Japanese encephalitis virus: an emerging and re-emerging virus in Australia
John S. Mackenzie A * and David T. Williams BA Faculty of Health Sciences, Curtin University, Unit 143, 6 Tighe Street, Jolimont, Perth, WA 6014, Australia.
B CSIRO Australian Centre for Disease Preparedness, Geelong, Vic., Australia.

John Mackenzie is an Emeritus Professor of Curtin University, and an Honorary Professor at The University of Queensland. His research has been largely concerned with vector-borne viruses, emerging zoonoses, and the One Health concept. He currently serves on WHO Emergency Committees on Polio and on COVID-19, and is a member of the FAO-UNEP-WHO-WOAH One Health High Level Expert Panel. He has been a member of the National Arbovirus and Malaria Advisory Committee since its inception. He is a former President of ASM, and Secretary-General of IUMS. |

Dr David Williams leads the Diagnosis and Mammalian Infectious Disease Research group at the CSIRO Australian Centre for Disease Preparedness in Geelong. David’s research interests include the detection, epidemiology and pathogenesis of emerging and exotic viruses that affect humans and animals. This work has focussed on mosquito-borne viruses such as Japanese encephalitis virus. David is a member of the National Arbovirus and Malaria Advisory Committee and the Public Health Laboratory Network Expert Review Panel on Japanese encephalitis. |
Microbiology Australia 43(4) 150-155 https://doi.org/10.1071/MA22050
Submitted: 11 October 2022 Accepted: 5 November 2022 Published: 1 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Japanese encephalitis virus (JEV) first emerged in the Torres Strait of north-eastern Australia in 1995, with three human cases, and widespread infection of pigs on a number of islands. The virus was shown to belong to genotype II. Further cases occurred in 1998, including the first case on mainland Australia on Cape York. A second genotype of JEV, genotype Ia, was reported in mosquitoes and pigs in 2000–04, possibly displacing genotype II. JEV re-emerged in Australia with a fatal human case on the Tiwi Islands, Northern Territory, in 2021, and shown to belong to genotype IV. This case was followed about a year later by a large outbreak of JE; first detected in piggeries in four states, Queensland, New South Wales, Victoria, and South Australia, resulting in reproductive losses affecting 80 piggeries and 42 human cases, with seven fatal cases. The wide geographic spread of cases suggested that the virus had been circulating for a number of months or even years prior to detection, and has led to significant concern that the virus will become endemic to Australia, in a similar ecology to Murray Valley encephalitis virus. Known competent mosquito vectors and ardeid birds, as maintenance hosts, occur in Australia, and it is probable that feral pigs will provide an additional wildlife reservoir of virus. Little is known of the properties of genotype IV, but it is expected to have a similar ecology and pathogenesis to other JEV genotypes.
Keywords: ardeid birds, Culex sp. mosquitoes, domestic piggeries, feral pigs, flaviviruses, Japanese encephalitis, Japanese encephalitis virus, JEV genotype IV, malformed and stillborn pigs, Torres Strait.
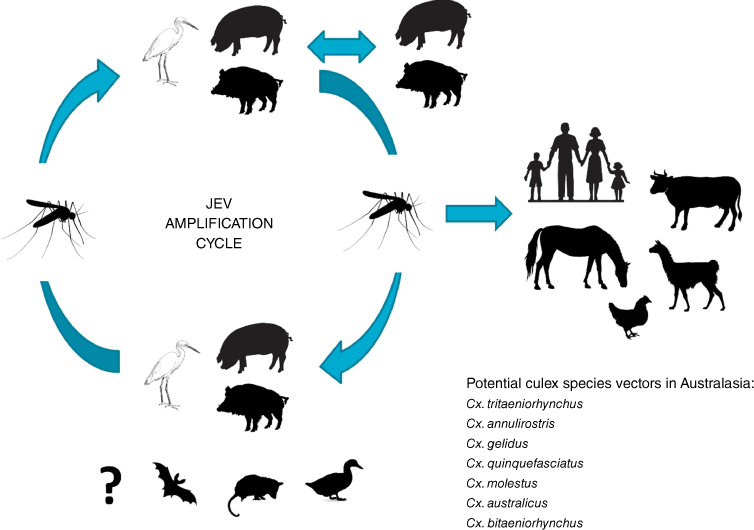
