‘The awesome power of yeast’ in Alzheimer’s disease research
Sudip DhakalSchool of Science, RMIT University, Bundoora, Vic. 3083, Australia. Email: sudip.dhakal@rmit.edu.au; dhakal.sudip001@gmail.com
Microbiology Australia 42(3) 130-133 https://doi.org/10.1071/MA21034
Submitted: 6 July 2021 Accepted: 7 August 2021 Published: 6 September 2021
Journal Compilation © The Authors 2021 Open Access CC BY-NC-ND, published (by CSIRO Publishing) on behalf of the ASM
Abstract
The difficulties in performing experimental studies related to diseases of the human brain have fostered a range of disease models from highly expensive and complex animal models to simple, robust, unicellular yeast models. Yeast models have been used in numerous studies to understand Alzheimer’s disease (AD) pathogenesis and to search for drugs targeting AD. Thanks to the conservation of fundamental eukaryotic processes including ageing and the availability of appropriate technological platforms, budding yeast are a simple model eukaryote to assist with understanding human cell biology, offering a platform to study human diseases. This article aims to provide insights from yeast models on the contributions of amyloid beta, a causative agent in AD, and recent research findings on AD chemoprevention.
Alzheimer’s disease (AD) is one of the most important progressive age-related neurodegenerative diseases. It accounts for the majority (60–80%) of dementia-related deaths in the elderly1. Until recently, AD drugs approved by FDA have been limited to the treatment of moderate symptoms2. In June 2021, Aducanumab, a monoclonal antibody acting against amyloid beta (Aβ), was approved by the FDA3,4. However, it is not likely to cure the neuronal loss found in AD5.
The major hallmarks of AD pathology include the presence of extracellular amyloid plaques, intracellular tau neurofibrillary tangles, accumulation of oxidative stress, loss of proteostasis, epigenetic changes, alteration in biometal distributions, lipid imbalances, mitochondrial dysfunction, genomic instability, chronic cellular stress, neuroinflammation, neuronal death, loss of synapses and cognitive deficits6. The complexity and difficulty of assessing the brain in living humans makes it difficult to perform experimental research to understand AD pathogenesis and has hindered exploration of therapeutic strategies.
Yeast models and bioassays to study AD
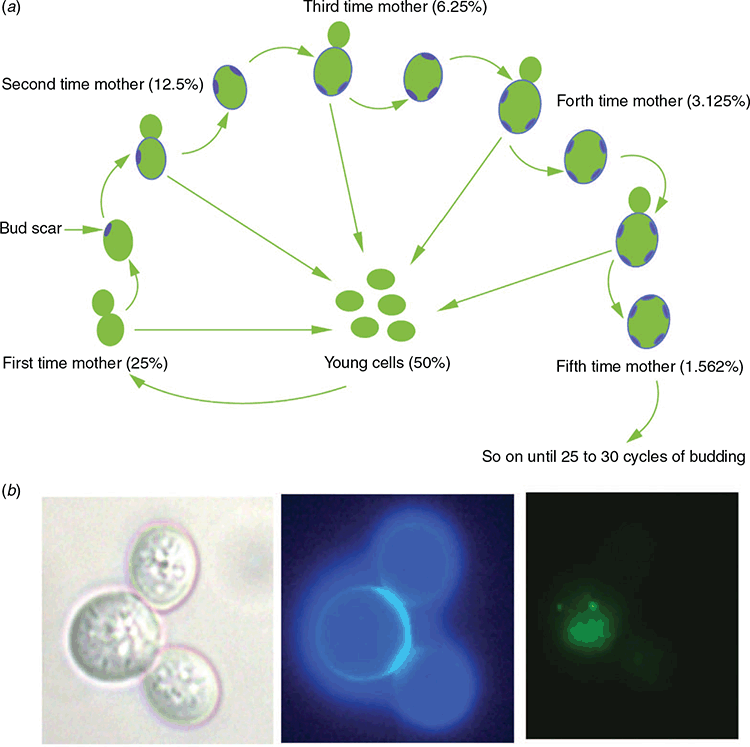
The “awesome power of yeast” has been hailed by numerous researchers, with at least five Nobel prizes awarded to yeast researchers in the last two decades. In studies initiated by Macreadie and colleagues, yeast has offered powerful contributions for studying AD pathogenesis and for finding novel therapeutic agents. There are many reasons for the preference of yeast models in AD research. The most important one stems from the conservation of the molecular mechanisms in yeast that inform about fundamental processes of human biology7. Energy metabolism, genetics, vesicle trafficking, cell division, protein homeostasis networks, lipid metabolism, stress response pathways and cell death pathways are some major processes that are conserved between humans and yeasts8. The different phases of yeast growth also allow