Vaccination for cytomegalovirus: when, where and how
Vijayendra Dasari A and Rajiv Khanna A BA Tumour Immunology Laboratory
QIMR Clive Berghofer Medical Research Institute
300 Herston Road
Brisbane, Qld 4006, Australia
B Tel: +61 7 3362 0385
Fax: +61 7 3845 3510
Email: rajiv.khanna@qimr.edu.au
Microbiology Australia 36(4) 175-178 https://doi.org/10.1071/MA15062
Published: 19 October 2015
Although following primary human cytomegalovirus (CMV) infection in many individuals no overt symptoms are observed, CMV came to medical attention due to its significant morbidity and mortality associated with congenital infection and immunosuppressed individuals. Congenital infection occurs following transplacental transmission during pregnancy as a result of primary infection, reactivation or re-infection with a different isolate. Estimates suggest at least a million cases of congenital CMV occur annually worldwide. Congenital infection is a leading cause of neurological complications such as mental retardation, cerebral palsy, developmental delay and seizure disorders and also causes permanent disabilities, such as hearing loss and vision impairment. In addition, other common manifestation of CMV infection are stillbirth, preterm delivery and intrauterine growth restriction (IUGR) and cardiovascular disease, which are risk factors for perinatal and lifetime morbidity. Recent reports have estimated that the economic costs to public health and families due to congenital CMV infection are immense, with direct annual costs of billions of dollars. An effective CMV vaccine that could prevent transplacental transmission, reduce CMV disease and CMV-associated stillbirths has been recognised as an urgent medical need. Over the past 40 years several CMV vaccine candidates have been evaluated in a series of clinical trials and found to be effective in preclinical and clinical studies. However, in spite of extensive efforts over many decades, successful licensure of an effective CMV vaccine formulation to prevent congenital CMV infection remains elusive.
The major target populations for a CMV vaccine to reduce congenital infection include women of reproductive age, infants, toddlers and adolescents. Children who attend day care represent a particularly important reservoir of CMV. Women of reproductive age exposed to children who are shedding virus in urine and saliva are 10 times more likely to seroconvert compared to women unexposed to virus shedding children1,2. In addition, the symptomatic congenital CMV rate is very high if women acquire primary infection or CMV virus reactivates during or just before pregnancy, whilst prior natural CMV infection provides protection from transplacental transmission3–6. In pregnant women following natural primary infection, viral replication is controlled by the emergence of antigen-specific CD4+, CD8+ and CD45RA+ effector memory T-cells7,8. Lower frequencies of CMV-specific CD4+ T-cells and CD45RA+ cells in mothers following primary infection is known to be associated with virus transmission to the fetus9,10. Based on these observations it can be hypothesised that emergence of higher frequencies of CMV-specific CD4+, CD8+ and CD45RA+ effector memory T-cells may be associated with control of viremia and prevention of transplacental transmission. Therefore, an effective CMV vaccine specially designed to induce CMV-specific CD4+, CD8+ and CD45RA+ effector memory T-cells in women of reproductive age could potentially decrease CMV transmission to the fetus and vaccination of infants, toddlers and adolescents could reduce the duration of viral shedding, which may reduce child-to-mother transmission.
Clinical evaluation of active and passive immunisation strategies against CMV in the context of congenital CMV
Live attenuated virus vaccines
The initial CMV vaccine trials based on an attenuated form of CMV isolates Towne and AD16911,12 induced humoral and cellular immune responses in immunocompromised solid organ transplant patients. However, a study conducted in young women with children attending group day care showed no reduction in the infection rate in Towne-vaccinated mothers compared to placebo. Thus the efficacy of the Towne vaccine against congenital CMV infection was questioned13. Subsequently several alternative approaches have been used to improve the efficacy of the live attenuated Towne vaccine, which includes generating recombinant chimeras by swapping lost genome segments of Towne vaccine with the less attenuated Toledo strain. The evaluation of safety and immunogenicity of chimeras in CMV-naïve subjects is now in phase 1 clinical trials14. However, safety concerns raised by experts in the field during an FDA review in 1999 are the major confounders to vaccination with any live attenuated CMV vaccine14.
