The ‘other’ epidemic: canine ehrlichiosis in Australia
Peter Irwin A * and John Beadle BA Vector and Waterborne Pathogens Research Group, School of Veterinary Medicine, College of Science, Health, Engineering and Education, Murdoch University, Murdoch, WA 6150, Australia.
B All Creatures Veterinary Hospital Broome, PO Box 1752, Broome, WA 6725, Australia.

Peter Irwin is Emeritus Professor at Murdoch University. He has been studying tick-borne diseases of companion animals and wildlife for >30 years. |

John Beadle is a veterinarian and owner of a general practice in Broome, WA. His interests are 4WD remote camping, photography, cycling, yoga, orthopaedic surgery, astronomy, skiing, and hiking. |
Microbiology Australia 43(4) 156-159 https://doi.org/10.1071/MA22053
Submitted: 30 October 2022 Accepted: 1 December 2022 Published: 16 December 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Canine monocytic ehrlichiosis (Ehrlichia canis infection) is a serious tick-transmitted disease of dogs that was considered exotic to Australia until recently. The disease was first reported across northern and central Australia in 2020, with significant canine morbidity and mortalities observed at indigenous communities where dog numbers are high, ticks are superabundant, and tick prevention is scant. The date and location of the incursion are unknown, yet comparative genomic analysis suggest the Australian E. canis may have originated from Asia or the Middle East. Veterinarians nationwide are on alert for this notifiable disease since cases have been reported in southern locations as a consequence of moving infected dogs from endemic areas. Acute infections in dogs respond favourably to doxycycline therapy, however chronic disease results in bone marrow failure and death. Tick prophylaxis is key to preventing canine ehrlichiosis and is best achieved using products that repel and kill ticks before they attach. Although reports exist of E. canis as a zoonosis, there is no evidence that the strain involved in the current Australian outbreak poses risk to humans.
Keywords: canine, dog, Ehrlichia, Ehrlichia canis, Rhipicephalus linnaei, Rhipicephalus sanguineus, tick, vector-borne disease.
Introduction
Canine monocytic ehrlichiosis (CME) is a tick-transmitted disease of domestic dogs and wild canids caused by Ehrlichia canis (E. canis), a Gram-negative, intracellular bacterium belonging to the family Anaplasmataceae.1 The organism, initially named Rickettsia canis, was first described in dogs in Algeria,2 and later came to prominence during the Vietnam War in the 1960–70s when scores of military working dogs died of what was referred to as ‘tropical canine pancytopenia’.3–5 Today, CME is recognised as a common illness affecting dogs in tropical and subtropical regions of the world with the exception, until recently, of Australia.
In May 2020, in Kununurra, northern Western Australia (WA), dogs with significant fever, malaise, and haemorrhagic diatheses were diagnosed with E. canis infection, sparking a national veterinary biosecurity alert.6 Despite its designation as a notifiable disease, the swift introduction of movement restrictions for dogs, and the unfolding COVID-19 pandemic during 2020 (that imposed travel limitations on the general public, including dog owners), CME was confirmed to have spread throughout northern WA and the Northern Territory, with high morbidity and mortalities reported, especially among dogs at indigenous communities.7
Biological and clinical aspects
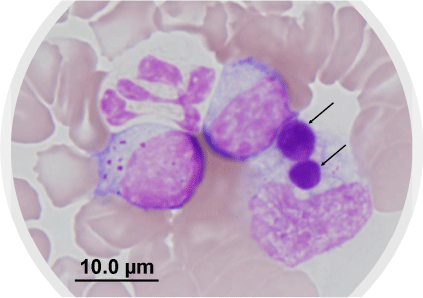
The biological and clinical features of E. canis infection have been studied extensively for many years (outside Australia). The vector is the brown dog tick, Rhipicephalus linnaei, previously named Rhipicephalus sanguineus sensu lato (‘tropical strain’).8,9 As the latter name implies, this tick is enzootic throughout warm and humid climates, including northern and central Australia, and is well adapted to survival in dwellings and kennel environments.10 Transmission of E. canis to the dog occurs rapidly, within 3 h of tick attachment,11 and pleomorphic organisms may be observed as intracytoplasmic inclusions in circulating monocytes, either singly or in compact colonies (morulae) (Fig. 1).5 It is known from experimental studies that E. canis infection in dogs progresses through acute, sub-clinical, and chronic (terminal) phases,12 and clinical illness develops in dogs approximately 1–3 weeks post-infection. Typical clinical signs reported globally include lethargy, weakness, anorexia, fever, pale membranes, panting, bleeding diathesis, hepatosplenomegaly, and dehydration.8,13 Despite the severe nature of some cases of acute E. canis infection, including fatalities, many dogs in endemic areas overseas appear to tolerate infection without developing overt clinical disease.8
|