us to understand chronological and replicative lifespans9. Stationary phase yeast cells can mimic terminally differentiated human neurons with several neuronal features conserved in these cells. Most yeast species divide by budding. When a budding yeast mother cell produces a bud, chitin-rich bud scars are left behind on the cell surface (Figure 1a). Staining of these bud scars provides an excellent method to differentiate the old and young cells (Figure 1b), which is instrumental to understand the ageing process. In addition, the availability of several analytical platforms to analyse single cells or large populations, robust growth, facile genetic modification and the existence of numerous yeast species and gene deletion libraries improves our ability to use them as models for several chronic diseases including AD8.
|
It is now clear that the protein homeostasis network is involved in the pathogenesis of AD, and proteostasis failure a major cause of AD. Proteostasis failure in AD is present at all levels of the protein quality control system inside a cell including unfolded protein response, ubiquitin proteasome system and autophagy5. Some of the most important evidence depicting the proteostasis failure are the presence of misfolded proteins, calcium dyshomeostasis, defective proteaphagy, impaired mitophagy, mutations of ubiquitin, oxidation of deubiquitinating enzymes, vacuolar-ATPase assembly defects and presence of several types of uncleared autophagic vesicles containing the toxic amyloid beta (Aβ) protein in the neurons of AD patients5. Most importantly, the persistent misfolded proteins and protein aggregates of Aβ and tau in AD patients continuously activate the heat stress response in the neuronal cells11: Aβ and tau are the proteins most associated with AD and are likely to be the causative agents. Meanwhile, the chronic stress response activation and inability of the protective stress response to recover the cells from detrimental effects of these toxic proteins render cells prone to activation of destructive mechanisms12. Disrupted proteostasis and protein aggregation is common in ageing yeast, similar to that of ageing neurons. Considering such similarities with human cells, several yeast assays have been developed to monitor these fundamental eukaryotic processes or to find chemicals that can protect cells from the toxicity of protein misfolding and ageing.
A number of assays have been developed to study autophagy in yeast13. Yeast expressing amyloid precursor protein, Aβ, GFP-Aβ and tau protein have been engineered to study the effects of these proteins on cells8,14,15. Such yeasts have shown that Aβ causes mitochondrial dysfunction, and increases intracellular reactive oxygen species, cell stress and cell death15,16. Interestingly, ageing yeast cells harboured Aβ while young cells removed it, mimicking what is observed to happen to Aβ in human ageing (Figure 1b). Being able to evaluate the turnover of GFP-Aβ measured by its fluorescence has revolutionized the drug screening process17. The punctate patterns of the GFP-Aβ fusion protein observed in the microscopic images (Figure 1b) illustrate the aggregating nature of the fusion protein and provide evidence of the utility of the yeast model15.
