronglyosis is a disease caused by migration of larvae of the rat lungworm Angiostrongylus cantonensis1–5. It generally occurs after a dog, typically (but not invariably) a young dog, ingests slugs or snails (Fig. 2). Anecdotal evidence from Sydney and Brisbane indicates infection of non-native rats (Norwegian and black rats) with this parasite is widespread15,16. Accordingly, mollusc intermediate hosts have a very high probability of harbouring infective larvae. Thus when taking a history, enquiring whether rats, snails and slugs are present in the dog’s environment is pertinent, as often owners will have seen the patient ingest molluscs. The most characteristic feature of these cases is the exaggerated pain response to palpation and manipulation of the tail base, hind limbs and spine5. The worst cases experience excruciating pain. Signs tend to ascend, so some dogs present for nuchal rigidity referable to neck pain from parasitic meningitis, while the hind limb weakness changes neurologically from a LMN type to a mixed upper motor neuron (UMN) and lower motor neuron paresis. Later, a variety of other neurologic abnormalities, including cranial nerve palsies, may develop5. The prognosis with treatment is usually favourable, although some cases are left with residual neural deficits which occasionally result in euthanasia1–3,5.
|
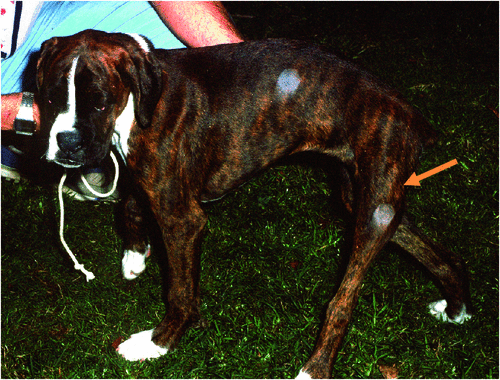
Neosporosis is a protozoan disease caused by Neospora caninum6–11, an apicomplexan closely related to Toxoplasma gondii (the cause of toxoplasmosis)11 and Sarcocystis neurona (the cause of equine protozoan myelitis, a disease of horses in North America)17,18. Neospora caninum has a very similar life cycle to T. gondii; however, the dog, coyote, wolf or dingo (all genetically the same species of canid) are definitive hosts for N. caninum, whereas the cat is the definitive host for T. gondii11 and the opossum for S. neurona17. Neosporosis is mostly seen in young dogs (typically less than 6 months) infected via their dams, most likely in late pregnancy, during parturition or in the early neonatal period during lactation6,7; the exact mechanism is not established. After a variable latent period, pups develop signs of an ascending radiculomyelopathy, initially manifest as LMN-type hind limb weakness. If this is not diagnosed in a timely manner, the spinal cord and nerve roots subserving the hind limbs are damaged irreparably, resulting in rigidity and contracture of the pelvic limbs (Fig. 3). Spinal hyperaesthesia is usually not a conspicuous feature, although some cases have myalgia and lameness due to concurrent protozoan myositis. Pups with neosporosis can be completely cured if therapy is started early; delayed therapy results in permanent and severe neurologic deficits.
|
What clinical features are most helpful in distinguishing neural angiostrongyliasis and neosporosis? Neosporosis appears to be more common in Boxers and Labradors than other breeds, early in the disease LMN features predominate (viz. reduced muscle tone and myotatic reflexes in the hind limbs) and hyperaesthesia is not prominent. In contrast, hyperaesthesia is the conspicuous feature of NA, and the neurologic picture changes from LMN to UMN as larvae migrate “up” the spinal cord towards the brain. In both diseases, multiple littermates can be affected. In NA this is because littermates may share an environment where rats and molluscs are abundant, whereas in neosporosis siblings are affected as infection is vertically transmitted6,7. Both diseases are more common in large breeds5,6. Time of presentation can be helpful for NA, as this condition tends to be more common in autumn5, although cases can occur in any month. Finally, geography can be an important consideration because to date NA has not been reported in Melbourne, Adelaide or Perth, although it occurs from Sydney all the way north along the coast of eastern Australia1–5,15,16.
