Cytomegalovirus infection and pathogenesis in the human placenta
Lenore Pereira A B , Takako Tabata A and Matthew Petitt AA Department of Cell and Tissue Biology, University of California San Francisco, San Francisco, CA 94143, USA
B Tel: 415 476 8248
Email: Lenore.Pereira@UCSF.edu
Microbiology Australia 36(4) 171-174 https://doi.org/10.1071/MA15061
Published: 27 October 2015
Human cytomegalovirus (HCMV) is the most common cause of congenital viral infection. Affected children can have permanent neurological complications, including hearing loss, visual impairment and mental retardation1–3. In Australia, 57% of women are seronegative and at risk for primary infection and transmission of virus to the fetus during pregnancy4. Despite its public health significance, the specific molecular and cellular basis of HCMV replication in the human placenta and pathogenesis associated with poor clinical outcome are unknown. Direct fetal infection is involved in severe cases of neuropathology and infection of the placenta can impair its development and functions resulting in a hypoxic environment5–8 and stillbirth6,9,10. Gestational age at the time of infection is an important determinant of outcome. The rates of virus transmission increase from 30% in first trimester to over 70% in third trimester suggesting different mechanisms for overcoming the placental barrier2. Remarkable insights into viral pathogenesis factors that function in the tissue environment have been gained by studying congenitally infected placentas and explants infected by clinical strains ex vivo. Together these studies revealed that direct infection of specialised placental cells and paracrine factors contribute to impaired development and functional defects.
Research on congenital HCMV infection has been hindered by the strict species specificity of the human virus. No animal model recapitulates the development and architecture of the human placenta, a hematogenous organ that survives by maternal tolerance of the fetal hemiallograft and performs critical functions throughout pregnancy. Currently, diagnostic indicators for primary and recurrent maternal HCMV infection have not been identified, nor are there any accepted treatments to prevent transmission. Development of a vaccine is in the early stages, long delayed by a poor understanding of the parameters of immune protection and routes of virus spread across the placenta9,11–13.
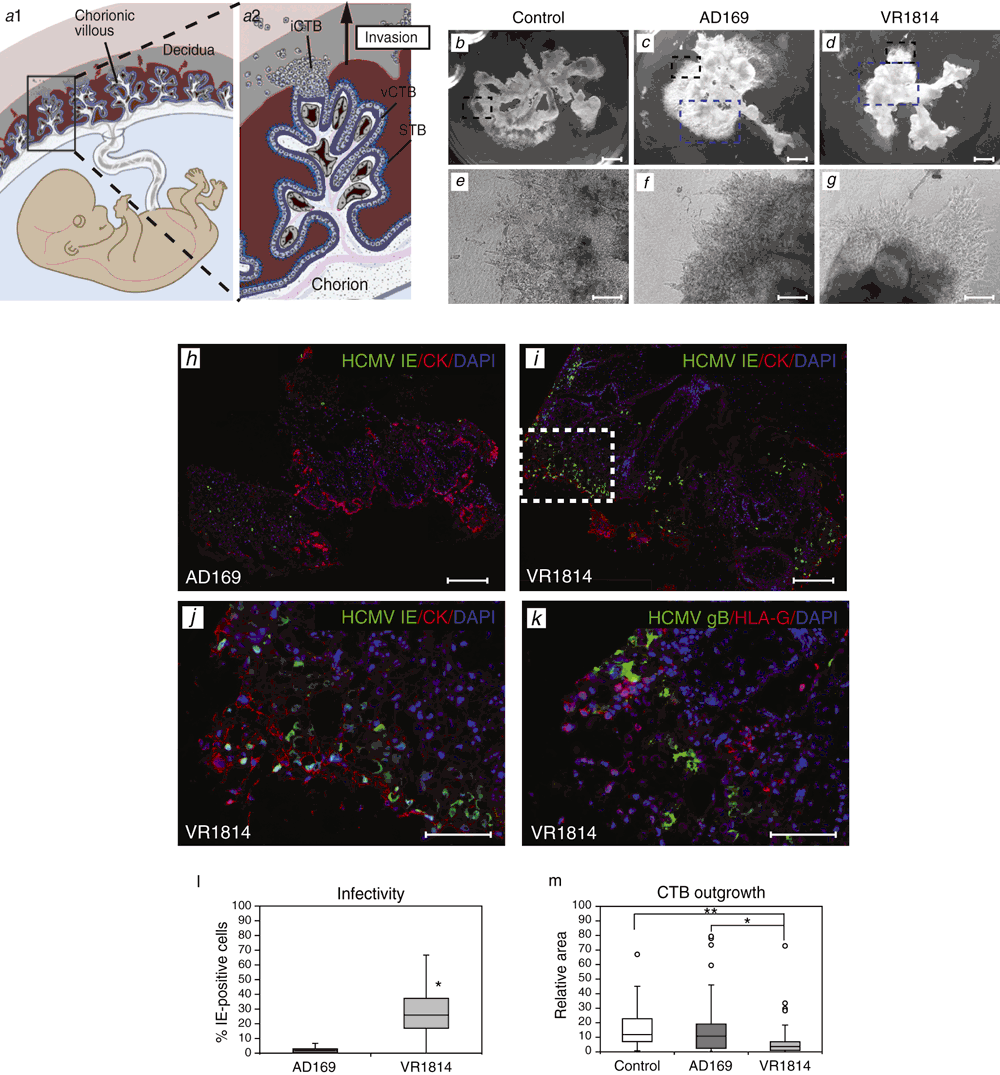
As shown in Figure 1, the human placenta is composed of chorionic villi bathed in maternal blood and villi that anchor the placenta in the uterine wall (decidua), attaching the fetus to the mother (panel a1). The individual chorionic villus contains a connective core with blood vessels that carry substances to the fetal circulation (panel a2). Placentation is a stepwise process whereby villus cytotrophoblasts (vCTB) attached to the basement membrane as a polarised epithelium leave the membrane to differentiate along one of two independent pathways depending on their location. In floating villi, they fuse to form a multinucleate synctiotrophoblast (STB) covering attached at one end to the tree-like fetal portion of the placenta. The rest of the villus floats in a stream of maternal blood, which optimises exchange of substances between the maternal and fetal circulation. In the pathway that gives rise to anchoring villi, cytotrophoblasts aggregate into cell columns of non-polarised mononuclear cells that attach to and then invade the uterine wall (iCTBs).