Japanese encephalitis virus (JEV) is the major vaccine-preventable cause of human encephalitis in Asia,1 with a geographic range extending from eastern, south-eastern and southern Asia. In 1995, it moved into Oceania for the first time, causing infections in Papua New Guinea (PNG) and the Torres Strait (TS) of northern Australia.2 It is a vector-borne flavivirus, and a member of the Japanese encephalitis serological complex, that includes West Nile (Kunjin subtype) and Murray Valley encephalitis viruses in Australia.3 JEV exists in zoonotic transmission cycles between ardeid birds (maintenance or reservoir hosts), such as herons and egrets, and pigs (amplifying hosts) and Culex sp. mosquitoes, particularly Culex tritaeniorhynchus, although other species such as Cx. gelidus, Cx. vishnui and Cx. fuscocephala are also important vectors in specific areas, with Cx. annulirostris the probable major vector species in Australia (Fig. 1).4
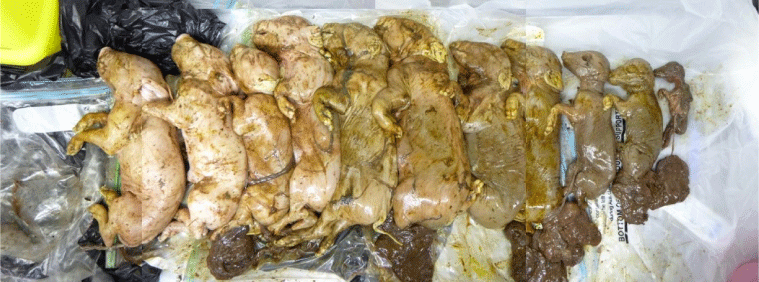
JEV causes occasional disease in humans, horses and pigs,5 and rare cases of fatal disease in other species. Infection in horses is generally asymptomatic, but very occasionally it can lead to a severe neurological disease. Horses and humans are recognised as ‘dead-end’ hosts as viraemia levels are not high enough to infect mosquitoes. JEV is an important disease of pigs. Although infection of adult pigs is generally asymptomatic but with a measurable viraemia, it is an important cause of reproductive disease resulting in still-births, malformations and mummified foetuses (Fig. 2), and surviving piglets often display fatal neurological symptoms. Vector-free transmission between pigs has also been demonstrated through virus shedding in oro-nasal secretions.6 In humans, most cases are asymptomatic, but between 1:30 and 1:300 cases depending on endemicity and past exposures, can lead to clinical disease, usually a severe meningomyeloencephaltitis with 25% mortality and 50% of survivors suffering neuropsychiatric sequelae.7 Some patients present with a poliovirus-like flaccid paralysis.7 Where JEV reaches a new geographic area, adults and children are affected, but adults over 60 years of age are more likely to have severe symptomatic disease.7
Virus genotypes
It is believed that JEV evolved from an ancestral virus in the Indonesia-Malaysia region,8,9 and subsequently diverged into five genotypes I, II, III, IV, and V.8–12 Genotype I has been further divided into Ia and Ib.13 Phylogenetic studies showed that genotype V is the oldest genotype, followed by IV, III, II, and I as the youngest. In general, GIb, and GIII are considered to be largely associated with seasonal, epidemic disease in temperate areas, whereas GIa, GII and GIV are associated with endemic disease in tropical areas of south-east Asia.14,15 Over the past 2–3 decades, GIb has replaced GIII in temperate areas.13–16 The two most rarely detected genotypes have been GIV and GV. GV was isolated initially in Singapore in 1952,12 but not seen again for 57 years until 2009 when an isolate was reported from Tibet, China,17 and further isolates reported from Korea.18 GIV was first described from mosquitoes trapped in various parts of Indonesia between 1980 and 1981,11,19 but was not seen again for 36 years until isolated in Bali from pigs in 201720 and Cx. vishnui mosquitoes in 2019.21 Also in 2019, an Australian tourist became infected with JEV GIV in Bali and later died in Brisbane.22 Thus, the geographic range of GIV viruses was previously considered to be confined to Indonesia.