Bioassays to find chemoprotective agents against AD
Yeasts have not only been used as a platform to understand how Aβ and tau proteins are involved in cellular destruction, but have also been used to study drugs and bioactive compounds that can prevent detrimental effects caused by Aβ and tau17–19. For example, simvastatin (the best chemopreventative for AD20) has been shown to reduce levels of both GFP-Aβ and native Aβ from yeast cells. This reduction was independent of the effect of statins on lowering ergosterol, a functional equivalent of cholesterol in yeast17. It is considered that simvastatin might act on other pathways in addition to that involving cholesterol biosynthesis. One such mechanism that is of high importance is protein prenylation21. Importantly, protein prenylation is involved in regulating autophagy, inflammatory responses, oxidative stress and synaptic/cognitive function22. In addition, to determine the stress response activation, a stress reporter yeast has been developed23. The reporter yeast is designed such that the mCherry fluorescent protein is expressed under control of a heat shock promoter23. The promoter is activated once the heat shock factor 1 (HSF1) transcription factor was translocated into the nucleus as a result of stress, thus activating the heat stress response. This yeast model could be an important method for rapid screening of compounds that can induce stress response and simultaneously decrease levels of Aβ in cells. In addition, when used in conjunction with other assays such as reactive oxygen species (ROS) measurement, toxicity assays and growth inhibition assays, yeasts may provide unprecedented benefits. So far, the results obtained from such studies have been promising and have demonstrated that these assays could lead to excellent discovery of novel therapeutics17–19,24.
Bioassays to find compounds that enhance Aβ toxicity
Engineered yeast have also been used to identify compounds that exhibit toxic synergies with Aβ. In separate recent studies, both tyramine and aluminium exacerbated Aβ toxicity by enhancing the ROS inside cells25,26. Aluminium is the most abundant neurotoxic metal present in our surroundings including our food and consumer products. Although the toxic effect of aluminium in AD has been proposed for decades with limited evidence, the recent yeast study illustrated its ability to enhance Aβ toxicity suggesting its potential role in AD25. Similarly, tyramine and Aβ’s synergistic toxicity indicates tyramine’s potential role in AD26. It is difficult to perform similar studies to understand the involvement of biogenic amines in humans as they are present in very low amount and they are rapidly metabolized. In contrast, the yeast models hint at the potential role of trace amines in AD. Furthermore, trace amine associated receptor (TAAR) signalling could also be involved in AD as it has been found to be associated with various molecular pathways involved in AD27.
Mitochondrial dysfunction and impaired mitophagy are common features of AD28. The fact that mammalian cells are not able to survive with defective mitochondria makes it impossible for studies involving investigations on mitochondrial health. On the contrary, the ability of yeasts to grow without functional mitochondria allows discovery of chemicals or biomolecules that directly affect mitochondria, its turnover and biogenesis9. For example, the respiratory growth (growth supported by mitochondria) of the yeast cells was inhibited more in GFP-Aβ transformant yeast cells compared to those in GFP transformants in the presence of tyramine and aluminium indicating increased mitochondrial and mitophagy defects in these cells25,26. These studies illustrated that yeast provided a unique platform to investigate compounds that affect mitochondrial health, which is impossible in higher eukaryotes.
The potential role of these novel modifiers of Aβ toxicity, that are present in human body, can be identified using the yeast models. This opens up avenues for new dimensions in AD research25,26. These studies are important because the sporadic nature of AD suggests factors in addition to Aβ may be involved in AD. These factors could be compounds or elements discovered in recent studies using yeast models.
Conclusion
In summary, yeast models have provided a very attractive platform to study the toxic proteins involved in AD and compounds that can modify the effects of these proteins. Intriguingly, yeast mitochondrial function is not essential for yeast survival, so it is the only available model that can grow with defective mitochondria enabling studies on drugs that specifically target mitochondrial health. Despite the enormous potential of yeast as model for AD research, the lack of proper nervous system, endocrine system, circulatory system, immune system, and other systems that are present in humans limit its applications. However, with careful manipulation of the yeast genome and appropriate alterations in culture conditions it is possible to mimic the human cellular environment. Importantly, yeasts have the potential to enable us to answer significant research questions about AD including those related to cell–cell communication. New dimensions in AD pathogenesis and pathology along with the discovery of novel bioactive compounds beneficial for prevention or cure of AD are being discovered thanks to “the awesome power of yeast”.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] (2021) 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 17, 327–406.| 2021 Alzheimer’s disease facts and figures.Crossref | GoogleScholarGoogle Scholar | 33756057PubMed |
[2] Abeysinghe, A.A.D.T. et al. (2020) Alzheimer’s disease; a review of the pathophysiological basis and therapeutic interventions. Life Sci. 256, 117996.
| Alzheimer’s disease; a review of the pathophysiological basis and therapeutic interventions.Crossref | GoogleScholarGoogle Scholar |
[3] Schneider, L. (2020) A resurrection of aducanumab for Alzheimer’s disease. Lancet Neurol. 19, 111–112.
| A resurrection of aducanumab for Alzheimer’s disease.Crossref | GoogleScholarGoogle Scholar | 31978357PubMed |
[4] Mahase, E. (2021) FDA approves controversial Alzheimer’s drug despite uncertainty over effectiveness. BMJ 373, 373.