|
Our studies of HCMV infection in the human placenta revealed important differences in infection between cell types15–19 and maternal immune status7,8,16,20. Studies of primary human placental cells and tissue models have identified molecular pathways that impair trophoblast differentiation17–19,21–24. Patterns of viral proteins in infected placentas suggest modulation of infection in early gestation5,25–27 and formation of structural defects during pregnancy7,8,18,23.
Congenital HCMV infection can result in intrauterine growth restriction (IUGR), which is found in conjunction with changes in the placental architecture. Specific pathology includes fibrinoids that occlude the villous surface, avascular villi and arrested differentiation of trophoblasts7,8,20,28. Together these changes contribute to impaired transport functions, even without virus transmission to the fetus. A hypoxic environment evolves that up-regulates the vascular endothelial growth factor, its receptor and a soluble form, which is elevated in amniotic fluid and cord blood of infected babies7,8.
We have utilised placental villus explants as a model to investigate the early steps in HCMV infection and found tissue effects not anticipated by studies in primary cells and have begun to identify viral pathogenesis factors for the human placenta29. Specifically, we discovered that a clinical strain (VR1814) undermines the formation of cell columns in anchoring villi, but an attenuated laboratory strain (AD169) lacking a segment of the viral genome does not. These divergent abilities to replicate in cytotrophoblasts in villus explants were not observed in isolated cells infected with these viruses. In the placenta model system, the uninfected controls developed robust cell columns and anchoring villi of cytotrophoblasts that aggregated and attached the explants to the substrate (Figure 1b, e). Surprisingly, explants infected with the attenuated strain formed normal-size anchoring villi indistinguishable from controls (Figure 1c, f). In contrast, explants infected with the clinical strain formed spindly cell columns composed largely of individual cytotrophoblasts that migrated on top of instead of invading the substrate (Figure 1d, g). Analysis of cytotrophoblasts within the placental villi revealed that the attenuated strain infected few cells as indicated by low expression of the viral immediate-early (IE) IE1&2 proteins (Figure 1h) and failed to make gB, a late viral protein that signifies productive infection (not shown). In contrast, many cytotrophoblasts infected with the clinical strain expressed IE1&2 proteins (Figure 1i inset, j). At late times, gB was made confirming viral replication and HLA-G was down-regulated (Figure 1k), suggesting infected cells could become targets of natural killer cells in the decidua30.