Japanese encephalitis virus emergence in Europe, Africa and Oceania
JEV, like many other flaviviruses, has a propensity to spread and establish in new areas. In the 1990s, JEV emerged in parts of Oceania and South-Western Asia, with virus isolations from PNG, Papua Province (Indonesia), the TS of northern Australia, and Pakistan.23 More recently, JEV has been detected in the bone marrow of passerine birds24 and in Cx. pipiens mosquitoes25 in Italy and in a patient who was co-infected with yellow fever in Angola.26 There is considerable concern that JEV might spread further in Europe27 and into the west coast of North America.28
JEV first appeared in the TS in 1995, where it caused three human cases on Badu, an island in the central TS.29 JEV belonging to GIV was isolated from two asymptomatic human cases. In addition, pigs were found to have seroconverted on at least nine other central and northern TS islands.29 Cx. annulirostris mosquitoes were shown to be the major vector in the outbreak, from which eight isolates of JEV were obtained.30 JE vaccine was offered to the inhabitants of the TS islands.31 Later studies showed that the virus had originated from PNG; identical strains of JEV were isolated from Cx. sitiens subgroup mosquitoes trapped in Western Province,32 and human cases of JE were observed at a mission hospital near Kiunga in 1997–98,33 and Port Moresby in 2004.34 The first case of JE was also reported from Irian Jaya (now Papua) in 1997,35 and several local inhabitants of Timika were found to be seropositive to JEV.36 JEV GII occurred again in 1998 in an unvaccinated child on Badu and the first clinical case on mainland Australia at the mouth of the Mitchell River in Cape York.37 Sentinel pigs on Badu and Cape York seroconverted, and JEV GII was isolated from 3 pigs at Seisa in the Northern Peninsula Area (NPA) at the tip of Cape York.37,38 A total of 42 JEV isolations were obtained from mosquitoes on Badu, 41 of which were isolated from Cx. annulirostris and one from Aedes vigilax, but no isolate was obtained from mosquitoes trapped on Cape York.39 It has been postulated that the movement of JEV from PNG into Badu and Cape York may have been due to wind-borne mosquitoes during cyclonic weather patterns.40
JEV re-emerged in 2000, but virus isolates from mosquitoes and pigs belonged to a different genotype, GI,39,41 later confirmed as GIa.14 Further incursions were observed annually between 2001 and 2005 on Badu through sentinel pig seroconversions and mosquito trapping, and JEV was detected on mainland Australia in Cape York again in 2004, with sentinel pig seroconversions and virus isolation from mosquitoes.38,42 Sentinel pigs were discontinued in the TS at the end 2005, largely for safety reasons, and from the NPA in 2012.38 Sporadic or opportunistic surveillance activities have been carried out by Northern Australian Quarantine Service (NAQS) over the past decade and a half, and serological evidence of JEV activity was reported in pigs and horses in the TS (2005, 2010, 2012, 2013, 2014, 2016, 2017 and 2019) and the NPA (2020 and 2021) (data collected from the NAQS reports in the Australian Animal Health Quarterly, Quarterly Statistics), but no human infections have been reported. It should be noted that testing was performed on single samples only, and serological data cannot distinguish viral genotypes. Nevertheless, they indicate that JEV was either endemic in the TS, or frequently re-introduced from PNG. These results indicate the ongoing risk of JEV to northern Australia.
The re-emergence of Japanese encephalitis virus in Australia
A fatal case of JE occurred in March 2021 in an inhabitant from the Tiwi Islands.43,44 Sequence studies confirmed that it was JEV belonging to genotype IV, the first time this genotype was acquired outside Indonesia. It was also the second known fatal human infection due to GIV, the first having been reported in an Australian tourist to Bali.22 The Tiwi Island case was the sentinel case for a subsequent outbreak in early 2022 that was initially detected by the appearance of mummified or malformed foetuses, still births (Fig. 2) and liveborn piglets with neurological signs in piggeries in Queensland, New South Wales, and Victoria.45 A human case of encephalitis was confirmed in Queensland, and with other possible cases under investigation, Australia’s Acting Chief Medical Officer formally declared the JE outbreak ‘A Communicable Disease Incident of National Significance’ on 4 March 2022.46 The outbreak spread widely in pig farms in Queensland, New South Wales, Victoria and South Australia, affecting over 80 piggeries,47 and 42 human cases were reported, seven of which were fatal.48 JEV GIV was detected in feral pigs from Northern Territory,49,50 Queensland50 and South Australia51 either by serology or virus isolation.