Two tests are most helpful in differentiating between these infections. NA is accompanied by eosinophilic meningoencephalitis, so finding eosinophilic pleocytosis in CSF is strongly suggestive of this aetiology1–5. The diagnosis can be confirmed by demonstrating antibodies against A. cantonensis in CSF using an ELISA4,5 developed by Rogan Lee at Westmead Hospital. In dogs, CSF is generally collected from the cisterna magna under general anaesthesia. Myelography, computed tomography myelography or MR imaging can be conducted at the time of CSF collection. To date, MR studies have not been helpful in the specific diagnosis of NA in canine patients (in contrast to human cases where the presence of larval migration “trails” can be sometimes detected9). However, imaging studies exclude most other diagnostic possibilities. Neosporosis is normally diagnosed using an immunofluorescent antibody test (IFAT); by the time dogs are presented, they have elevated serum antibody titres against this protozoan. The IFAT is only available in laboratories in Perth (VetPath) and Launceston (Mt. Pleasant Laboratory) and assays are typically only run once or twice a week, so there is invariably some delay in obtaining a result. In neosporosis, CSF cytology shows a mixed pleocytosis, often with a prominent neutrophilic component and very rarely zoites have been recorded in CSF after cytocentrifugation6,7.
The conundrum for the vet is that treatments of these two diseases are quite different. Neosporosis is treated with antimicrobials effective against apicomplexan protozoans, traditionally trimephoprim-sulphadiazine, pyremethamine and clindamycin6,7. Usually two of the three drugs are selected. The combination of sulphadiazine and pyremethamine has a theoretical advantage due to sequential attacks on the parasite’s folic acid pathway. In the future, drugs such as toltrazuril and ponazuril may also prove to be effective18, the latter having become the drug of choice for treating equine protozoan myelitis.
In contrast, NA is traditionally treated with corticosteroids to dampen the eosinophilic inflammatory response until the larvae die (because the dog is the “wrong” host)5. Perhaps we should be braver and actually kill the larva using an anthelmintic after starting aggressive corticosteroid therapy, choosing a drug regimen that kills larvae slowly using albendazole or fenbendazole, to avoid a sudden release of metazoan antigens. Such an approach has been used in human patients in Asia19 and more recently in two patients at Westmead Children’s Hospital20. Dogs with neosporosis treated with corticosteroids deteriorate dramatically. Accordingly, many veterinarians treat suspect cases (such as “Honey”) with anti-protozoan drugs while awaiting serology for neosporosis, or “bite the bullet” and collect CSF to look for eosinophilic pleocytosis, if owners are agreeable. Where owners do not permit or cannot afford the full investigation, a presumptive diagnosis of NA is made after excluding neosporosis by serology or a treatment trial; corticosteroids are then administered.
It is tantalising to speculate why clinical neosporosis is apparently more prevalent in Australia than in other countries. One possibility is that it is more common to feed dogs uncooked kangaroo and beef meat in Australia (sold as “pet mince” at supermarkets) compared to other countries. Such fresh meat might contain viable cysts of both N. caninum (and T. gondii) sufficient to establish subclinical disease in the bitch, which would be transmitted subsequently to pups.
It is also fascinating that the only reports of NA in dogs have been from Australia, even though the parasite exists throughout Asia, the Pacific islands and in some southern states of the USA. This condition is behaving as an emerging infectious disease in Australia, having spread from south eastern Queensland where it was first reported by Mason in the early 1970s1,2, reaching Sydney in 19913 and becoming progressively more common there16. Nineteen new canine cases have been diagnosed in a single referral centre in Sydney over the past 4 years (E. Thrift, A. Lam and G. Child, personal communication). It is of great interest that NA has been reported in a wide range of species other than the dog - horses21, multiple animals in zoological collections (especially primates)22,23, macrobats24, possums25 and tawny frogmouths25,26. From a population ecology standpoint, impact on tawny frog mouths is greater than for any other species25,26.