In contrast, clinical data recorded by one of us (JB, unpublished observations) in Broome, WA during 2020–2021, reveals a severe clinical syndrome in dogs diagnosed with CME during the first 6–9 months after the disease was recognised. Epistaxis was often profound (Fig. 2a), and body temperatures as high as 41.5°C were not uncommon. Some dogs presented with dyspnoea and fever, but no bleeding, and in others cachexia was often severe and rapid in onset. Dogs with marked pancytopenia on haematology rapidly progressed to sepsis and uncontrollable bleeding within a few days, and some dogs presented with blindness due to retinal haemorrhages. Another group developed generalised dependant oedema, often months after the acute illness, characterised by marked swelling of the face, neck and limbs (Fig. 2b) and, in some cases, the scrotum (Fig. 2c).
A fascinating historical comparison can be drawn between these severe clinical descriptions in the tropical maritime climate of Broome and those reported by veterinarians in similar climatic conditions managing the disease in military working dogs (MWD) brought into Southeast Asia from Europe and the US during the conflicts of the 1960s and 1970s.14 Reports then described a highly fatal epizootic tropical canine pancytopenia, the aetiology of which was not immediately apparent, even raising concerns about environmental toxins and ionising radiation.3 Some of the worst clinical manifestations, including the development of oedema, are less commonly reported since the dramatic descriptions from Indochina 50 years ago. We believe that the clinical parallels between Vietnam in the 1960s and Broome in 2020 deserve to be recorded, and presumably reflect the sudden and overwhelming exposure of immunologically naïve dogs to large numbers of ticks infected with E. canis. Systemic inflammation and widespread vasculitis are the underlying pathological processes of acute CME, with the development of myelosuppression in some cases.15
Due to its close taxonomic/phylogenetic relationship with rickettsial organisms, E. canis was treated originally with tetracyclines,14 but doxycycline is now the most widely used antimicrobial for CME.16,17 Evidence for its antimicrobial efficacy comes from in vitro studies that indicate E. canis is highly susceptible to doxycycline, requiring a very low minimum inhibitory concentration (MIC) (0.03 µg/mL).18 There is generally a good clinical response to a 28 day course of doxycycline (and other supportive care, as indicated) in the acute phase, however some dogs remain bacteraemic (therefore infectious to ticks) for unpredictably variable periods of time during the course of antimicrobial drug treatment, and in a few cases after cessation of doxycycline.19,20
Microbial considerations and molecular genetics
Evidence for strain variation comes from research into variable genes of E. canis predominantly comprising genetic loci that encode major immunoreactive proteins.21 At least four glycoproteins (gp19, gp36, gp140, gp200) have been characterised molecularly and major continuous species-specific epitopes have been mapped to acidic serine-rich tandem repeat proteins (TRP).22,23 Genetic variations have been described through analysis of these TRPs, demonstrating different amino acid sequences among E. canis strains from different geographical areas.24 Ehrlichia canis TRP36 is a species-specific antigen with different tandem repeat sequences among E. canis strains and variations in the TRP36 gene are used widely to describe strains of E. canis worldwide, and at least four clades/genogroups of TRP36 have been described.25
Recently, the full genome of E. canis in Australia was sequenced,26 providing information about the strain and its phylogenetic relationship with overseas isolates. Ehrlichia canis sequenced from ticks and blood samples collected from dogs with CME were found to be highly conserved across large geographical distances,26 consistent with the assumption of a recent introduction of the disease into the country. Analysis demonstrated that the Australian E. canis belongs to the Taiwan genotype, that is widespread throughout Asia and the Middle East.26
Prevention of ehrlichiosis in dogs
Until the arrival of CME, the most serious tick-associated disorder afflicting dogs in Australia was tick paralysis, resulting from envenomation by the eastern Australian paralysis tick, Ixodes holocyclus. In contrast to the rapid transmission of E. canis from brown dog ticks described above,11 the neurotoxins injected by I. holocyclus do not take effect until 3–4 days after tick attachment. Understanding this difference in timing is crucial with respect to the use of acaricides (tick prevention products). Topically acting synthetic pyrethroids (e.g. flumethrin) exert their protective effect through repellency (and kill), thus preventing tick attachment, and are proven to protect dogs against transmission of E. canis.27 The other major class of acaricides currently in use in companion animal practice, the isoxazolines, are systemically acting, requiring the tick to feed in order to take effect.28 This works well to prevent tick paralysis but does not protect dogs from acquiring CME, although isoxazoline products stop onward transmission of E. canis from an infected dog, and have proven a valuable adjunctive treatment to reduce tick burdens where dogs are housed.