Two successful vaccines are available for human use; IMOJEV, a live attenuated chimeric vaccine comprising the yellow fever virus 17D vaccine strain as the backbone, and the pre-membrane and envelope genes of the GIII Chinese live attenuated JEV vaccine virus, SA-14-14-2 strain52 and JESPECT, an adjuvanted inactivated Vero cell-grown SA-14-14-2 virus.53 Both vaccines have been shown to protect against the different JEV genotypes. In Australia, those most at risk of infection and for whom vaccination is recommneded include piggery workers, abattoir workers, laboratory workers, hunters of feral pigs, people living nearby to piggeries or wetlands, certain remote Aboriginal communities in northern Australia, and campers and fishers and others involved in outdoor activities near lakes, lagoons and wetlands. There are no vaccines currently available in Australia for pigs or horses. However, inactivated and live attenuated veterinary vaccines based on GIII strains produced by overseas manufacturers may have application in Australia following assessment and regulatory approval. In piggeries, it is expected that vaccination will focus on sows to reduce reproductive losses, rather than pork production animals. Vaccination has not been widely used for the latter due to the costs associated with immunising large numbers of young pigs, high turnover of animals, and the short period for effective immunisation.54
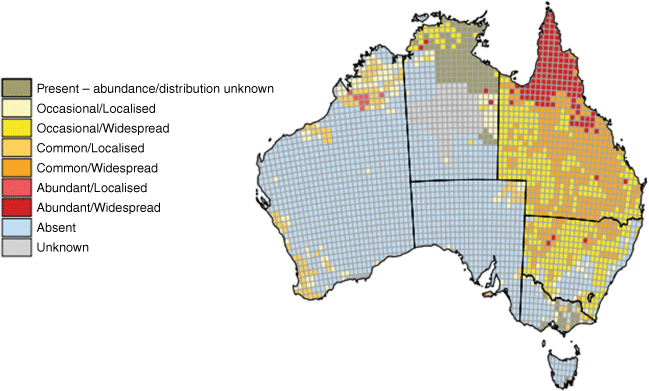
JEV GIV was the third genotype to emerge in Australia, but unlike the earlier GII and GIa incursions between 1995 and 2004, little is known about the properties and pathogenesis of JEV GIV viruses. From knowledge gained over many years in Asia with the more common JEV GI, GII and GIII genotypes, and more limited knowledge from the TS, it is assumed the major mosquito vector in Australia is Culex annulirostris, although a number of other vector species may also be involved.55 Similarly, the vertebrate hosts in Australia are expected to include ardeid birds as maintenance hosts, and feral and domestic pigs as amplifying hosts, but it is possible other hosts could play a role, such as magpie geese, ducks, and flying foxes. Marsupials appear to be less likely to be involved, although viraemia levels suggested possums could play a role in urban and peri-urban environments.56 Feral pigs are expected to be a major vertebrate host because of their abundance and wide distribution (Fig. 3). A number of other questions related to the properties of GIV viruses remain to be determined, including their virulence and neuro-invasiveness compared to other genotypes; their antigenic profile and the sensitivity and specificity of current diagnostic tests; and the sensitivity of current surveillance methods and the need for improved surveillance systems.
The geographic extent of the outbreak is still not fully understood, and is based largely on the location of infected piggeries, human cases and some limited feral pig studies in New South Wales, Queensland, Northern Territory, and South Australia. Further information has recently come from a relatively small human serological survey carried out in five towns in NSW, all of which had evidence of JEV-infected mosquitoes earlier in the year. There was evidence of JEV infection in approximately one in 11 of the 1048 participants, indicating that JEV was prevalent in those areas and suggesting that a relatively large number of people may have been infected.57
The close antigenic relationships between flaviviruses can make serology-based diagnostic interpretation complex and difficult.58 Improved surveillance methods and procedures are needed to provide early warning of possible virus activity. The origin and route by which the JEV GIV virus reached Australia and where it entered northern Australia remains to be determined, although it may be difficult to obtain an absolute answer. Most evidence would suggest that the virus probably came from a focus of activity in the Indonesian archipelago or PNG through either wind-blown mosquitoes or vagrant or migratory birds. It is probable that the 2022 outbreak was driven in part by the increased rainfall and flooding due to the La Niña weather patterns in 2020–21 and 2021–22 providing conditions for increased mosquito breeding and attracting waterbirds to many additional feeding grounds. There is ongoing concern that JEV GIV will become endemic in Australia, and evidence from the widespread nature of the outbreak suggests that the virus may already be endemic, but this may become clearer as we enter the summer months of 2022–23. The current third year of La Niña weather pattern is therefore of considerable concern.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] Campbell, GL et al.. (2011) Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ 89, 766–774E.| Estimated global incidence of Japanese encephalitis: a systematic review.Crossref | GoogleScholarGoogle Scholar |
[2] Mackenzie JS et al. (2007) Japanese encephalitis virus: the geographic distribution, incidence, and spread of a virus with a propensity to emerge in new areas. In Emerging Viruses in Human Populations (Tabor E, ed.). pp. 201–268. Elsevier.