[5] Dhakal, S. and Macreadie, I. (2020) Protein homeostasis networks and the use of yeast to guide interventions in Alzheimer’s disease. Int. J. Mol. Sci. 21, 8014.
| Protein homeostasis networks and the use of yeast to guide interventions in Alzheimer’s disease.Crossref | GoogleScholarGoogle Scholar |
[6] Dhakal, S. et al. (2019) Dietary polyphenols: a multifactorial strategy to target Alzheimer’s disease. Int. J. Mol. Sci. 20, 5090.
| Dietary polyphenols: a multifactorial strategy to target Alzheimer’s disease.Crossref | GoogleScholarGoogle Scholar |
[7] Khurana, V. and Lindquist, S. (2010) Modelling neurodegeneration in Saccharomyces cerevisiae: why cook with baker’s yeast? Nat. Rev. Neurosci. 11, 436–449.
| Modelling neurodegeneration in Saccharomyces cerevisiae: why cook with baker’s yeast?Crossref | GoogleScholarGoogle Scholar | 20424620PubMed |
[8] Mcdonald, J.B. et al. (2020) Yeast contributions to Alzheimer’s disease. J. Human Clin. Genet. 2, 1–19.
| Yeast contributions to Alzheimer’s disease.Crossref | GoogleScholarGoogle Scholar |
[9] Seynnaeve, D. et al. (2018) Recent insights on Alzheimer’s disease originating from yeast models. Int. J. Mol. Sci. 19, 1947.
| Recent insights on Alzheimer’s disease originating from yeast models.Crossref | GoogleScholarGoogle Scholar |
[10] Macreadie, I. and Luu, Y.N. (2018) How yeast can inform us about healthy aging. Open J. Soc. Sci. 6, 24–31.
| How yeast can inform us about healthy aging.Crossref | GoogleScholarGoogle Scholar |
[11] San Gil, R. et al. (2017) The heat shock response in neurons and astroglia and its role in neurodegenerative diseases. Mol. Neurodegener. 12, 65.
| The heat shock response in neurons and astroglia and its role in neurodegenerative diseases.Crossref | GoogleScholarGoogle Scholar | 28923065PubMed |
[12] Richter, K. et al. (2010) The heat shock response: life on the verge of death. Mol. Cell 40, 253–266.
| The heat shock response: life on the verge of death.Crossref | GoogleScholarGoogle Scholar | 20965420PubMed |
[13] Torggler, R. et al. (2017) Assays to monitor autophagy in Saccharomyces cerevisiae. Cells 6, 23.
| Assays to monitor autophagy in Saccharomyces cerevisiae.Crossref | GoogleScholarGoogle Scholar |
[14] Vandebroek, T. et al. (2005) Identification and isolation of a hyperphosphorylated, conformationally changed intermediate of human protein tau expressed in yeast. Biochemistry 44, 11466–11475.
| Identification and isolation of a hyperphosphorylated, conformationally changed intermediate of human protein tau expressed in yeast.Crossref | GoogleScholarGoogle Scholar | 16114883PubMed |
[15] Caine, J. et al. (2007) Alzheimer’s Aβ fused to green fluorescent protein induces growth stress and a heat shock response. FEMS Yeast Res. 7, 1230–1236.
| Alzheimer’s Aβ fused to green fluorescent protein induces growth stress and a heat shock response.Crossref | GoogleScholarGoogle Scholar | 17662055PubMed |
[16] Chen, X. and Petranovic, D. (2015) Amyloid-β peptide-induced cytotoxicity and mitochondrial dysfunction in yeast. FEMS Yeast Res. 15, fov061.
