Cervicovaginal microbiota and women’s health outcomes
Ciara J Bryant A , Catherine Burke A and Wilhelmina M Huston A BA School of Life Sciences, Faculty of Science, University of Technology Sydney, 15 Broadway, Ultimo, NSW 2007, Australia
B Tel.: +61 2 9514 3449; Email: Wilhelmina.Huston@uts.edu.au
Microbiology Australia 42(2) 65-68 https://doi.org/10.1071/MA21022
Submitted: 2 April 2021 Accepted: 30 April 2021 Published: 19 May 2021
Journal Compilation © The Authors 2021 Open Access CC BY-NC-ND, published (by CSIRO Publishing) on behalf of the ASM
Abstract
The human cervicovaginal microbiome has an important role in the health and homoeostasis of the female reproductive tract. A eubiotic microbiome is typically dominated with lactic acid producing bacteria and is categorised into five community state types. Issues arise when the microbiome becomes dysbiotic, with the microbial composition shifting to contain a greater relative abundance of strict and facultative anaerobes. This shift will lead to several adverse changes in the vaginal environment including compromised epithelial cells, cell death, inflammation, and greater susceptibility to infection. These changes are associated with various adverse outcomes including infections, preterm birth, and infertility. In this review, we discuss how the cervicovaginal microbiome influences these outcomes and possible future directions of treatment and research.
Introduction
The human microbiome is a unique collection of microorganisms that colonises the body and has an important role in health and homeostasis. The cervicovaginal microbiome is particularly distinctive as it is frequently dominated by Lactobacillus with decreased diversity of other bacteria, unlike what is seen in other sites such as the gut1. The cervicovaginal microbiome is extremely important to the host tissue as it maintains an acidic environment, preventing pathogenic colonisation, and modulates inflammation by cross-kingdom signalling1. Thus, the composition of the cervicovaginal microbiome plays an important role in health outcomes for women, particularly in relation to vaginal infection, pregnancy, and fertility.
The eubiotic microbiome
Early culture-based studies identified Lactobacillus as the dominant bacteria in the vaginal microbiome and recognised that it may play a key role in maintaining the health of the female reproductive tract2. Molecular-based techniques, including relatively recent next generation sequencing, have been used to obtain an in-depth understanding of vaginal flora and to classify microbiota into broad profiles termed community state types (CST)3,4. Four CSTs are dominated by a species of Lactobacillus; Lactobacillus crispatus (CST I), L. gasseri (CST II), L. iners (CST III) and L. jensenii (CST V). CST IV is characterised by various strict and facultative anaerobes and is typified by the absence of a dominant Lactobacillus species. The CSTs have varying levels of stability and transitions between CSTs are associated with composition, menstrual cycle, and sexual activity4. Lactobacillus produces lactic acid, maintaining vaginal pH at ≤4.5, promoting a selective environment for acid tolerant bacteria whilst suppressing pathogenic colonisation (Figure 1). Lactic acid has an immunomodulatory function, acting directly on epithelial cells to promote an anti-inflammatory response via the production of interleukin (IL)-1 receptor antagonist, as well as promoting the production of pro-inflammatory mediators and antimicrobial peptides (Figure 1)5.