[3] Mackenzie, JS et al.. (2002) Japanese encephalitis as an emerging virus: the emergence and spread of Japanese encephalitis virus in Australasia. Curr Top Microbiol Immunol 267, 49–73.
| Japanese encephalitis as an emerging virus: the emergence and spread of Japanese encephalitis virus in Australasia.Crossref | GoogleScholarGoogle Scholar |
[4] Vaughn, DW and Hoke Jr, CH (1992) The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol Rev 14, 197–221.
| The epidemiology of Japanese encephalitis: prospects for prevention.Crossref | GoogleScholarGoogle Scholar |
[5] Mansfield, KL et al.. (2017) Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet Microbiol 201, 85–92.
| Japanese encephalitis virus infection, diagnosis and control in domestic animals.Crossref | GoogleScholarGoogle Scholar |
[6] Ricklin, ME et al.. (2016) Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat Commun 7, 10832.
| Vector-free transmission and persistence of Japanese encephalitis virus in pigs.Crossref | GoogleScholarGoogle Scholar |
[7] Solomon, T and Vaughn, DW (2002) Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol 267, 171–194.
| Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections.Crossref | GoogleScholarGoogle Scholar |
[8] Solomon, T et al.. (2003) Origin and evolution of Japanese encephalitis virus in southeast Asia. J Virol 77, 3091–3098.
| Origin and evolution of Japanese encephalitis virus in southeast Asia.Crossref | GoogleScholarGoogle Scholar |
[9] Gao, X et al.. (2015) Insights into the evolutionary history of Japanese encephalitis virus (JEV) based on whole-genome sequences comprising the five genotypes. Virol J 12, 43.
| Insights into the evolutionary history of Japanese encephalitis virus (JEV) based on whole-genome sequences comprising the five genotypes.Crossref | GoogleScholarGoogle Scholar |
[10] Chen, W-R et al.. (1990) Genetic variation of Japanese encephalitis virus in nature. J Gen Virol 71, 2915–2922.
| Genetic variation of Japanese encephalitis virus in nature.Crossref | GoogleScholarGoogle Scholar |
[11] Chen, W-R et al.. (1992) A new genotype of Japanese encephalitis virus from Indonesia. Am J Trop Med Hyg 47, 61–69.
| A new genotype of Japanese encephalitis virus from Indonesia.Crossref | GoogleScholarGoogle Scholar |
[12] Uchil, PD and Satchidanandam, V (2001) Phylogenetic analysis of Japanese encephalitis virus: envelope gene-based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am J Trop Med Hyg 65, 242–251.
| Phylogenetic analysis of Japanese encephalitis virus: envelope gene-based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent.Crossref | GoogleScholarGoogle Scholar |
[13] Schuh, AJ et al.. (2014) Dynamics of the emergence and establishment of a newly dominant genotype of Japanese encephalitis virus throughout Asia. J Virol 88, 4522–4532.
| Dynamics of the emergence and establishment of a newly dominant genotype of Japanese encephalitis virus throughout Asia.Crossref | GoogleScholarGoogle Scholar |
[14] Schuh, AJ et al.. (2013) Phylogeography of Japanese encephalitis virus: genotype is associated with climate. PLoS Negl Trop Dis 7, e2411.
| Phylogeography of Japanese encephalitis virus: genotype is associated with climate.Crossref | GoogleScholarGoogle Scholar |
[15] Gao, X et al.. (2019) Changing geographic distribution of Japanese encephalitis virus genotypes, 1935–2017. Vector Borne Zoonotic Dis 19, 35–44.