Since the attenuated and clinical strains exhibited markedly distinct levels of infection in placental explants, the differences were quantified by counting the number of cytotrophoblasts expressing IE1 protein in the cell columns and anchoring villi (Figure 1l). AD169-infected explants contained a median of 2% infected cytotrophoblasts with a 5% maximum. In contrast, VR1814-infected placental villi contained a median of 26% infected cells with a 67% maximum. To quantify the effects on development of anchoring villi, we measured the sizes of villi formed by measuring the areas covered by the villous outgrowths (Figure 1m). Control explants and those infected with AD169 were comparable whereas explants infected with VR1814 formed significantly smaller villi less than 10% the size of controls. Together, the results showed that a clinical strain expressed pathogenesis factors that promote infection of cell column cytotrophoblasts and impair functions of cells that form anchoring villi, reducing their size in explants.
Important insights into virus replication in the tissue environment were also obtained using xenografts of human placental villi implanted under the kidney capsules of Scid-hu mice10. Our immunohistological analysis revealed differences in the ability of pathogenic and attenuated HCMV strains to impair cytotrophoblast invasion, blood vessel remodelling and the development of a lymphatic vasculature29. Moreover, cytokines important for lymphangiogenesis dysregulated by the clinical strain but not by the attenuated strain have functional effects in villus xenografts. These findings emphasise the critical importance of examining infection in the intact human tissues in order to understand viral effects on the developing placenta.
Future studies of viral replication in the natural tissue environment of the human placenta could provide insights into HCMV pathogenesis factors including tropism genes that modulate viral entry and enable the spread of infection impairing placental development.
Acknowledgements
This work was supported by grants from the National Institutes of Health RO1AI046657, R56AI073752, RO1AI21420, R56AI21739 (L.P.) and HD061890 (T.T.). We thank June Fang-Hoover and Mirhan Kapidzik for excellent technical assistance.
References
[1] Boppana, S.B. et al. (2001) Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N. Engl. J. Med. 344, 1366–1371.| Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M3kvFejtw%3D%3D&md5=035d1440452daca9662e1fd2356783cfCAS | 11333993PubMed |
[2] Cannon, M.J. and Pellett, P.E. (2005) Risk of congenital cytomegalovirus infection. Clin. Infect. Dis. 40, 1701–1702.
| Risk of congenital cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 15889375PubMed |
[3] Pass, R.F. et al. (2006) Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J. Clin. Virol. 35, 216–220.
| Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome.Crossref | GoogleScholarGoogle Scholar | 16368262PubMed |
[4] Basha, J. et al. (2014) Congenital cytomegalovirus infection is associated with high maternal socio-economic status and corresponding low maternal cytomegalovirus seropositivity. J. Paediatr. Child Health 50, 368–372.
| Congenital cytomegalovirus infection is associated with high maternal socio-economic status and corresponding low maternal cytomegalovirus seropositivity.Crossref | GoogleScholarGoogle Scholar | 24593837PubMed |
[5] Fisher, S. et al. (2000) Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J. Virol. 74, 6808–6820.
| Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXkvFCmt78%3D&md5=149f24ac07c6790e8fa3cddeb726b792CAS | 10888620PubMed |
[6] Gabrielli, L. et al. (2012) Congenital cytomegalovirus infection: patterns of fetal brain damage. Clin. Microbiol. Infect. 18, E419–E427.
| Congenital cytomegalovirus infection: patterns of fetal brain damage.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38fntVKrsw%3D%3D&md5=aaf84d8b7a9e2a36f57b4c23b56b428dCAS | 22882294PubMed |
[7] Maidji, E. et al. (2010) Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection. Am. J. Pathol. 177, 1298–1310.
| Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1KmsbzK&md5=83e1a5e447e98aeb7988d620a6470c22CAS | 20651234PubMed |
[8] Pereira, L. et al. (2014) Intrauterine growth restriction caused by underlying congenital cytomegalovirus infection. J. Infect. Dis. 209, 1573–1584.