Zoonotic implications
Ehrlichia canis is related to several members of the Anaplasmataceae that are recognised to be zoonotic, including E. chaffeensis, E. ewingii and E. muris eauclairensis,1 and there is evidence for the zoonotic potential of E. canis from molecular and serological testing in Central and South America.29 However, to date there have been only small numbers of people with confirmed E. canis infections in South America and there are no reports of infection in humans that implicate the Asian/Taiwan genogroup of E. canis. It therefore seems unlikely that E. canis will be of public health concern in Australia.
Implications for Australia
It is fortunate and, indeed, somewhat surprising, that Australia remained free of CME for so long, considering the abundant population of brown dog ticks across the north. Prior to 2020 there were occasional reports of E. canis-positive serological tests in dogs from northern and central Australia, yet follow-up investigations using PCR testing were negative. Seropositivity in these individuals was attributed to false positive results, likely cross-reactions with the closely related Anaplasma platys, also associated with the same tick vector.30 Mandatory pre-import screening (comprising immunofluorescence antibody test by registered laboratories) was established in September 1995,30 and dogs testing positive were prohibited entry into the country.
Regardless, current surveillance data indicate that infected dogs (or ticks) have been detected in most states and territories, and the disease is now (2022) regarded as endemic. There is no hope of eradication, so veterinarians will need to add CME to the differential diagnosis of canine illnesses in northern Australia and in travelled dogs, and institute prompt treatment when cases are diagnosed.
Data availability
The data that support this study cannot be publicly shared due to ethical or privacy reasons and may be shared upon reasonable request to the corresponding author if appropriate.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] Rar, V and Golovljova, I (2011) Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect Genet Evol 11, 1842–1861.| Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review.Crossref | GoogleScholarGoogle Scholar |
[2] Donatien, A and Lestoguard, F (1935) Existence en Algerie d’une Rickettsia du chien. Bull Soc Pathol Exot 28, 418–419.
[3] Nims, RM et al.. (1971) Epizootiology of tropical canine pancytopenia in Southeast Asia. J Am Vet Med Assoc 158, 53–63.
[4] Seamer, J and Snape, T (1972) Ehrlichia canis and tropical canine pancytopenia. Res Vet Sci 13, 307–314.
| Ehrlichia canis and tropical canine pancytopenia.Crossref | GoogleScholarGoogle Scholar |
[5] Huxsoll, DL (1976) Canine ehrlichiosis (tropical canine pancytopenia): a review. Vet Parasitol 2, 49–60.
| Canine ehrlichiosis (tropical canine pancytopenia): a review.Crossref | GoogleScholarGoogle Scholar |
[6] Shilton , C et al.. (2020) Detection of Ehrlichia canis in WA and the NT. The Scope 1, 12–16.
