New diagnostics and methods of assessing pregnant women at risk of cytomegalovirus
Tiziana Lazzarotto A , Liliana Gabrielli B and Roberta Rizzo CA Department of Specialised, Experimental, and Diagnostic Medicine, Operative Unit of Clinical Microbiology, Laboratory of Virology, St Orsola-Malpighi University Hospital, University of Bologna, Via Massarenti 9, 40138 Bologna, Italy, Tel: +39 051 214 3360, Fax: +39 051 307397, Email: tiziana.lazzarotto@unibo.it
B Operative Unit of Clinical Microbiology, Laboratory of Virology, St Orsola-Malpighi University Hospital, Bologna, Italy
C Department of Medical Sciences, Section of Microbiology and Medical Genetics, University of Ferrara, Ferrara, Italy
Microbiology Australia 36(4) 167-170 https://doi.org/10.1071/MA15060
Published: 19 October 2015
Human cytomegalovirus (CMV) infection can occur in pregnant women by primary infection or by non-primary infection, namely by either reactivation of the latent virus or reinfection with a different strain1. In all cases the mother can transmit the virus to the fetus through the placenta2,3. In the diagnosis of primary CMV infection, the gold standard is maternal seroconversion to CMV-specific antibodies. Currently, women are not routinely screened for CMV before conception or during pregnancy, thus CMV seroconversion is infrequently documented1. Lastly, serological diagnosis of non-primary CMV infection is very difficult and very often unreliable since no optimal diagnostic methods are currently available. Today, the fetal compartment can be only studied by amniocentesis and ultrasound examination for the diagnosis and prognosis of CMV infection and generally, invasive diagnostic protocol can be only suggested to pregnant women with evidence of primary CMV infection acquired early in gestation and in case of abnormal findings suggestive of congenital infection1. Therefore, a correct maternal diagnosis makes so that invasive prenatal diagnosis is only offered in selected cases. This report points out how a CMV-screening program combined with an advanced diagnostic protocol performed on pregnant women could identify those at high risk of transmitting the virus to their fetus. Furthermore, we evaluated the possible role of soluble HLA-G (sHLA-G) molecules detected in maternal and fetal samples in order to more accurately assess a greater risk of CMV-transmission and fetal/neonatal injury.
Diagnosis of maternal CMV infection
Testing for anti-CMV IgM antibodies is the most widely used and appropriate procedure for screening pregnant women4. Anti-CMV IgM antibodies are a good indicator of acute or recent infection, however it is not always correlated with active infection4–6. The rate of CMV-IgM detection by screening test ranged from 3–5.7%7–9, however only 7.5% of IgM-positive women have a congenitally infected fetus/newborn1. Consequently, all pregnant women with a positive screening CMV-IgM test should be offered advanced diagnostic testing as early as possible in pregnancy (before week 12–16 of gestation). Anti-CMV IgG avidity testing10–13, CMV-IgG and IgM immunoblotting (IB)14–16, microneutralisation assay17, and detection of viral DNA in blood, saliva and urine samples18–20 with Real Time PCR are currently the most reliable advanced procedures in order to identify all pregnant women who can transmit CMV infection.
The value of advanced diagnostic tests for identifying women at high risk of transmitting the virus
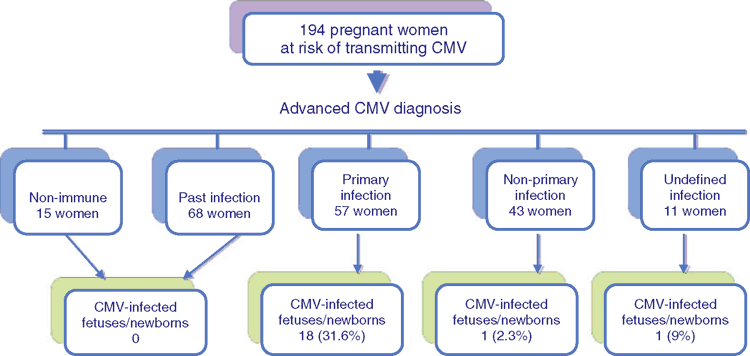
Since 1994, pregnant women with test results showing seroconversion or IgM screening positivity were referred to our centre for further analysis19. In 2014, we performed a check up on 194 pregnant women during a 6 month period. Out of these 194 cases 104 (53.6%) did not know their CMV serostatus before pregnancy. At the time of recruitment, the patients were in the first or second trimester of pregnancy, except 28 who were in the third trimester (range 6–38 weeks gestation, median 14).