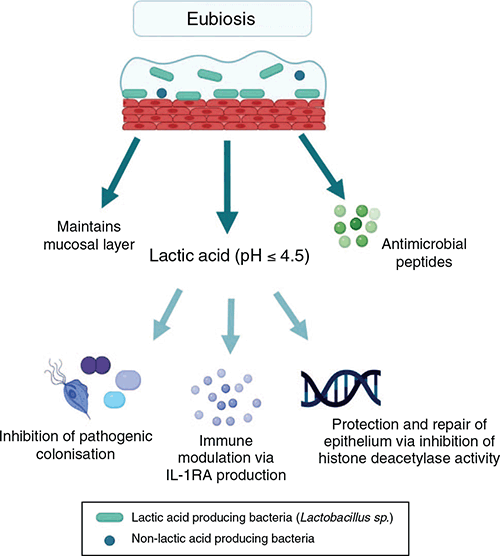
|
The dysbiotic microbiome
Dysbiosis is defined as a change in microbiota composition relative to the community of commensal bacteria seen in a healthy state6. There are no specific bacteria universally seen in dysbiosis but it is frequently associated with increased relative abundance of Gardnerella, Prevotella, and Atopbium7,8. This shift in composition results in a decrease in lactic acid, with an increase in short chain fatty acids, amines, and pH (Figure 2)9. Dysbiosis is also associated with several detrimental changes in the cervicovaginal environment including alterations in the cytoskeleton, increased cell death, an imbalance in the concentration of antimicrobial peptides and increased production of pro-inflammatory cytokines (Figure 2)10. These changes are thought to leave the tissue susceptible to infection and inflammation.
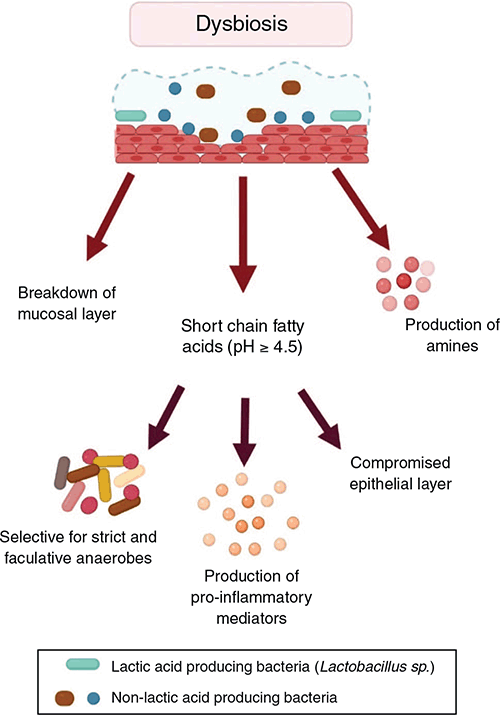
|
Bacterial vaginosis
Bacterial vaginosis (BV) is the most common vaginal infection, characterised by dysbiosis and the associated metabolomic changes. BV often is asymptomatic, but women may experience symptoms such as discoloured vaginal discharge, and a ‘fishy’ odour. The prevalence of BV is variable between different populations but worldwide prevalence is approximately 30%, with prevalence in Australia considerably lower at 4.7%11. Treatment with oral or intravaginal antibiotics is only recommend for women experiencing symptoms. However, after treatment, reoccurrence is common with up to 60-80% of women experiencing reoccurrence within 12 months after treatment12. Recent research has now shifted to investigating the variables associated with reoccurrence, specifically microbiota composition, to improve the treatment outcomes for women with BV13.
Sexually transmitted infections
Dysbiosis of the cervicovaginal microbiota is known to increase the risk of acquiring a sexually transmitted infection (STI). Numerous longitudinal studies have determined that high microbiota diversity increases the risk of acquiring an infection14. A possible mechanism that increases susceptibility may be an inflammatory response to diverse bacteria. Gosmann et al.15 investigated the association between the microbiome, inflammation, and human immunodeficiency virus (HIV)-acquisition in a prospective cohort study of South Africa women. They determined that women with polymicrobial microbiomes dominated with anaerobes, had increased activated mucosal CD4+ T cells, and four-fold greater risk of HIV infection. They suggested that the target cells were responding to the microbial diversity, which in turn increase host susceptibility15. A similar response is also hypothesised to be involved in human papillomavirus infection, but is yet to be investigated16. Another mechanism involved in the susceptibility is the modulation of cellular functions. Ceccarani et al.17 investigated the changes in the microbiome and metabolome during Chlamydia infection. In comparison to a healthy state, they showed clear changes in composition occurred during infection, specifically a decrease in lactic acid. Similarly, Edwards et al.18 showed D (-) lactic acid produced by the microbiome may prevent cellular proliferation, protecting against Chlamydia infection. They suggested that a eubiotic microbiome modulates cell function preventing Chlamydia infection in vitro. These studies support that via direct metabolic profiles and cross talk involved in host cell responses, the cervicovaginal microbiome influences the risk of STI acquisition.