[7] Australian Government Department of Agriculture, Fisheries and Forestry (2022) Ehrlichiosis in dogs. https://www.agriculture.gov.au/biosecurity-trade/pests-diseases-weeds/animal/ehrlichiosis-in-dogs
[8] Little, SE (2010) Ehrlichiosis and anaplasmosis in dogs and cats. Vet Clin North Am Small Anim Pract 40, 1121–1140.
| Ehrlichiosis and anaplasmosis in dogs and cats.Crossref | GoogleScholarGoogle Scholar |
[9] Šlapeta, J et al.. (2021) The “tropical lineage” of the brown dog tick Rhipicephalus sanguineus sensu lato identified as Rhipicephalus linnaei (Audouin, 1826). Int J Parasitol 51, 431–436.
| The “tropical lineage” of the brown dog tick Rhipicephalus sanguineus sensu lato identified as Rhipicephalus linnaei (Audouin, 1826).Crossref | GoogleScholarGoogle Scholar |
[10] Dantas-Torres, F (2010) Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors 3, 26.
| Biology and ecology of the brown dog tick, Rhipicephalus sanguineus.Crossref | GoogleScholarGoogle Scholar |
[11] Fourie, JJ et al.. (2013) Transmission of Ehrlichia canis by Rhipicephalus sanguineus ticks feeding on dogs and on artificial membranes. Vet Parasitol 197, 595–603.
| Transmission of Ehrlichia canis by Rhipicephalus sanguineus ticks feeding on dogs and on artificial membranes.Crossref | GoogleScholarGoogle Scholar |
[12] Harrus, S (2015) Perspectives on the pathogenesis and treatment of canine monocytic ehrlichiosis (Ehrlichia canis). Vet J 204, 239–240.
| Perspectives on the pathogenesis and treatment of canine monocytic ehrlichiosis (Ehrlichia canis).Crossref | GoogleScholarGoogle Scholar |
[13] Harrus, S et al.. (1997) Canine monocytic ehrlichiosis: a retrospective study of 100 cases, and an epidemiological investigation of prognostic indicators for the disease. Vet Rec 141, 360–363.
| Canine monocytic ehrlichiosis: a retrospective study of 100 cases, and an epidemiological investigation of prognostic indicators for the disease.Crossref | GoogleScholarGoogle Scholar |
[14] Walker, JS et al.. (1970) Clinical and clinicopathologic findings in tropical canine pancytopenia. J Am Vet Med Assoc 157, 43–55.
[15] De Castro, MB et al.. (2004) Experimental acute canine monocytic ehrlichiosis: clinicopathological and immunopathological findings. Vet Parasitol 119, 73–86.
| Experimental acute canine monocytic ehrlichiosis: clinicopathological and immunopathological findings.Crossref | GoogleScholarGoogle Scholar |
[16] Mylonakis, ME et al.. (2019) An update on the treatment of canine monocytic ehrlichiosis (Ehrlichia canis). Vet J 246, 45–53.
| An update on the treatment of canine monocytic ehrlichiosis (Ehrlichia canis).Crossref | GoogleScholarGoogle Scholar |
[17] Neer, TM et al.. (2002) Consensus statement on ehrlichial disease of small animals from the infectious disease study group of the ACVIM. J Vet Intern Med 16, 309–315.
| Consensus statement on ehrlichial disease of small animals from the infectious disease study group of the ACVIM.Crossref | GoogleScholarGoogle Scholar |
[18] Branger, S et al.. (2004) Evaluation of antibiotic susceptibilities of Ehrlichia canis, Ehrlichia chaffeensis, and Anaplasma phagocytophilum by real-time PCR. Antimicrob Agents Chemother 48, 4822–4828.
| Evaluation of antibiotic susceptibilities of Ehrlichia canis, Ehrlichia chaffeensis, and Anaplasma phagocytophilum by real-time PCR.Crossref | GoogleScholarGoogle Scholar |
[19] Harrus, S et al.. (1998) Therapeutic effect of doxycycline in experimental subclinical canine monocytic ehrlichiosis: evaluation of a 6-week course. J Clin Microbiol 36, 2140–2142.
| Therapeutic effect of doxycycline in experimental subclinical canine monocytic ehrlichiosis: evaluation of a 6-week course.Crossref | GoogleScholarGoogle Scholar |
[20] Schaefer, JJ et al.. (2007) Tick acquisition of Ehrlichia canis from dogs treated with doxycycline hyclate. Antimicrob Agents Chemother 51, 3394–3396.