We tested blood, saliva and urine samples obtained from all 194 women and were able to identify five different groups using serological and virological advanced diagnosis. In the first group of 15 non-immune pregnant women, all samples were positive/borderline for CMV-IgG or IgM with the screening assays. After using the advanced tests, we obtained in all cases negative results with IB-CMV IgG and IgM antibodies, undetectable IgG-avidity and CMV-DNA negative in all body fluids.
In the second group of 68 patients with past infection, the screening tests identified borderline/positive results for IgM antibodies in all 68 cases. After using advanced testing, we obtained negative results in all cases for IB-IgM antibodies and CMV-DNA in all body fluids. Moreover, we found high avidity in all cases.
In the third group, advanced diagnosis was able to identify 57 pregnant women with primary CMV infection. Also in this group the screening test was IgM positive or borderline and the IB confirmed this specific IgM-positivity for CMV. IgG-avidity in all 57 samples was low/moderate and in 52 out of 57 patients (91.2%) viral DNA in whole blood, saliva and urine samples was detected.
In particular, we found CMV-DNA in 35 whole blood samples out of 52 (61.4%) and the number of copies ranged from <500 to 8700 copies/mL. Higher rates of positivity were detected in the saliva and urine samples, 75.4% and 64.9%, respectively. The range varied from <500 to a maximum of 44 000 copies/mL of saliva and <500 to 9700 copies/mL of urine. Positive viral detection and viral load in whole blood, saliva and urine were not associated with a greater risk of infection and/or fetal/neonatal injury.
The fourth group included 43 pregnant women with non-primary CMV infection. IB confirmed the positive results for IgM in 40 out of 43 cases; in the remaining three patients we were able to prove the diagnosis of non-primary CMV infection with the detection of viral DNA in urine and saliva. In 43 pregnant women with non-primary CMV infection, we observed a good sensitivity of virological tests in saliva and urine samples (48.8% and 41.9%, respectively) and very low sensitivity in whole blood samples (9.3%). When considering overall virological results, we found viral DNA in body fluids in 65% of patients with non-primary CMV infection. The DNA levels were very low in all body fluids, ranging from <500 to 900 copies/mL in urine and less than 500 copies/mL in both whole blood and saliva.
Finally in the last group, the advanced diagnosis investigation confirmed active CMV infection, however we were not able to identify the kind of infection, hence the reason why this group included 11 pregnant women with undefined CMV infections. The incidence of congenital CMV infection in the 5 groups of pregnant women classified with advanced diagnostic protocol is shown in Figure 1.
|
Although generally the diagnosis of CMV infection remains complex, major goals have been achieved in recent years including maternal diagnosis with serological and virological tests. In particular, the use of advanced serological diagnosis has proven to be reliable in assessing pregnant women at risk of CMV infection. Likewise, virological diagnosis is also reliable and can support the serological diagnosis of primary, past and undefined CMV infection, as well as playing a role in the diagnosis of non-primary CMV infection.
Interaction between HLA-G expression and CMV infection during pregnancy
In order to improve the identification of i) pregnant women who transmit the virus to their fetus and ii) CMV-infected and compromised fetuses, we studied the expression of soluble isoform of HLA-G (sHLA-G) during CMV infection in maternal blood and amniotic fluid samples.
HLA-G is a non-classical HLA class I antigen characterised by a low allelic polymorphism, compared with the HLA class21,22. The HLA-G antigen is a tolerogenic molecule that acts on cells of both innate and adaptive immunity23,24. Interestingly, HLA-G expression by cytotrophoblasts is down-modulated by CMV infection25, while it is up-modulated in peripheral blood cells, with possible functional consequences in pregnancy immuno-regulation26,27.