Subunit vaccines
Another vaccine strategy is based on a subunit vaccine that was developed initially by Chiron by combining recombinant glycoprotein B (gB) with an oil-in-water adjuvant, MF59. The efficacy of gB-MF59 vaccine was recently evaluated in a Phase II, double-blind, randomised, placebo-controlled trial, in seronegative women of child bearing age15. The vaccine showed reduction in the incidence of primary maternal infection by 50% in the vaccinated group compared to the placebo group. However, the protection was not durable and it was predominantly observed within the first year after immunisation. Subsequent testing of gB-MF59 vaccine in seropositive women showed a significant boost in neutralising antibody titers and CD4+ T-cell responses16. Nevertheless, whether such boosting will provide protection against reactivation or reinfection with a different isolate in women with pre-existing immunity is not yet known.
Passive immunisation
In addition to active immunisation strategies, passive immunisation strategies based on administration of anti-CMV immune globulin to women at high risk of transmitting CMV to the fetus also have been explored in clinical research. Initial observations suggest that CMV hyperimmunoglobulin (HIG) could inhibit viral spread in vitro17,18, restore placental health in mothers during primary infection19 and lead to the regression of fetal cerebral ultrasound abnormalities20. A prospective study carried out in mothers with confirmed primary infection demonstrated that monthly intravenous administration of HIG can decrease mother-to-fetus transmission significantly, from 40% to 16% and the risk of congenital disease from 50% to 3%21. Several retrospective studies have suggested that CMV HIG can reduce intrauterine transmission of CMV22 and can protect against poor outcomes in infants23,24. However, in a recent randomised trial, HIG treatment did not significantly modify the course of primary CMV infection during the pregnancy25. Therefore, in our perspective more randomised studies are required to draw a firm conclusion on the efficacy of HIG therapy.
CMV vaccines in preclinical studies
Several additional proof-of-concept studies of various candidate vaccines have also been evaluated in clinical and preclinical studies in recent years14,26. Vical/Astellas developed a vaccine (CyMVectin™) to target congenital CMV using plasmids that encode gB and pp65 formulated with Vaxfectin adjuvant. The preclinical data from this study presented at the 5th International Congenital CMV Conference (2015) held in Brisbane showed that CyMVectin™ has the potential to induce neutralising antibodies against both fibroblasts and epithelial cells. AplhaVax developed a dual alphavirus replicon that expresses the CMV antigens gB plus an IE1-pp65 fusion protein and has evaluated its safety and immunogenicity in Phase I clinical trials. Following vaccination all vaccine recipients developed polyfunctional CD4+ and CD8+ T-cell responses and neutralising antibody response27,28. Furthermore, several alternative vaccine strategies have shown promising results in emerging preclinical evaluations26. These include a recombinant modified vaccinia Ankara (MVA) expressing three immunodominant CMV antigens pp65, IE1 and IE2 as a fusion protein, a dense body vaccine consisting of non-infectious, replication-defective particles formed during the replication of CMV, polyepitope vaccines comprising a replication-deficient adenoviral vector for the expression of gB antigenic domain-1 or the extracellular domain of the gB protein and 46 HLA class I and II restricted T-cell epitopes or recombinant gB and polyepitope protein formulated with TLR4 and TLR9 agonists29. However, the safety and immunogenicity of these vaccine candidates is yet to be determined in advanced clinical studies in the context of congenital CMV.
Extensive studies in humans have revealed that the gH/gL/UL128–131A pentameric complex is the most important antigenic complex for neutralising antibodies especially to restrict the entry of CMV into epithelial and endothelial cells30. High titers of neutralising antibodies are thought to protect against transmission by blocking receptor-mediated transplacental transmission of CMV and by reducing viral replication31,32. Therefore, it will be interesting to investigate the potential role of pentameric glycoprotein complex-specific humoral responses in both primary and recurrent infections in pregnant women.