| Changing geographic distribution of Japanese encephalitis virus genotypes, 1935–2017.Crossref | GoogleScholarGoogle Scholar |
[16] Pan, XL et al.. (2011) Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J Virol 85, 9847–9853.
| Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia.Crossref | GoogleScholarGoogle Scholar |
[17] Li, MH et al.. (2011) Genotype V Japanese encephalitis virus is emerging. PLoS Negl Trop Dis 5, e1231.
| Genotype V Japanese encephalitis virus is emerging.Crossref | GoogleScholarGoogle Scholar |
[18] Takhampunya, R et al.. (2011) Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol J 8, 449.
| Emergence of Japanese encephalitis virus genotype V in the Republic of Korea.Crossref | GoogleScholarGoogle Scholar |
[19] Schuh, AJ et al.. (2013) Genetic diversity of Japanese encephalitis virus isolates obtained from the Indonesian archipelago between 1974 and 1987. Vector Borne Zoonotic Dis 13, 479–488.
| Genetic diversity of Japanese encephalitis virus isolates obtained from the Indonesian archipelago between 1974 and 1987.Crossref | GoogleScholarGoogle Scholar |
[20] Kuwata, R et al.. (2020) Distribution of Japanese encephalitis virus, Japan and Southeast Asia, 2016–2018. Emerg Infect Dis 26, 125–128.
| Distribution of Japanese encephalitis virus, Japan and Southeast Asia, 2016–2018.Crossref | GoogleScholarGoogle Scholar |
[21] Faizah, AN et al.. (2021) Identification and isolation of Japanese encephalitis virus Genotype IV from Culex vishnui collected in Bali, Indonesia in 2019. Am J Trop Med Hyg 105, 813–817.
| Identification and isolation of Japanese encephalitis virus Genotype IV from Culex vishnui collected in Bali, Indonesia in 2019.Crossref | GoogleScholarGoogle Scholar |
[22] Pyke, AT et al.. (2020) A case of Japanese encephalitis with a fatal outcome in an Australian who traveled from Bali in 2019. Trop Med Infect Dis 5, 133.
| A case of Japanese encephalitis with a fatal outcome in an Australian who traveled from Bali in 2019.Crossref | GoogleScholarGoogle Scholar |
[23] Mackenzie JS et al. (2006) Japanese encephalitis virus: an example of a virus with a propensity to emerge and spread in new geographic areas. In Emerging Viruses in Human Populations. Perspectives in Medical Virology (Tabor E, ed.). Vol. 16, pp. 201–268. Elsevier, Amsterdam.
| Crossref |
[24] Platonov, AE et al.. (2012) Does the Japanese encephalitis virus (JEV) represent a threat for human health in Europe? Detection of JEV RNA sequences in birds collected in Italy. Euro Surveill 17, 20241.
| Does the Japanese encephalitis virus (JEV) represent a threat for human health in Europe? Detection of JEV RNA sequences in birds collected in Italy.Crossref | GoogleScholarGoogle Scholar |
[25] Ravanini, P et al.. (2012) Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy. Euro Surveill 17, 20221.
| Japanese encephalitis virus RNA detected in Culex pipiens mosquitoes in Italy.Crossref | GoogleScholarGoogle Scholar |
[26] Simon-Loriere, E et al.. (2017) Autochthonous Japanese encephalitis with yellow fever coinfection in Africa. N Engl J Med 376, 1483–1485.
| Autochthonous Japanese encephalitis with yellow fever coinfection in Africa.Crossref | GoogleScholarGoogle Scholar |
[27] European Centre for Disease Prevention and Control. Factsheet about Japanese encephalitis. https://www.ecdc.europa.eu/en/japanese-encephalitis/facts
[28] Oliveira, ARS et al.. (2020) Perspectives regarding the risk of introduction of the Japanese encephalitis virus (JEV) in the United States. Front Vet Sci 7, 48.
| Perspectives regarding the risk of introduction of the Japanese encephalitis virus (JEV) in the United States.Crossref | GoogleScholarGoogle Scholar |
[29] Hanna, JN et al.. (1996) An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med J Aust 165, 256–260.