| Intrauterine growth restriction caused by underlying congenital cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXnt1CmtLg%3D&md5=fe270b2b7d97aa348ac72318112cd191CAS | 24403553PubMed |
[9] Pass, R.F. et al. (2009) Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 360, 1191–1199.
| Vaccine prevention of maternal cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjtlersbk%3D&md5=37fbcd143155580003a2aef0c548f566CAS | 19297572PubMed |
[10] Red-Horse, K. et al. (2006) Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J. Clin. Invest. 116, 2643–2652.
| Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVKgu7rJ&md5=4509ff83e57bf29877d67dcc99e422c6CAS | 16998586PubMed |
[11] Fu, T.M. et al. (2014) Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease. Vaccine 32, 2525–2533.
| Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXlvVWhu78%3D&md5=b62d1a68b008305c36b082988d452b6fCAS | 24681264PubMed |
[12] McCormick, A.L. and Mocarski, E.S. (2015) The immunological underpinnings of vaccinations to prevent cytomegalovirus disease. Cell. Mol. Immunol. 12, 170–179.
| The immunological underpinnings of vaccinations to prevent cytomegalovirus disease.Crossref | GoogleScholarGoogle Scholar | 25544503PubMed |
[13] Wang, D. and Fu, T.M. (2014) Progress on human cytomegalovirus vaccines for prevention of congenital infection and disease. Curr. Opin. Virol. 6, 13–23.
| Progress on human cytomegalovirus vaccines for prevention of congenital infection and disease.Crossref | GoogleScholarGoogle Scholar | 24632198PubMed |
[14] Maltepe, E. et al. (2010) The placenta: transcriptional, epigenetic, and physiological integration during development. J. Clin. Invest. 120, 1016–1025.
| The placenta: transcriptional, epigenetic, and physiological integration during development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXksVChs7w%3D&md5=16a22eb92f336f5e837581717badac4eCAS | 20364099PubMed |
[15] Maidji, E. et al. (2002) Transmission of human cytomegalovirus from infected uterine microvascular endothelial cells to differentiating/invasive placental cytotrophoblasts. Virology 304, 53–69.
| Transmission of human cytomegalovirus from infected uterine microvascular endothelial cells to differentiating/invasive placental cytotrophoblasts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XpsFyhtL0%3D&md5=4ab3b457f601818c6023a716cc0b1f62CAS | 12490403PubMed |
[16] Pereira, L. et al. (2003) Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J. Virol. 77, 13301–13314.
| Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpslSms7s%3D&md5=9da0c4f219eed80b09d8dbf43631e4efCAS | 14645586PubMed |
[17] Tabata, T. et al. (2007) Cytotrophoblasts infected with a pathogenic human cytomegalovirus strain dysregulate cell–matrix and cell–cell adhesion molecules: a quantitative analysis. Placenta 28, 527–537.
| Cytotrophoblasts infected with a pathogenic human cytomegalovirus strain dysregulate cell–matrix and cell–cell adhesion molecules: a quantitative analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXksV2mtrs%3D&md5=f32ac3c429758c0cd4cc4f58c90eb786CAS | 16822542PubMed |
[18] Tabata, T. et al. (2015) Human cytomegalovirus infection interferes with the maintenance and differentiation of trophoblast progenitor cells of the human placenta. J. Virol. 89, 5134–5147.
| Human cytomegalovirus infection interferes with the maintenance and differentiation of trophoblast progenitor cells of the human placenta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXntFajs7g%3D&md5=79cc7bf2fa70b76f8039ae8c5828ad50CAS | 25741001PubMed |
[19] Zydek, M. et al. (2014) HCMV infection of human trophoblast progenitor cells of the placenta is neutralized by a human monoclonal antibody to glycoprotein B and not by antibodies to the pentamer complex. Viruses 6, 1346–1364.
| HCMV infection of human trophoblast progenitor cells of the placenta is neutralized by a human monoclonal antibody to glycoprotein B and not by antibodies to the pentamer complex.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1GrtrrI&md5=b2ed8e118c4c69e770817bc08d1ab9b3CAS | 24651029PubMed |
[20] Nozawa, N. et al. (2009) Cytomegalovirus-specific, high-avidity IgG with neutralizing activity in maternal circulation enriched in the fetal bloodstream. J. Clin. Virol. 46, S58–S63.