Major barriers in the development of effective CMV vaccines
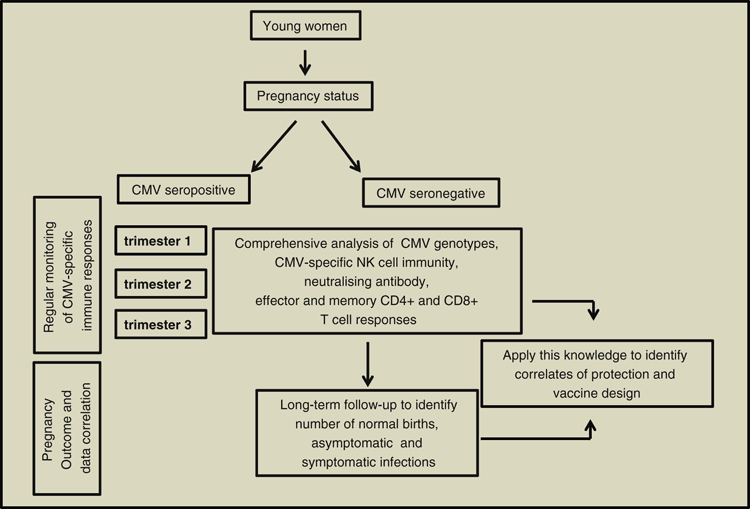
Conventional CMV vaccine approaches that target a single genotype may induce only partial protection due to high levels of CMV genomic variation and recombination within infected populations33,34. Frequent recurrence and transmission in largely CMV seropositive individuals are the major confounders for CMV vaccine development. Despite the promising results from CMV-HIG trials, the mode of action of these antibodies in limiting transplacental transmission of CMV in high-seroprevalence population remains to be determined. Finally, understanding the immune parameters that effectively protect from transplacental transmission of CMV in pregnant women as a result of primary infection, reactivation or re-infection need to be delineated for the development of an effective CMV vaccine (Figure 1).
Acknowledgements
We thank Dr Corey Smith for critically reviewing this article. RK is supported by a NHMRC Senior Principal Research Fellowship. CMV vaccine studies described in this manuscript are currently supported through an NHMRC Development Grant.
References
[1] Hyde, T.B. et al. (2010) Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev. Med. Virol. 20, 311–326.| Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV.Crossref | GoogleScholarGoogle Scholar | 20645278PubMed |
[2] Cannon, M.J. et al. (2012) Awareness of and behaviors related to child-to-mother transmission of cytomegalovirus. Prev. Med. 54, 351–357.
| Awareness of and behaviors related to child-to-mother transmission of cytomegalovirus.Crossref | GoogleScholarGoogle Scholar | 22465669PubMed |
[3] Revello, M.G. et al. (2006) Preconceptional primary human cytomegalovirus infection and risk of congenital infection. J. Infect. Dis. 193, 783–787.
| Preconceptional primary human cytomegalovirus infection and risk of congenital infection.Crossref | GoogleScholarGoogle Scholar | 16479511PubMed |
[4] Fowler, K.B. et al. (2003) Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 289, 1008–1011.
| Maternal immunity and prevention of congenital cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 12597753PubMed |
[5] Ross, S.A. et al. (2006) Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J. Pediatr. 148, 332–336.
| Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity.Crossref | GoogleScholarGoogle Scholar | 16615962PubMed |
[6] Plotkin, S.A. (2003) Natural vs vaccine-acquired immunity to cytomegalovirus. JAMA 290, 1709.
| Natural vs vaccine-acquired immunity to cytomegalovirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnvVyiurY%3D&md5=d13466eaf499ce0c2cef90b1e717eda3CAS | 14519704PubMed |
[7] Lilleri, D. et al. (2008) Human cytomegalovirus-specific memory CD8+ and CD4+ T cell differentiation after primary infection. J. Infect. Dis. 198, 536–543.
| Human cytomegalovirus-specific memory CD8+ and CD4+ T cell differentiation after primary infection.Crossref | GoogleScholarGoogle Scholar | 18590456PubMed |
[8] Lilleri, D. et al. (2007) Development of human cytomegalovirus-specific T cell immunity during primary infection of pregnant women and its correlation with virus transmission to the fetus. J. Infect. Dis. 195, 1062–1070.
| Development of human cytomegalovirus-specific T cell immunity during primary infection of pregnant women and its correlation with virus transmission to the fetus.Crossref | GoogleScholarGoogle Scholar | 17330798PubMed |
[9] Sylwester, A.W. et al. (2005) Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202, 673–685.
| Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVSjs7zE&md5=c2e7c2af934e68e5980f5c22f45dcd92CAS | 16147978PubMed |
[10] Gyulai, Z. et al. (2000) Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-Exon4, gB, pp150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs. J. Infect. Dis. 181, 1537–1546.
| Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-Exon4, gB, pp150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktlGhtL4%3D&md5=6095dffd37862aeddaece317a08a83a4CAS | 10823751PubMed |
[11] Plotkin, S.A. et al. (1984) Prevention of cytomegalovirus disease by Towne strain live attenuated vaccine. Birth Defects Orig. Artic. Ser. 20, 271–287.