In this study, sHLA-G levels in serum and amniotic fluid samples were assayed in triplicate as previously reported28,29, using an enzyme-linked immunosorbent assay (ELISA) and the monoclonal antibody MEM-G9 (Exbio), which recognises HLA-G molecules, in β2-microglobulin associated form. The intra-assay coefficient of variation (CV) was 1.4% and the inter-assay CV was 4.0%; the limit of sensitivity was 1.0 ng/mL.
We have an interim analysis of a clinical prospective trial which is enrolling 400 pregnant women suspected at routine CMV testing to have active CMV infection. Here, we report the results obtained from a first cohort of 166 pregnant women. At the moment of serological-virological advanced diagnosis, we evaluated sHLA-G levels in 171 serum samples of 55 pregnant women with primary CMV infection, 31 with non-primary, 69 with past infection, and 11 CMV-uninfected. The median levels of sHLA-G in pregnant women with primary were higher in comparison with non-primary CMV infection (45.16 ng/mL v. 10.58 ng/mL, P = 0.005; Student’s t-test). Furthermore, we observed lower median levels of sHLA-G serum between past CMV infected and uninfected women (14.68 ng/mL and 6.71 ng/mL, respectively). When we analysed the levels of sHLA-G in plasma samples from 55 primarily infected pregnant, considering transmitter and non-transmitter mothers, we did not find any statistical correlation (P = 0.72; Student’s t-test).
Finally, we analysed 25 amniotic fluid samples collected during amniocentesis (20–21 weeks gestation)19 from pregnant women with primary CMV infection arising before 14 weeks gestation. The comparison of the levels of sHLA-G between transmitter and non-transmitter mother was not statistically significant (P = 0.38; Student’s t-test).
The limited sample size does not permit firm conclusions, however our preliminary results suggest that sHLA-G detected in maternal plasma samples might be an additional biomarker of CMV infection that could be considered in combination with currently used serological and virological markers.
Conclusion
The laboratory diagnosis of CMV infection proves to be a reliable tool, provided that pregnant women are checked from the first weeks of gestation. Moreover, the use of advanced serological and virological maternal tests allow clinicians to identify women who are at higher risk of transmitting CMV to their fetus; however, they do not identify the infected fetuses, therefore making it necessary to offer prenatal diagnosis.
Nevertheless, major limitations of prenatal diagnosis of CMV should be acknowledged; amniocentesis is an invasive procedure and positive results of amniotic fluid tests do not discriminate between infected fetuses and compromised fetuses. For this reason, researchers continue to work on the prognosis factors for the CMV disease. All in all, our very preliminary results in this study suggest that sHLA-G could be a sensitive marker in order to monitor CMV infection during pregnancy.
Acknowledgements
This work was partially supported by grants Ricerca Finalizzata 2010 from the Italian Ministry of Health, Rome (Italy), ‘A. Liberati’ 2010–2012 Young Scientists Award, Regione Emilia Romagna (Italy). We thank our Linguistic Consultant, Lucy Scioscia, for editing the English language text.
References
[1] Lazzarotto, T. et al. (2004) Congenital cytomegalovirus infection: recent advances in the diagnosis of maternal infection. Hum. Immunol. 65, 410–415.| Congenital cytomegalovirus infection: recent advances in the diagnosis of maternal infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXksVCjsbs%3D&md5=85318020bb9621b016cabbc45386e4abCAS | 15172439PubMed |
[2] Gabrielli, L. et al. (2001) Complete replication of human cytomegalovirus in explants of first trimester human placenta. J. Med. Virol. 64, 499–504.
| Complete replication of human cytomegalovirus in explants of first trimester human placenta.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MvhvVamuw%3D%3D&md5=e55f01ce511ce7a95d03fbab7baa586bCAS | 11468735PubMed |
[3] Fisher, S. et al. (2000) Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J. Virol. 74, 6808–6820.
| Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXkvFCmt78%3D&md5=149f24ac07c6790e8fa3cddeb726b792CAS | 10888620PubMed |
[4] De Paschale, M. et al. (2010) Positive predictive value of anti-HCMV IgM as an index of primary infection. J. Virol. Methods 168, 121–125.
| Positive predictive value of anti-HCMV IgM as an index of primary infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptlWktrw%3D&md5=b43566e3bae36ec8389f8ead2222c708CAS | 20470827PubMed |
[5] Lazzarotto, T. et al. (1997) Search for cytomegalovirus-specific immunoglobulin M: comparison between a new western blot, conventional western blot, and nine commercially available assays. Clin. Diagn. Lab. Immunol. 4, 483–486.
| 1:CAS:528:DyaK2sXkvFymt7o%3D&md5=7ee658e3e53b5f22d06269f1a824ba58CAS | 9220169PubMed |
[6] De Carolis, S. et al. (2010) False-positive IgM for CMV in pregnant women with autoimmune disease: a novel prognostic factor for poor pregnancy outcome. Lupus 19, 844–849.
| False-positive IgM for CMV in pregnant women with autoimmune disease: a novel prognostic factor for poor pregnancy outcome.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3czntFyiuw%3D%3D&md5=b683279966740848f3611cd1786fe7afCAS | 20305050PubMed |
[7] Munro, S.C. et al. (2005) Diagnosis of and screening for cytomegalovirus infection in pregnant women. J. Clin. Microbiol. 43, 4713–4718.
| Diagnosis of and screening for cytomegalovirus infection in pregnant women.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVOrtLbK&md5=251d466089be2daf14860b6bf7fb9809CAS | 16145132PubMed |
[8] Picone, O. et al. (2009) A 2-year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG 116, 818–823.
| A 2-year study on cytomegalovirus infection during pregnancy in a French hospital.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MzjslWnsQ%3D%3D&md5=cde25bbcf85b61fa52f677a3aa5e93b3CAS | 19432571PubMed |
[9] Dollard, S.C. et al. (2011) National prevalence estimates for cytomegalovirus IgM and IgG avidity and association between high IgM antibody titer and low IgG avidity. Clin. Vaccine Immunol. 18, 1895–1899.
| National prevalence estimates for cytomegalovirus IgM and IgG avidity and association between high IgM antibody titer and low IgG avidity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsV2qtbzJ&md5=a58571e940b743723862d7bca0dfa082CAS | 21918114PubMed |
[10] Grangeot-Keros, L. et al. (1997) Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary infection in pregnant women. J. Infect. Dis. 175, 944–946.
| Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary infection in pregnant women.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s3ktVKqtw%3D%3D&md5=95ed7a8a3fe26b962ea672cd04c42408CAS | 9086155PubMed |
[11] Lazzarotto, T. et al. (2000) Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Viral Immunol. 13, 137–141.
| Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhvVejsro%3D&md5=67b7535b38c36fda6f1f09b7653e579aCAS | 10733175PubMed |
[12] Kanengisser-Pines, B. et al. (2009) High cytomegalovirus IgG avidity is a reliable indicator of past infection in patients with positive IgM detected during the first trimester of pregnancy. J. Perinat. Med. 37, 15–18.
| High cytomegalovirus IgG avidity is a reliable indicator of past infection in patients with positive IgM detected during the first trimester of pregnancy.Crossref | GoogleScholarGoogle Scholar | 18673093PubMed |
[13] Guisasola, M.E. et al. (2010) Comparison of IgG avidity assays in the confirmation of the diagnosis of cytomegalovirus primary infection. APMIS 118, 991–993.
| Comparison of IgG avidity assays in the confirmation of the diagnosis of cytomegalovirus primary infection.Crossref | GoogleScholarGoogle Scholar | 21091781PubMed |
[14] Enders, G. et al. (2013) The value of CMV IgG avidity and immunoblot for timing the onset of primary CMV infection in pregnancy. J. Clin. Virol. 56, 102–107.