| Amyloid-β peptide-induced cytotoxicity and mitochondrial dysfunction in yeast.Crossref | GoogleScholarGoogle Scholar | 26152713PubMed |
[17] Dhakal, S. et al. (2019) Simvastatin efficiently reduces levels of Alzheimer’s amyloid beta in yeast. Int. J. Mol. Sci. 20, 3531.
| Simvastatin efficiently reduces levels of Alzheimer’s amyloid beta in yeast.Crossref | GoogleScholarGoogle Scholar |
[18] Chen, X. et al. (2020) FMN reduces amyloid-β toxicity in yeast by regulating redox status and cellular metabolism. Nat. Commun. 11, 867.
| FMN reduces amyloid-β toxicity in yeast by regulating redox status and cellular metabolism.Crossref | GoogleScholarGoogle Scholar | 32054832PubMed |
[19] Porzoor, A. et al. (2015) Anti-amyloidogenic properties of some phenolic compounds. Biomolecules 5, 505–527.
| Anti-amyloidogenic properties of some phenolic compounds.Crossref | GoogleScholarGoogle Scholar | 25898401PubMed |
[20] Wolozin, B. et al. (2007) Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 5, 20.
| Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease.Crossref | GoogleScholarGoogle Scholar | 17640385PubMed |
[21] Carabott, M. et al. (2021) Lipids, statins and susceptibility to SARS-CoV-2 and influenza A viruses. Microbiol. Aust. 42, 87–91.
| Lipids, statins and susceptibility to SARS-CoV-2 and influenza A viruses.Crossref | GoogleScholarGoogle Scholar |
[22] Hottman, D.A. and Li, L. (2014) Protein prenylation and synaptic plasticity: implications for Alzheimer’s disease. Mol. Neurobiol. 50, 177–185.
| Protein prenylation and synaptic plasticity: implications for Alzheimer’s disease.Crossref | GoogleScholarGoogle Scholar | 24390573PubMed |
[23] Luu, Y.N. and Macreadie, I. (2018) Development of convenient system for detecting yeast cell stress, including that of amyloid beta. Int. J. Mol. Sci. 19, 2136.
| Development of convenient system for detecting yeast cell stress, including that of amyloid beta.Crossref | GoogleScholarGoogle Scholar |
[24] Bharadwaj, P.R. et al. (2012) Latrepirdine (dimebon) enhances autophagy and reduces intracellular GFP-Aβ42 levels in yeast. J. Alzheimers Dis. 32, 949–967.
| Latrepirdine (dimebon) enhances autophagy and reduces intracellular GFP-Aβ42 levels in yeast.Crossref | GoogleScholarGoogle Scholar | 22903131PubMed |
[25] McDonald, J.B. et al. (2021) A toxic synergy between aluminium and amyloid beta in yeast. Int. J. Mol. Sci. 22, 1835.
| A toxic synergy between aluminium and amyloid beta in yeast.Crossref | GoogleScholarGoogle Scholar | 33673244PubMed |
[26] Dhakal, S. and Macreadie, I. (2020) Tyramine and amyloid beta 42: a toxic synergy. Biomedicines 8, 145.
| Tyramine and amyloid beta 42: a toxic synergy.Crossref | GoogleScholarGoogle Scholar |
[27] Dhakal, S. and Macreadie, I. (2021) Potential contributions of trace amines in Alzheimer’s disease and therapeutic prospects. Neural Regen. Res. 16, 1394–1396.
| Potential contributions of trace amines in Alzheimer’s disease and therapeutic prospects.Crossref | GoogleScholarGoogle Scholar | 33318424PubMed |
[28] Bell, S.M. et al. (2021) Mitochondrial dysfunction in Alzheimer’s disease: a biomarker of the future? Biomedicines 9, 63.
| Mitochondrial dysfunction in Alzheimer’s disease: a biomarker of the future?Crossref | GoogleScholarGoogle Scholar | 33440662PubMed |
Biography
Sudip Dhakal is a PhD researcher and a tutor/instructor at School of Science, RMIT University, whose research focuses on investigating therapeutic strategies against Alzheimer’s disease using yeast models.