| An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995.Crossref | GoogleScholarGoogle Scholar |
[30] Ritchie, SA et al.. (1997) Isolation of Japanese encephalitis virus from Culex annulirostris in Australia. Am J Trop Med Hyg 56, 80–84.
| Isolation of Japanese encephalitis virus from Culex annulirostris in Australia.Crossref | GoogleScholarGoogle Scholar |
[31] Hanna, J et al.. (1996) Vaccination against Japanese encephalitis in the Torres Strait. Comm Dis Intell 20, 188–191.
| Vaccination against Japanese encephalitis in the Torres Strait.Crossref | GoogleScholarGoogle Scholar |
[32] Johansen, CA et al.. (2000) Isolation of Japanese encephalitis virus from mosquitoes (Diptera: Culicidae) collected in the Western Province of Papua New Guinea, 1997–1998. Am J Trop Med Hyg 62, 631–638.
| Isolation of Japanese encephalitis virus from mosquitoes (Diptera: Culicidae) collected in the Western Province of Papua New Guinea, 1997–1998.Crossref | GoogleScholarGoogle Scholar |
[33] Mackenzie JS, et al. (2002) Japanese encephalitis as an emerging virus: the emergence and spread of Japanese encephalitis virus in Australasia. In Japanese Encephalitis and West Nile Viruses. Current Topics in Microbiology and Immunology (Mackenzie JS et al., eds). Vol. 267, pp. 49–73. Springer, Berlin, Heidelberg.
| Crossref |
[34] Hanson, JP et al.. (2004) Japanese encephalitis acquired near Port Moresby: implications for residents and travellers to Papua New Guinea. Med J Aust 181, 282.
| Japanese encephalitis acquired near Port Moresby: implications for residents and travellers to Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
[35] Spicer, PE (1997) Japanese Encephalitis in Western Irian Jaya. J Travel Med 4, 146–147.
| Japanese Encephalitis in Western Irian Jaya.Crossref | GoogleScholarGoogle Scholar |
[36] Spicer, PE et al.. (1999) Antibodies to Japanese encephalitis virus in human sera collected from Irian Jaya. Follow-up of a previously reported case of Japanese encephalitis in that region. Trans R Soc Trop Med Hyg 93, 511–514.
| Antibodies to Japanese encephalitis virus in human sera collected from Irian Jaya. Follow-up of a previously reported case of Japanese encephalitis in that region.Crossref | GoogleScholarGoogle Scholar |
[37] Hanna, JN et al.. (1999) Japanese encephalitis in north Queensland, Australia, 1998. Med J Aust 170, 533–536.
| Japanese encephalitis in north Queensland, Australia, 1998.Crossref | GoogleScholarGoogle Scholar |
[38] van den Hurk, AF et al.. (2019) Japanese encephalitis virus in Australia: from known known to known unknown. Trop Med Infect Dis 4, 38.
| Japanese encephalitis virus in Australia: from known known to known unknown.Crossref | GoogleScholarGoogle Scholar |
[39] Pyke, AT et al.. (2001) The appearance of a second genotype of Japanese encephalitis virus in the Australasian region. Am J Trop Med Hyg 65, 747–753.
| The appearance of a second genotype of Japanese encephalitis virus in the Australasian region.Crossref | GoogleScholarGoogle Scholar |
[40] Ritchie, SA and Rochester, W (2001) Wind-blown mosquitoes and introduction of Japanese encephalitis into Australia. Emerg Infect Dis 7, 900–903.
| Wind-blown mosquitoes and introduction of Japanese encephalitis into Australia.Crossref | GoogleScholarGoogle Scholar |
[41] Johansen, CA et al.. (2004) Flavivirus isolations from mosquitoes collected from Saibai Island in the Torres Strait, Australia, during an incursion of Japanese encephalitis virus. Med Vet Entomol 18, 281–287.
| Flavivirus isolations from mosquitoes collected from Saibai Island in the Torres Strait, Australia, during an incursion of Japanese encephalitis virus.Crossref | GoogleScholarGoogle Scholar |
[42] Van Den Hurk, AF et al.. (2006) Short report: the first isolation of Japanese encephalitis virus from mosquitoes collected from mainland Australia. Am J Trop Med Hyg 75, 21–25.