| The value of CMV IgG avidity and immunoblot for timing the onset of primary CMV infection in pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1OksrjO&md5=e199b5b2e456d9c0915c11c8603690e2CAS | 23153820PubMed |
[15] Lazzarotto, T. et al. (1998) Development of a new cytomegalovirus (CMV) immunoglobulin M (IgM) immunoblot for detection of CMV-specific IgM. J. Clin. Microbiol. 36, 3337–3341.
| 1:CAS:528:DyaK1cXntFOit70%3D&md5=7d340b3d9a337c16eba14d3dd51fdd79CAS | 9774589PubMed |
[16] Rajasekariah, H. et al. (2013) Improving diagnosis of primary cytomegalovirus infection in pregnant women using immunoblots. J. Med. Virol. 85, 315–319.
| Improving diagnosis of primary cytomegalovirus infection in pregnant women using immunoblots.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVCksrbK&md5=7ae233affbcf9d0d692a5029152c24d3CAS | 23171986PubMed |
[17] Eggers, M. et al. (2000) Combination of microneutralization and avidity assays: improved diagnosis of recent primary human cytomegalovirus infection in single serum sample of second trimester pregnancy. J. Med. Virol. 60, 324–330.
| Combination of microneutralization and avidity assays: improved diagnosis of recent primary human cytomegalovirus infection in single serum sample of second trimester pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2Fps1OgtQ%3D%3D&md5=98b79b2d7014bdb7a0a488177a4de903CAS | 10630965PubMed |
[18] Kourí, V. et al. (2010) Diagnosis and screening for cytomegalovirus infection in pregnant women in Cuba as prognostic markers of congenital infection in newborns: 2007–2008. Pediatr. Infect. Dis. J. 29, 1105–1110.
| Diagnosis and screening for cytomegalovirus infection in pregnant women in Cuba as prognostic markers of congenital infection in newborns: 2007–2008.Crossref | GoogleScholarGoogle Scholar | 20622711PubMed |
[19] Lazzarotto, T. et al. (2008) New advances in the diagnosis of congenital cytomegalovirus infection. J. Clin. Virol. 41, 192–197.
| New advances in the diagnosis of congenital cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXitVensbo%3D&md5=73db54c15b977447096fd7db91108b47CAS | 18054840PubMed |
[20] Revello, M.G. et al. (2014) Cytomegalovirus DNAemia in pregnant women. J. Clin. Virol. 61, 590–592.
| Cytomegalovirus DNAemia in pregnant women.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsl2gsr3O&md5=0b53f99eac7d3ca6976908549976c4b7CAS | 25457128PubMed |
[21] Lin, A. et al. (2007) Modulation of HLA expression in human cytomegalovirus immune evasion. Cell. Mol. Immunol. 4, 91–98.
| 17484802PubMed |
[22] Yan, W.H. et al. (2009) Induction of both membrane-bound and soluble HLA-G expression in active human cytomegalovirus infection. J. Infect. Dis. 200, 820–826.
| Induction of both membrane-bound and soluble HLA-G expression in active human cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFChsLzP&md5=dc8c99d2ce1883029834a19b855712c3CAS | 19619059PubMed |
[23] Rizzo, R. et al. (2011) The importance of HLA-G expression in embryos, trophoblast cells, and embryonic stem cells. Cell. Mol. Life Sci. 68, 341–352.
| The importance of HLA-G expression in embryos, trophoblast cells, and embryonic stem cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntVeitQ%3D%3D&md5=ca399e9ecb985d59e9c54e6c53e13db0CAS | 21080028PubMed |
[24] Rizzo, R. et al. (2009) Soluble human leukocyte antigen-G isoforms in maternal plasma in early and late pregnancy. Am. J. Reprod. Immunol. 62, 320–338.
| Soluble human leukocyte antigen-G isoforms in maternal plasma in early and late pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVKhu7zJ&md5=cddbf0c7b78d388f679ff432bc404494CAS | 19811467PubMed |
[25] Onno, M. et al. (2000) Modulation of HLA-G antigens expression by human cytomegalovirus: specific induction in activated macrophages harboring human cytomegalovirus infection. J. Immunol. 164, 6426–6434.