Pregnancy
The composition of the cervicovaginal microbiome has been associated with increased risk of adverse outcomes in pregnancy such as preterm birth. Preterm birth is defined as either a live or still birth after 20 weeks’ gestation but before 37 weeks19. During pregnancy, hormonal changes alter the composition of the cervicovaginal microbiota resulting in an increased abundance of Lactobacillus. Several studies have shown that women with a diverse, non-Lactobacillus dominated microbiome are at a greater risk of preterm birth20,21. However, there is no defined profile of bacteria associated with adverse outcomes and results from each study greatly vary due to the population and study design. Kosti et al.22 recently conducted meta-analysis to address these issues and created a microbial signature associated with preterm birth. They successfully identified a lack of Lactobacillus as a predictor of preterm birth, alongside several species that had been previously reported. Interestingly, they identified an association between preterm birth and the presence of Olsenella and Clostridium sensu stricto, which had not been previously reported22. Overall, these promising results show the potential for novel diagnostics that could guide interventions to improve pregnancy outcomes for women at risk.
Infertility treatment
Infertility is defined as the inability to attain a clinical pregnancy after 12 months of regular unprotected intercourse19. In vitro fertilisation (IVF) is now the most common procedure used to treat a range of infertility issues23. However, in Australia, the success rate of IVF procedures is reported as approximately 30% with little improvement over the past 5 years24. Poor outcomes of IVF have been associated with the composition of the cervicovaginal microbiome in several studies, although these studies often have a small sample size and mixed quality of methodologies. Initially Hyman et al.20 associated diverse vaginal bacteria with poor IVF outcomes and suggested that the composition of the microbiome at the time of embryo transfer may be an important factor in the success of IVF treatment. Since this initial study there have been several others that have associated increased diversity of cervicovaginal microbiota and the presence of specific bacteria, with IVF failure25–27. However, there is no defined profile of microbiota associated with poor outcomes in IVF treatment, mostly due to the lack of larger studies. To understand the pathogenesis of this relationship, Fu et al.28 conducted a study to assess changes in the microbiome and metabolome in association with the outcomes of IVF failure. They determined that there was a lack of key metabolites necessary for embryo development and implantation such as glycerophospholipids and benzopyran in those who experienced IVF failure, and in turn these metabolite differences were associated with different compositions of microbiota. While this study shows some promising results, the pathophysiology involved in this relationship is yet to be fully explored.
Future directions
It is clear the cervicovaginal microbiome plays a key role in health outcomes for women, with dysbiosis commonly observed in a range of adverse events. However, the mechanisms underlying these relationships are not well understood. Future microbiome and metabolome models will provide a method of representing these interactions in vitro. Delgado-Diaz et al.29 used key metabolites associated with a Lactobacillus dominated microbiome and BV-associated microbiome to model the response of cells. This showed the immunomodulatory effect of lactic acid, but also showed that a lack of lactic acid and high concentrations of short chain fatty acids would stimulate increased production of pro-inflammatory cytokines. Thus, the approach of using an in vitro model is a promising method to better understand the microbiome and host cell interplay at a molecular level. Furthermore, large studies are necessary to determine predictive biomarkers of adverse outcomes, and to inform development of treatments such as probiotics for targeted treatment of the microbiome.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] García-Velasco, J.A. et al. (2020) The reproductive microbiome – clinical practice recommendations for fertility specialists. Reprod. Biomed. Online 41, 443–453.| The reproductive microbiome – clinical practice recommendations for fertility specialists.Crossref | GoogleScholarGoogle Scholar | 32753361PubMed |
[2] Redondo-Lopez, V. et al. (1990) Emerging role of lactobacilli in the control and maintenance of the vaginal bacteria microflora. Rev. Infect. Dis. 12, 856–872.