[43] Waller, C et al.. (2022) Japanese encephalitis in Australia — a sentinel case. N Engl J Med 387, 661–662.
| Japanese encephalitis in Australia — a sentinel case.Crossref | GoogleScholarGoogle Scholar |
[44] Sikazwe, C et al.. (2022) Molecular detection and characterisation of the first Japanese encephalitis virus belonging to genotype IV in Australia. PLoS Negl Trop Dis , .
[45] Australian Department of Agriculture, Fisheries, and Forestry (2022) Japanese encephalitis detected in eastern Australia. 1 March. https://www.awe.gov.au/about/news/media-releases/japanese-encephalitis-virus (accessed 30 September 2022)
[46] Australian Department of Health and Aged Care (2022) Japanese encephalitis virus situation declared a Communicable Disease Incident of National Significance. 4 March. https://www.health.gov.au/news/japanese-encephalitis-virus-situation-declared-a-communicable-disease-incident-of-national-significance (accessed 1 October 2022)
[47] Australian Department of Agriculture, Fisheries and Forestry (2022) Japanese encephalitis. Updated 29 July. https://www.agriculture.gov.au/biosecurity-trade/pests-diseases-weeds/animal/japanese-encephalitis (accessed 1 October 2022)
[48] Australian Department of Health and Aged Care (2022) Japanese encephalitis virus (JEV). Current status, 19 July. Japanese encephalitis virus (JEV) | Australian Government Department of Health and Aged Care (accessed 1 October 2022)
[49] Northern Territory Government (2022) Japanese encephalitis in animals. Updated 19 July. https://nt.gov.au/industry/agriculture/livestock/animal-health-and-diseases/japanese-encephalitis-in-animals#:~:text=Northern% 20Territory,and% 20Cox% 2DDaly% 20unincorporated% 20areas (accessed 30 September 2022)
[50] National Feral Pig Action Plan. Japanese encephalitis virus (JEV) Action Plan Fact Sheet. https://feralpigs.com.au/wp-content/uploads/2022/06/JEV-Factsheet-27-June-2022.pdf (accessed 10 October 2022)
[51] South Australia Arid Lands Landscape Board. Notifiable diseases detected in feral pigs. Across the Outback, 2022, Issue 97, October, p. 11. https://www.landscape.sa.gov.au/saal/news-resources/publications (accessed 10 October 2022)
[52] Appaiahgari, MB and Vrati, S (2012) Clinical development of IMOJEV®—a recombinant Japanese encephalitis chimeric vaccine (JE-CV). Expert Opin Biol Ther 12, 1251–1263.
| Clinical development of IMOJEV®—a recombinant Japanese encephalitis chimeric vaccine (JE-CV).Crossref | GoogleScholarGoogle Scholar |
[53] Firbas, C and Jilma, B (2015) Product review on the JE vaccine IXIARO. Hum Vaccin Immunother 11, 411–420.
| Product review on the JE vaccine IXIARO.Crossref | GoogleScholarGoogle Scholar |
[54] Igarashi, A (2002) Control of Japanese encephalitis in Japan: immunization of humans and animals, and vector control. Curr Top Microbiol Immunol 267, 139–152.
| Control of Japanese encephalitis in Japan: immunization of humans and animals, and vector control.Crossref | GoogleScholarGoogle Scholar |
[55] van den Hurk, AF et al.. (2022) The emergence of Japanese encephalitis virus in Australia in 2022: existing knowledge of mosquito vectors. Viruses 14, 1208.
| The emergence of Japanese encephalitis virus in Australia in 2022: existing knowledge of mosquito vectors.Crossref | GoogleScholarGoogle Scholar |
[56] Daniels P et al. (2000) Assessment of the potential of Australian fauna as maintenance or amplifying hosts of Japanese encephalitis (JE) virus. In Report to the Northern Australian Quarantine Strategy. CSIRO Animal Health Laboratory, Geelong, Australia. pp. 1–3.
[57] New South Wales Health Department. Summary of NSW Japanese encephalitis virus serosurvey results. https://www.health.nsw.gov.au/environment/pests/vector/Documents/jev-serosurvey-report.pdf (accessed 5 November 2022)
[58] Pham, D et al.. (2022) Emergence of Japanese encephalitis in Australia: a diagnostic perspective. Pathology 54, 669–677.
| Emergence of Japanese encephalitis in Australia: a diagnostic perspective.Crossref | GoogleScholarGoogle Scholar |