| Modulation of HLA-G antigens expression by human cytomegalovirus: specific induction in activated macrophages harboring human cytomegalovirus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktFWltLo%3D&md5=fb2dc552510726045fe044d8c687b061CAS | 10843698PubMed |
[26] Ponte, M. et al. (1999) Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proc. Natl. Acad. Sci. USA 96, 5674–5679.
| Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjtFCntLg%3D&md5=7a18baab0216a774cfe5fd8a372007cfCAS | 10318943PubMed |
[27] Rajagopalan, S. and Long, E.O. (1999) A human histocompatibility leucocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 189, 1093–1100.
| A human histocompatibility leucocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXitlemtrg%3D&md5=ec555e314f7b0531d093606cc4cd640bCAS | 10190900PubMed |
[28] Rizzo, R. et al. (2007) Soluble HLA-G molecules in follicular fluid: a tool for oocyte selection in IVF? J. Reprod. Immunol. 74, 133–142.
| Soluble HLA-G molecules in follicular fluid: a tool for oocyte selection in IVF?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXkvFGgsb4%3D&md5=c9128b7f58fd63e1a265492adc21b66fCAS | 17399800PubMed |
[29] Fainardi, E. et al. (2007) Soluble HLA-G molecules are released as HLA-G5 and not as soluble HLA-G1 isoforms in CSF of patients with relapsing-remitting multiple sclerosis. J. Neuroimmunol. 192, 219–225.
| Soluble HLA-G molecules are released as HLA-G5 and not as soluble HLA-G1 isoforms in CSF of patients with relapsing-remitting multiple sclerosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhsVSmu7fK&md5=285311a8b5a334abd8ba80b475b48411CAS | 17997167PubMed |
Biographies
Professor Tiziana Lazzarotto is Associate Professor of Microbiology and Clinical Microbiology, Department of Specialised, Experimental, and Diagnostic Medicine, School of Medicine and Surgery at the Alma Mater Studiorum University of Bologna (Italy). She heads the Laboratory of Virology. She has made significant contributions in the study of: (1) the immune response during cytomegalovirus (CMV) infection in immunocompetent and immunocompromised subjects; (2) the management of CMV during pregnancy and neonatal period; and (3) the management of CMV infection in solid organ/haematopoietic stem cell transplant recipients. She is a member of the ‘European Congenital CMV Initiative’ and coordinator of a multidisciplinary network ‘Infectious Diseases in Obstetrics-Gynecology and Neonatology’ of the six Italian Scientific Societies. She has published more than 130 papers in International peer-reviewed Journals.
Dr Liliana Gabrielli is a Medical Doctor at the Operative Unit of Microbiology, St Orsola-Malpighi University Hospital, Bologna (Italy). She has expertise in the field of virology with specific reference to diagnosis and monitoring of herpes viruses infection in both immunocompromised and immunocompetent individuals. Her research focuses on intrauterine transmission of cytomegalovirus (CMV) and in particular on the correlation between inflammatory infiltrate and tissue damage and between placental and brain damage in fetuses congenitally infected. She also studied congenital CMV-related damage in the inner ear, especially fetal cochlear infection, in order to understand the pathophysiological mechanisms of sensorineural hearing loss. She has published more than 35 papers in international peer-reviewed journals.
Dr Roberta Rizzo is Assistant Professor in Microbiology at the University of Ferrara (Italy) and she is supervising several projects in the field of immunological tolerance in pregnancy, autoimmunity, viral and bacterial infections. During her career she made important researches to unravelling networks of immunological tolerance during pregnancy, first by evidencing the importance of HLA-G molecules in embryo implantation and pregnancy outcome. More recently, she started working in the field of host immune response towards microbiological infections elucidating the role of NK cell KIR receptors in the control of herpesvirus infections. She has published more than 70 papers in international peer-reviewed journals.