From a human perspective NA is zoonotic. It occurs in patients who (accidently or purposely!) eat slugs, snails or planarians. The most celebrated Australian case was a silly chap who ate a slug on a dare at a bucks night13. Far more serious is the threat posed to young infants, who have the propensity to put anything into their mouths, including molluscs. An immature immune system and small spinal cord means the neurologic impact on children to be far worse than in adult patients, potentially resulting in death or permanent sequelae. For this reason, we should do much more - as scientists, doctors, veterinarians and parents – to (i) alert people to the dangers of permitting rats and molluscs to accumulate in the environment, (ii) be diligent supervising children in the backyard and (iii) educate chefs and anyone preparing salads using fresh vegetables about the need to remove slugs and snails and their mucus trails by assiduous washing. From a veterinary perspective, we need more funded research to develop anthelmintic strategies that prevent infection of dogs. Long acting moxidectin formulations given to prevent heartworm seem the most promising line for further studies5.
Although to the best of our knowledge neosporosis has never been reported in human patients, recent evidence of infections in sheep27, rhinos28 and carnivorous marsupials29 suggest that a human case will turn up sooner or later! It would be a terrible irony if the patient was a vet.
References
[1] Mason, K.V. (1987) Canine neural angiostrongyliasis: the clinical and therapeutic features of 55 natural cases. Aust. Vet. J. 64, 201–203.| Canine neural angiostrongyliasis: the clinical and therapeutic features of 55 natural cases.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c%2FkvFektA%3D%3D&md5=ccc2d1fb0d4922cc86ace720e86cbe97CAS |
[2] Mason, K.V. et al. (1976) Granulomatous encephalomyelitis of puppies due to Angiostrongylus cantonensis. Aust. Vet. J. 52, 295.
| Granulomatous encephalomyelitis of puppies due to Angiostrongylus cantonensis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2s%2FitFagsQ%3D%3D&md5=f10e8a2a3179de9e3a137229786acd8cCAS |
[3] Collins, G.H. et al. (1992) Angiostronglyosis in dogs in Sydney. Aust. Vet. J. 69, 170–171.
| Angiostronglyosis in dogs in Sydney.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s%2FnsVyntA%3D%3D&md5=7e252988bdcbab49233fcee92402fdb8CAS |
[4] Lunn, J. et al. (2003) Antemortem diagnosis of canine neural angiostronglyosis using ELISA. Aust. Vet. J. 81, 128–131.
| Antemortem diagnosis of canine neural angiostronglyosis using ELISA.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c7osVCgtA%3D%3D&md5=72c3cdd87ce87f16a2b3805ac46fb070CAS |
[5] Lunn, J. et al. (2012) Twenty two cases of canine neural angiostronglyosis in eastern Australia (2002–2005) and a review of the literature. Parasites Vectors 5, 70.
| Twenty two cases of canine neural angiostronglyosis in eastern Australia (2002–2005) and a review of the literature.Crossref | GoogleScholarGoogle Scholar |
[6] Dubey, J.P. et al. (2007) Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 20, 323–367.
| Epidemiology and control of neosporosis and Neospora caninum.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2s3itVajsg%3D%3D&md5=60f67810ba8322ccaf6cd00f3b480947CAS |
[7] Reichel, M.P. et al. (2007) Neosporosis and hammondiosis in dogs. J. Small Anim. Pract. 48, 308–312.
| Neosporosis and hammondiosis in dogs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2szjsFOqsg%3D%3D&md5=c88f837544559155e51fee2b9c03e617CAS |
[8] King, J.S. et al. (2010) Australian dingoes are definitive hosts of Neospora caninum. Int. J. Parasitol. 40, 945–950.
| Australian dingoes are definitive hosts of Neospora caninum.Crossref | GoogleScholarGoogle Scholar |
[9] King, J.S. et al. (2012) Oocysts and high seroprevalence of Neospora caninum in dogs living in remote Aboriginal communities and wild dogs in Australia. Vet. Parasitol. 187, 85–92.