| Emerging role of lactobacilli in the control and maintenance of the vaginal bacteria microflora.Crossref | GoogleScholarGoogle Scholar | 2237129PubMed |
[3] Ravel, J. et al. (2011) Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 108, 4680–4687.
| Vaginal microbiome of reproductive-age women.Crossref | GoogleScholarGoogle Scholar | 20534435PubMed |
[4] Gajer, P. et al. (2012) Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4, 132ra52.
| Temporal dynamics of the human vaginal microbiota.Crossref | GoogleScholarGoogle Scholar | 22553250PubMed |
[5] Hearps, A.C. et al. (2017) Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 10, 1480–1490.
| Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition.Crossref | GoogleScholarGoogle Scholar | 28401934PubMed |
[6] Petersen, C. and Round, J.L. (2014) Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 16, 1024–1033.
| Defining dysbiosis and its influence on host immunity and disease.Crossref | GoogleScholarGoogle Scholar | 24798552PubMed |
[7] Fredricks, D.N. et al. (2005) Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 353, 1899–1911.
| Molecular identification of bacteria associated with bacterial vaginosis.Crossref | GoogleScholarGoogle Scholar | 16267321PubMed |
[8] Srinivasan, S. et al. (2012) Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 7, e37818.
| Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria.Crossref | GoogleScholarGoogle Scholar | 22719852PubMed |
[9] Vitali, B. et al. (2015) Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 34, 2367–2376.
| Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis.Crossref | GoogleScholarGoogle Scholar | 26385347PubMed |
[10] Borgdorff, H. et al. (2016) Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 9, 621–633.
| Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier.Crossref | GoogleScholarGoogle Scholar | 26349657PubMed |
[11] Fethers, K.A. et al. (2009) Early sexual experiences and risk factors for bacterial vaginosis. JID 200, 1662–1670.
| Early sexual experiences and risk factors for bacterial vaginosis.Crossref | GoogleScholarGoogle Scholar | 19863439PubMed |
[12] Coudray, M.S. and Madhivanan, P. (2020) Bacterial vaginosis—a brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 245, 143–148.
| Bacterial vaginosis—a brief synopsis of the literature.Crossref | GoogleScholarGoogle Scholar | 31901667PubMed |
[13] Xiao, B. et al. (2019) Association analysis on recurrence of bacterial vaginosis revealed microbes and clinical variables important for treatment outcome. Front. Cell. Infect. Microbiol. 9, 189.
| Association analysis on recurrence of bacterial vaginosis revealed microbes and clinical variables important for treatment outcome.Crossref | GoogleScholarGoogle Scholar | 31245300PubMed |
[14] Brotman, R.M. (2011) Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J. Clin. Invest. 121, 4610–4617.
| Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective.Crossref | GoogleScholarGoogle Scholar | 22133886PubMed |
[15] Gosmann, C. et al. (2017) Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 46, 29–37.
| Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women.Crossref | GoogleScholarGoogle Scholar | 28087240PubMed |
[16] Torcia, M.G. (2019) Interplay among vaginal microbiome, immune response and sexually transmitted viral infections. Int. J. Mol. Sci. 20, 266.
| Interplay among vaginal microbiome, immune response and sexually transmitted viral infections.Crossref | GoogleScholarGoogle Scholar |
[17] Ceccarani, C. et al. (2019) Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 9, 14095.
| Diversity of vaginal microbiome and metabolome during genital infections.Crossref | GoogleScholarGoogle Scholar | 31575935PubMed |
[18] Edwards, V.L. et al. (2019) The cervicovaginal microbiota-host interaction modulates Chlamydia trachomatis infection. MBio 10, e01548-19.