| 1:STN:280:DyaL2c3jt1CmsQ%3D%3D&md5=bed0b21fc2bae5dfcb77cb8c8e0393e4CAS | 6329367PubMed |
[12] Neff, B.J. et al. (1979) Clinical and laboratory studies of live cytomegalovirus vaccine Ad-169. Proc. Soc. Exp. Biol. Med. 160, 32–37.
| Clinical and laboratory studies of live cytomegalovirus vaccine Ad-169.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE1M7hs1ehsQ%3D%3D&md5=37c304e67391161a20808f72b708e3ebCAS | 217022PubMed |
[13] Adler, S.P. et al. (1995) Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J. Infect. Dis. 171, 26–32.
| Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M%2Fps1Grsw%3D%3D&md5=905e2590020ab2ee217ff77a1bd71f83CAS | 7798679PubMed |
[14] McCormick, A.L. and Mocarski, E.S. (2015) The immunological underpinnings of vaccinations to prevent cytomegalovirus disease. Cell. Mol. Immunol. 12, 170–179.
| The immunological underpinnings of vaccinations to prevent cytomegalovirus disease.Crossref | GoogleScholarGoogle Scholar | 25544503PubMed |
[15] Pass, R.F. et al. (2009) Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 360, 1191–1199.
| Vaccine prevention of maternal cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjtlersbk%3D&md5=37fbcd143155580003a2aef0c548f566CAS | 19297572PubMed |
[16] Sabbaj, S. et al. (2011) Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. J. Infect. Dis. 203, 1534–1541.
| Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXms1aisr8%3D&md5=f3f1534ed66985d6bc127444dfb6f3cdCAS | 21592981PubMed |
[17] Maidji, E. et al. (2010) Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection. Am. J. Pathol. 177, 1298–1310.
| Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1KmsbzK&md5=83e1a5e447e98aeb7988d620a6470c22CAS | 20651234PubMed |
[18] Weisblum, Y. et al. (2011) Modeling of human cytomegalovirus maternal-fetal transmission in a novel decidual organ culture. J. Virol. 85, 13204–13213.
| Modeling of human cytomegalovirus maternal-fetal transmission in a novel decidual organ culture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1Shs7bJ&md5=a4cd340815211907f22acd77f169a2d3CAS | 21976654PubMed |
[19] Schleiss, M.R. (2006) The role of the placenta in the pathogenesis of congenital cytomegalovirus infection: is the benefit of cytomegalovirus immune globulin for the newborn mediated through improved placental health and function? Clin. Infect. Dis. 43, 1001–1003.
| The role of the placenta in the pathogenesis of congenital cytomegalovirus infection: is the benefit of cytomegalovirus immune globulin for the newborn mediated through improved placental health and function?Crossref | GoogleScholarGoogle Scholar | 16983611PubMed |
[20] Nigro, G. et al. (2008) Regression of fetal cerebral abnormalities by primary cytomegalovirus infection following hyperimmunoglobulin therapy. Prenat. Diagn. 28, 512–517.
| Regression of fetal cerebral abnormalities by primary cytomegalovirus infection following hyperimmunoglobulin therapy.Crossref | GoogleScholarGoogle Scholar | 18509871PubMed |
[21] Nigro, G. et al. (2005) Passive immunization during pregnancy for congenital cytomegalovirus infection. N. Engl. J. Med. 353, 1350–1362.
| Passive immunization during pregnancy for congenital cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVGmtL3K&md5=972d98284a4ae342db016315d89c34dfCAS | 16192480PubMed |
[22] Buxmann, H. et al. (2012) Use of cytomegalovirus hyperimmunoglobulin for prevention of congenital cytomegalovirus disease: a retrospective analysis. J. Perinat. Med. 40, 439–446.
| Use of cytomegalovirus hyperimmunoglobulin for prevention of congenital cytomegalovirus disease: a retrospective analysis.Crossref | GoogleScholarGoogle Scholar | 22752777PubMed |
[23] Nigro, G. et al. (2012) Immunoglobulin therapy of fetal cytomegalovirus infection occurring in the first half of pregnancy–a case-control study of the outcome in children. J. Infect. Dis. 205, 215–227.