| Oocysts and high seroprevalence of Neospora caninum in dogs living in remote Aboriginal communities and wild dogs in Australia.Crossref | GoogleScholarGoogle Scholar |
[10] King, J.S. et al. (2011) Implications of wild dog ecology on the sylvatic and domestic life cycle of Neospora caninum in Australia. Vet. J. 188, 24–33.
| Implications of wild dog ecology on the sylvatic and domestic life cycle of Neospora caninum in Australia.Crossref | GoogleScholarGoogle Scholar |
[11] Reid, A.J. et al. (2012) Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. PLoS Pathog. 8, e1002567.
| Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XltVymu74%3D&md5=c9ea8f5cc48e0d0e82e9269d594c980cCAS |
[12] Cooke-Yarborough, C.M. et al. (1999) A fatal case of angiostronglyosis in an 11-month-old infant. Med. J. Aust. 170, 541–543.
| 1:STN:280:DyaK1MzisFelug%3D%3D&md5=06c19d5879151b1cc950ecc8ec3453f3CAS |
[13] Senanayake, S.N. et al. (2003) First report of human angiostrongyliasis reported in Sydney. Med. J. Aust. 179, 430–431.
[14] Hsieh, T. et al. (2001) Magnetic resonance imaging findings of eosinophilic meningoencephalitis caused by angiostronglyosis. Zhonghua Fang She Xue Za Zhi 26, 45–49.
[15] Yong, W.K. et al. (1981) Localized distribution of A. cantonensis among wild rat populations in Brisbane, Australia. S.E.A. J. Trop. Med. Pub. Hlth. 12, 608–609.
| 1:STN:280:DyaL383isVWhtQ%3D%3D&md5=18674729a2bc52493fb58cbbbdf6bf54CAS |
[16] Stokes, V.L.D. et al. (2007) Occurrence of Angiostrongylus species (Nematoda) in populations of Rattus rattus and Rattus fuscipes in coastal forests of south-eastern Australia. Aust. J. Zool. 55, 177–184.
| Occurrence of Angiostrongylus species (Nematoda) in populations of Rattus rattus and Rattus fuscipes in coastal forests of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
[17] Daft, B.M. et al. (2002) Sensitivity and specificity of western blot testing of cerebrospinal fluid and serum for diagnosis of equine protozoal myeloencephalitis in horses with and without neurologic abnormalities. J. Am. Vet. Med. Assoc. 221, 1007–1013.
| Sensitivity and specificity of western blot testing of cerebrospinal fluid and serum for diagnosis of equine protozoal myeloencephalitis in horses with and without neurologic abnormalities.Crossref | GoogleScholarGoogle Scholar |
[18] Furr, M. et al. (2001) Efficacy of ponazuril 15% oral paste as a treatment for equine protozoal myeloencephalitis. Vet. Ther. 2, 215–222.
| 1:STN:280:DC%2BD1MnhvVyjtw%3D%3D&md5=a29e3638a262e548107738297235abe9CAS |
[19] Chotmongkol, V. and Sawanyawisuth, K. (2002) Clinical manifestations and outcome of patients with severe eosinophilic meningoencephalitis presumably caused by Angiostrongylus cantonensis. S.E.A. J. Trop. Med. Pub. Health 33, 231–234.
[20] Britton, P. et al. (2011) Paediatric angiostrongyliasis – two severe concurrent cases from Sydney, Australia. Proceedings of the 7th World Congress of the World Society for Pediatric Infectious Diseases, Melbourne, Australia.
[21] Wright, J.D. (1991) Equine neural angiostronglyosis. Aust. Vet. J. 68, 58–60.
| Equine neural angiostronglyosis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M3it1Sluw%3D%3D&md5=22b211c038d726fd9cc77a9fd110fe59CAS |
[22] Higgins, D.P. et al. (1997) Neural angiostronglyosis in three captive rufous bettongs (Aepyprymnus rufescens). Aust. Vet. J. 75, 564–566.
| Neural angiostronglyosis in three captive rufous bettongs (Aepyprymnus rufescens).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2svjvVyluw%3D%3D&md5=6fa9f062c7fb8e7d7c3f079b07885e7cCAS |
[23] Carlisle, M.S. et al. (1998) Cerebrospinal angiostronglyosis in five captive tamarins (Sanguinus spp). Aust. Vet. J. 76, 167–170.