| The cervicovaginal microbiota-host interaction modulates Chlamydia trachomatis infection.Crossref | GoogleScholarGoogle Scholar | 31409678PubMed |
[19] Zegers-Hochschild, F. et al. (2009) International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil. Steril. 92, 1520–1524.
| International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009.Crossref | GoogleScholarGoogle Scholar | 19828144PubMed |
[20] Hyman, R.W. et al. (2014) Diversity of the vaginal microbiome correlates with preterm birth. Reprod. Sci. 21, 32–40.
| Diversity of the vaginal microbiome correlates with preterm birth.Crossref | GoogleScholarGoogle Scholar | 23715799PubMed |
[21] Stafford, G.P. et al. (2017) Spontaneous preterm birth is associated with differential expression of vaginal metabolites by lactobacilli-dominated microflora. Front. Physiol. 8, 615.
| Spontaneous preterm birth is associated with differential expression of vaginal metabolites by lactobacilli-dominated microflora.Crossref | GoogleScholarGoogle Scholar | 28878691PubMed |
[22] Kosti, I. et al. (2020) Meta-analysis of vaginal microbiome data provides new insights into preterm birth. Front. Microbiol. 11, 476.
| Meta-analysis of vaginal microbiome data provides new insights into preterm birth.Crossref | GoogleScholarGoogle Scholar | 32322240PubMed |
[23] Eichenberg, C. (2020) In vitro fertilization (IVF) J Repro Sci. , .
[24] Newman, J.E. et al. (2020) Assisted reproductive technology in Australia and New Zealand 2018, National Perinatal Epidemiology and Statistic Unit, Sydney.
[25] Haahr, T. et al. (2016) Abnormal vaginal microbiota may be associated with poor reproductive outcomes: a prospective study in IVF patients. Hum. Reprod. 31, 795–803.
| Abnormal vaginal microbiota may be associated with poor reproductive outcomes: a prospective study in IVF patients.Crossref | GoogleScholarGoogle Scholar | 26911864PubMed |
[26] Kitaya, K. et al. (2019) Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure. Mediators Inflamm. 2019, 4893437.
| Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure.Crossref | GoogleScholarGoogle Scholar | 31249472PubMed |
[27] Kong, Y. et al. (2020) The disordered vaginal microbiota is a potential indicator for a higher failure of in vitro fertilisation. Front. Med. 7, 217.
| The disordered vaginal microbiota is a potential indicator for a higher failure of in vitro fertilisation.Crossref | GoogleScholarGoogle Scholar |
[28] Fu, M. et al. (2020) Alterations in vaginal microbiota and associated metabolome in women with recurrent implantation failure. MBio 11, e03242-19.
| Alterations in vaginal microbiota and associated metabolome in women with recurrent implantation failure.Crossref | GoogleScholarGoogle Scholar | 32487762PubMed |
[29] Delgado-Diaz, D.J. et al. (2020) Distinct immune response elicited from cervicovaginal epithelial cells by lactic acid and short chain fatty acids associated with optimal and non-optimal and vaginal microbiota. Front. Cell. Infect. Microbiol. 9, 446.
| Distinct immune response elicited from cervicovaginal epithelial cells by lactic acid and short chain fatty acids associated with optimal and non-optimal and vaginal microbiota.Crossref | GoogleScholarGoogle Scholar | 31998660PubMed |
Biographies
Ciara J Bryant is a PhD candidate at the University of Technology Sydney. She has completed her honours degree in biomedical science and is continuing to investigate the factors involved in infertility and IVF failure in her PhD.
Catherine Burke is a Senior Lecturer at the University of Technology Sydney. She studies the human microbiome in a range of health and disease states. She was awarded her PhD in 2010 from the University of New South Wales.
Wilhelmina (Willa) M Huston is an Associate Professor at University of Technology Sydney, her research team investigates chlamydia and other STIS in the context of women’s health in order to assist with identification of improved diagnostics and therapies. She was awarded her PhD in 2004 from the University of Queensland. She was also awarded the 2020 ASM Frank Fenner Award.