| Immunoglobulin therapy of fetal cytomegalovirus infection occurring in the first half of pregnancy–a case-control study of the outcome in children.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs12gs7rI&md5=d6c584981b8099ea0f2850f7e7922c28CAS | 22140265PubMed |
[24] Visentin, S. et al. (2012) Early primary cytomegalovirus infection in pregnancy: maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age. Clin. Infect. Dis. 55, 497–503.
| Early primary cytomegalovirus infection in pregnancy: maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFagu7rN&md5=51efe83f7a3ce7f7a87beed2a785163fCAS | 22539662PubMed |
[25] Revello, M.G. et al. (2014) A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N. Engl. J. Med. 370, 1316–1326.
| A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXlvFGltrs%3D&md5=4150ce903a9dd117d1ad46e1ffd54a03CAS | 24693891PubMed |
[26] Dasari, V. et al. (2013) Recent advances in designing an effective vaccine to prevent cytomegalovirus-associated clinical diseases. Expert Rev. Vaccines 12, 661–676.
| Recent advances in designing an effective vaccine to prevent cytomegalovirus-associated clinical diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXptFCgt7o%3D&md5=9f387e71e1b640eab882e1e0534a76a9CAS | 23750795PubMed |
[27] Reap, E.A. et al. (2007) Development and preclinical evaluation of an alphavirus replicon particle vaccine for cytomegalovirus. Vaccine 25, 7441–7449.
| Development and preclinical evaluation of an alphavirus replicon particle vaccine for cytomegalovirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFWhu7vN&md5=f701c2186da8563e387bee0ba1ffa6d0CAS | 17870214PubMed |
[28] Bernstein, D.I. et al. (2009) Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine 28, 484–493.
| Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFagtLrM&md5=c4cb620d8595b2cfb334165b27973ac2CAS | 19857446PubMed |
[29] Dasari, V. et al. (2014) Induction of innate immune signatures following polyepitope protein-glycoprotein B-TLR4&9 agonist immunization generates multifunctional CMV-specific cellular and humoral immunity. Hum. Vaccin. Immunother. 10, 1064–1077.
| Induction of innate immune signatures following polyepitope protein-glycoprotein B-TLR4&9 agonist immunization generates multifunctional CMV-specific cellular and humoral immunity.Crossref | GoogleScholarGoogle Scholar | 24463331PubMed |
[30] Fouts, A.E. et al. (2012) Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. J. Virol. 86, 7444–7447.
| Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVansLbF&md5=426d243da9f253cf98e56e6471ae0d49CAS | 22532696PubMed |
[31] Maidji, E. et al. (2006) Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am. J. Pathol. 168, 1210–1226.
| Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjslOhtLc%3D&md5=3e52b75b9d1fc1f8551ea9d3b4dbefa4CAS | 16565496PubMed |
[32] Pereira, L. et al. (2003) Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J. Virol. 77, 13301–13314.
| Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpslSms7s%3D&md5=9da0c4f219eed80b09d8dbf43631e4efCAS | 14645586PubMed |
[33] Ross, S.A. et al. (2011) Mixed infection and strain diversity in congenital cytomegalovirus infection. J. Infect. Dis. 204, 1003–1007.
| Mixed infection and strain diversity in congenital cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 21881114PubMed |
[34] Boppana, S.B. and Britt, W.J. (2014) Recent approaches and strategies in the generation of antihuman cytomegalovirus vaccines. Methods Mol. Biol. 1119, 311–348.
| Recent approaches and strategies in the generation of antihuman cytomegalovirus vaccines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXns1Sgurk%3D&md5=279bfef60678ba03141e7b87ff4c1e55CAS | 24639230PubMed |
Biographies
Vijayendra Dasari is a Post-Doctoral fellow in the Tumour Immunology Laboratory at QIMR Clive Berghofer Medical Research Institute, Brisbane, Austrlia. He obtained his PhD from the Griffith University, Nathan, Qld, Australia, where he worked on developing a polyepitope prophylactic vaccine against human cytomegalovirus. His research focuses on the vaccine development against human cytomegalovirus and Epstein–Barr virus.
Professor Rajiv Khanna is a group leader, Tumour Immunology Laboratory, QIMR Clive Berghofer Medical Research Institute, Brisbane, Australia, Co-ordinator for QIMR Berghofer Center for Immunotherapy and Vaccine Development and Senior Principal Research Fellow (NHMRC). He has over 25 years of research experience on herpes virus immunology, vaccine development and immunotherapy and he has published numerous articles.