| Cerebrospinal angiostronglyosis in five captive tamarins (Sanguinus spp).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3jvFyqug%3D%3D&md5=1fc917b8d7a895d5c3472192113b1930CAS |
[24] Barrett, J.L. et al. (2002) Neuro-angiostrongylosis in wild Black and Grey-headed flying foxes (Pteropus spp). Aust. Vet. J. 80, 554–558.
| Neuro-angiostrongylosis in wild Black and Grey-headed flying foxes (Pteropus spp).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38njvVOisw%3D%3D&md5=0325917c03819d21cf2314f84dfef662CAS |
[25] Ma, G. et al. (2013) Tawny frogmouths and brushtail possums as sentinels for Angiostrongylus cantonensis, the rat lungworm. Vet. Parasitol. , .
| Tawny frogmouths and brushtail possums as sentinels for Angiostrongylus cantonensis, the rat lungworm.Crossref | GoogleScholarGoogle Scholar |
[26] Gelis, S. et al. (2011) Neuroangiostrongyliasis and other parasites in tawny frogmouths (Podargus strigoides) in south-eastern Queensland. Aust. Vet. J. 89, 47–50.
| Neuroangiostrongyliasis and other parasites in tawny frogmouths (Podargus strigoides) in south-eastern Queensland.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7otVCltQ%3D%3D&md5=d513398e797f3a269a52680cc789df53CAS |
[27] Bishop, S. et al. (2010) The first report of ovine cerebral neosporosis and evaluation of Neospora caninum prevalence in sheep in New South Wales. Vet. Parasitol. 170, 137–142.
| The first report of ovine cerebral neosporosis and evaluation of Neospora caninum prevalence in sheep in New South Wales.Crossref | GoogleScholarGoogle Scholar |
[28] Sangster, C. et al. (2010) Neosporosis in an aborted Southern White Rhinoceros (Ceratotherium simum simum) foetus. J. Wildlife Zoo Med. 41, 725–728.
| Neosporosis in an aborted Southern White Rhinoceros (Ceratotherium simum simum) foetus.Crossref | GoogleScholarGoogle Scholar |
[29] King, J.S. et al. (2011) Extensive production of Neospora caninum tissue cysts in a carnivorous marsupial succumbing to experimental neosporosis. Vet. Res. 42, 75.
| Extensive production of Neospora caninum tissue cysts in a carnivorous marsupial succumbing to experimental neosporosis.Crossref | GoogleScholarGoogle Scholar |
Biographies
Richard Malik is a consultant in small animal medicine that has a special interest in infectious diseases of companion animals. He is particularly interested in viral diseases of cats, fungal diseases especially those caused by Cryptococcus species, mycobacteria, saprophytic pathogens such as Burholderia, Prototheca and Pythium and most recently parasitic diseases because of the influence of Jan Slapeta! Richard works for the Centre for Veterinary Education where he facilitates feline distance education programs, and develops life-long learning strategies for vets in practice.
Derek Spielman is a Lecturer in Veterinary Pathology at the University of Sydney. He has worked as a companion animal clinician, zoo and wildlife veterinarian and has a PhD in conservation genetics. His special interests are in wildlife pathology and the ecology and epidemiology of wildlife diseases. He is also interested in the pharmacokinetics of antibiotics in Australian marsupials. He is especially interested in the effects of neural angiostronglyosis on tawny frogmouths and possums.
Jan Šlapeta joined the Parasitology team in the Faculty of Veterinary science at the University of Sydney in 2007. He has a broad understanding of the biology of parasites of both medical and veterinary importance, as well as the diseases caused by them. He has experience in several research laboratories, including the NIH in the USA, the CNRS in France and the University of Technology in Sydney. Jan specialises in the molecular identification and the evolution of protozoan parasites. His diagnostic techniques and biodiversity studies have received worldwide interest. He has a particular interest in applications of molecular biology towards elucidating the unique properties of parasites of medical and veterinary importance.