| Tick acquisition of Ehrlichia canis from dogs treated with doxycycline hyclate.Crossref | GoogleScholarGoogle Scholar |
[21] Mavromatis, K et al.. (2006) The genome of the obligately intracellular bacterium Ehrlichia canis reveals themes of complex membrane structure and immune evasion strategies. J Bacteriol 188, 4015–4023.
| The genome of the obligately intracellular bacterium Ehrlichia canis reveals themes of complex membrane structure and immune evasion strategies.Crossref | GoogleScholarGoogle Scholar |
[22] Doyle, CK et al.. (2005) Molecular characterization of E. canis gp36 and E. chaffeensis gp47 tandem repeats among isolates from different geographic locations. Ann N Y Acad Sci 1063, 433–435.
| Molecular characterization of E. canis gp36 and E. chaffeensis gp47 tandem repeats among isolates from different geographic locations.Crossref | GoogleScholarGoogle Scholar |
[23] Zhang, X et al.. (2008) Genetic and antigenic diversities of major immunoreactive proteins in globally distributed Ehrlichia canis strains. Clin Vaccine Immunol 15, 1080–1088.
| Genetic and antigenic diversities of major immunoreactive proteins in globally distributed Ehrlichia canis strains.Crossref | GoogleScholarGoogle Scholar |
[24] Bezerra-Santos, MA et al.. (2021) Genetic variability of Ehrlichia canis TRP36 in ticks, dogs, and red foxes from Eurasia. Vet Microbiol 255, 109037.
| Genetic variability of Ehrlichia canis TRP36 in ticks, dogs, and red foxes from Eurasia.Crossref | GoogleScholarGoogle Scholar |
[25] Geiger, J et al.. (2018) Molecular Characterization of Tandem Repeat Protein 36 Gene of Ehrlichia canis Detected in Naturally Infected Dogs from Peru. Am J Trop Med Hyg 99, 297–302.
| Molecular Characterization of Tandem Repeat Protein 36 Gene of Ehrlichia canis Detected in Naturally Infected Dogs from Peru.Crossref | GoogleScholarGoogle Scholar |
[26] Neave, MJ et al.. (2022) Comparative genomic analysis of the first Ehrlichia canis detections in Australia. Ticks Tick Borne Dis 13, 101900.
| Comparative genomic analysis of the first Ehrlichia canis detections in Australia.Crossref | GoogleScholarGoogle Scholar |
[27] Stanneck, D and Fourie, JJ (2013) Imidacloprid 10%/flumethrin 4.5% collars (Seresto®, Bayer) successfully prevent long-term transmission of Ehrlichia canis by infected Rhipicephalus sanguineus ticks to dogs. Parasitol Res 112, 21–32.
| Imidacloprid 10%/flumethrin 4.5% collars (Seresto®, Bayer) successfully prevent long-term transmission of Ehrlichia canis by infected Rhipicephalus sanguineus ticks to dogs.Crossref | GoogleScholarGoogle Scholar |
[28] Burgio, F et al.. (2016) A comparative laboratory trial evaluating the immediate efficacy of fluralaner, afoxolaner, sarolaner and imidacloprid + permethrin against adult Rhipicephalus sanguineus (sensu lato) ticks attached to dogs. Parasit Vectors 9, 626.
| A comparative laboratory trial evaluating the immediate efficacy of fluralaner, afoxolaner, sarolaner and imidacloprid + permethrin against adult Rhipicephalus sanguineus (sensu lato) ticks attached to dogs.Crossref | GoogleScholarGoogle Scholar |
[29] Unver, A et al.. (2001) Molecular and antigenic comparison of Ehrlichia canis isolates from dogs, ticks, and a human in Venezuela. J Clin Microbiol 39, 2788–2793.
| Molecular and antigenic comparison of Ehrlichia canis isolates from dogs, ticks, and a human in Venezuela.Crossref | GoogleScholarGoogle Scholar |
[30] Mason, RJ et al.. (2001) Serological survey for Ehrlichia canis in urban dogs from the major population centres of northern Australia. Aust Vet J 79, 559–562.
| Serological survey for Ehrlichia canis in urban dogs from the major population centres of northern Australia.Crossref | GoogleScholarGoogle Scholar |