| Cytomegalovirus-specific, high-avidity IgG with neutralizing activity in maternal circulation enriched in the fetal bloodstream.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVGntLrN&md5=d21c809ec71acc85981940e07caf1295CAS | 19854676PubMed |
[21] Fournier, T. et al. (2011) PPARgamma and human trophoblast differentiation. J. Reprod. Immunol. 90, 41–49.
| PPARgamma and human trophoblast differentiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXos1ygsbw%3D&md5=28c5ae872e948e84282251dd2b19e069CAS | 21704384PubMed |
[22] Kauvar, L.M. et al. (2015) A high affinity native human antibody blocks human cytomegalovirus infection of diverse cell types. Antimicrob. Agents Chemother. , .
| A high affinity native human antibody blocks human cytomegalovirus infection of diverse cell types.Crossref | GoogleScholarGoogle Scholar | 25534746PubMed |
[23] Tabata, T. et al. (2008) Induction of an epithelial integrin αvβ6 in human cytomegalovirus-infected endothelial cells leads to activation of transforming growth factor-β1 and increased collagen production. Am. J. Pathol. 172, 1127–1140.
| Induction of an epithelial integrin αvβ6 in human cytomegalovirus-infected endothelial cells leads to activation of transforming growth factor-β1 and increased collagen production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXislOht78%3D&md5=b8dd9f647a464dfc8f79635663ebd1f2CAS | 18349127PubMed |
[24] Yamamoto-Tabata, T. et al. (2004) Human cytomegalovirus interleukin-10 downregulates metalloproteinase activity and impairs endothelial cell migration and placental cytotrophoblast invasiveness in vitro. J. Virol. 78, 2831–2840.
| Human cytomegalovirus interleukin-10 downregulates metalloproteinase activity and impairs endothelial cell migration and placental cytotrophoblast invasiveness in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXitlSntLw%3D&md5=850d4ca33b8056a499a218eeb39080c4CAS | 14990702PubMed |
[25] Maidji, E. et al. (2007) Developmental regulation of human cytomegalovirus receptors in cytotrophoblasts correlates with distinct replication sites in the placenta. J. Virol. 81, 4701–4712.
| Developmental regulation of human cytomegalovirus receptors in cytotrophoblasts correlates with distinct replication sites in the placenta.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkvFChtr4%3D&md5=68560729cfdb1e491006400ba8292341CAS | 17314173PubMed |
[26] Maidji, E. et al. (2006) Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am. J. Pathol. 168, 1210–1226.
| Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjslOhtLc%3D&md5=3e52b75b9d1fc1f8551ea9d3b4dbefa4CAS | 16565496PubMed |
[27] McDonagh, S. et al. (2004) Viral and bacterial pathogens at the maternal-fetal interface. J. Infect. Dis. 190, 826–834.
| Viral and bacterial pathogens at the maternal-fetal interface.Crossref | GoogleScholarGoogle Scholar | 15272412PubMed |
[28] McDonagh, S. et al. (2006) Patterns of human cytomegalovirus infection in term placentas: a preliminary analysis. J. Clin. Virol. 35, 210–215.
| Patterns of human cytomegalovirus infection in term placentas: a preliminary analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjvVKgsg%3D%3D&md5=f9bf2b4d19f5d0c6fa84e1647f39c483CAS | 16386950PubMed |
[29] Tabata, T. et al. (2012) Cytomegalovirus impairs cytotrophoblast-induced lymphangiogenesis and vascular remodeling in an in vivo human placentation model. Am. J. Pathol. 181, 1540–1559.
| Cytomegalovirus impairs cytotrophoblast-induced lymphangiogenesis and vascular remodeling in an in vivo human placentation model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslOltbvM&md5=e7955d7d2c9a1a349ca5359a9d9aed12CAS | 22959908PubMed |
[30] Co, E.C. et al. (2013) Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy. Biol. Reprod. 88, 155.
| Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy.Crossref | GoogleScholarGoogle Scholar | 23553431PubMed |
Biographies
Lenore Pereira, PhD, is a Professor of Microbiology, and Takako Tabata, PhD, and Matthew Petitt, PhD, are Research Virologists in the Department of Cell and Tissue Biology at the University of California, San Francisco. Their studies focus on understanding the biology of human cytomegalovirus infection at the uterine-placental interface.


