Morphogenesis and pathogenesis: control of cell identity in a dimorphic pathogen
Hayley E Bugeja A and Alex Andrianopoulos A BA Genetics, Genomics and Development
School of BioSciences
The University of Melbourne
Vic. 3010, Australia
B Tel: +61 3 8344 5164, Fax: +61 3 8344 5139, Email: alex.a@unimelb.edu.au
Microbiology Australia 36(2) 95-97 https://doi.org/10.1071/MA15031
Published: 19 March 2015
Fungal pathogens span all major phylogenetic groupings within the fungal kingdom, infecting animals, plants and other fungi. Intrinsic to their ability to infect a host and survive host defense mechanisms is the capacity to produce the appropriate cell type. The link between morphogenesis and pathogenesis is clear for a number of pathogenic fungi that undergo a phase transition known as dimorphism (or dimorphic switching)1. Dimorphic fungi are able to alternate between multicellular filamentous growth, characterised by highly polarised hyphal growth, and unicellular growth with yeast cells dividing by budding or fission. This trait is strongly linked with virulence in the important human pathogens Blastomyces dermatitidis, Candida albicans, Coccidioides immitis/posadasii, Histoplasma capsulatum, Paracoccidioides brasiliensis/luttzii, Talaromyces marneffei (formerly named Penicillium marneffei) and Sporothrix schenckii1. Uncovering the mechanisms that control morphogenesis during dimorphic switching and the physiological properties of the hyphal and yeast cell types is crucial to understanding pathogenicity.
Prevalent in South-East Asia and the surrounding regions, T. marneffei causes a deadly systemic infection in immunocompromised hosts2,3. The rapid rise in T. marneffei infections associated with the worldwide HIV pandemic led to it being described as an AIDS-defining pathogen3. While there are sporadic reports of T. marneffei infections in ‘immunocompetent hosts’ the immune status has not been adequately tested in these cases, and the term ‘immunocompetent’ is often used interchangeably (and incorrectly) in these reports with HIV negative status. The ecological niche of T. marneffei is unclear, but there is a strong association with a number of bamboo rat species in endemic areas3,4. T. marneffei is unique as the only member of the very large Eurotiales order that can undergo a dimorphic switch, and the only ‘Penicillium’ species within this order known to be a pathogen5,6.
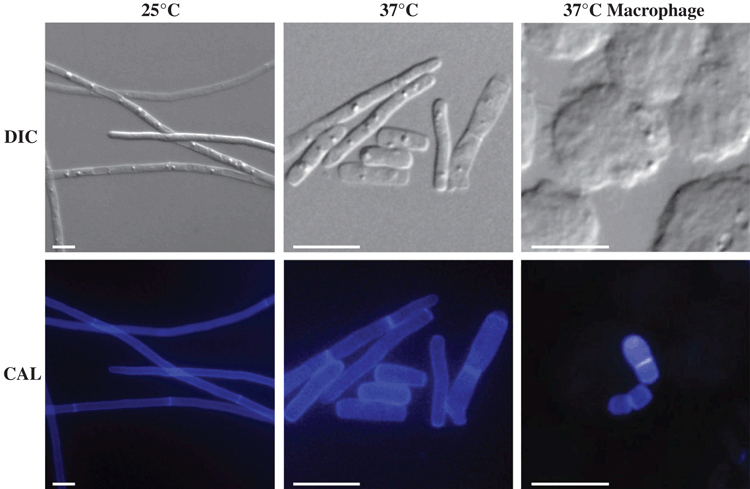
As for many dimorphic pathogens, temperature is a key trigger for the dimorphic transition (Figure 1). At 25°C, T. marneffei produces multinucleate filamentous hyphal networks (mycelia) by highly polarised apical growth, subapical cell branching and uncoupled nuclear and cellular division. Specialised differentiated aerial hyphae known as conidiophores generate uninucleate conidia, the most likely infectious agent. At 37°C, uninucleate fission yeast cells, which represent the pathogenic form, are produced via coupling of nuclear and cellular division and complete cell separation at centrally located double septae. In the host the yeast cells of T. marneffei reside within phagocytes, predominantly macrophages, subverting the killing activity of these cells and proliferating within them5.
Genetics studies in T. marneffei aimed at dissecting the roles of cell signalling and polarity determinants have identified many highly conserved factors including p21-activated kinases (PAKs) and Ras-superfamily small GTPases (Ras/Rho/Cdc42/Rac)7,8. In T. marneffei, PAKs are key regulators of the temperature-dependent response. Mutants in pakA fail to germinate at 37°C either in vitro or in host cells9. A second PAK also exists in many fungi and it has been shown in T. marneffei that PakB is essential for yeast cell morphogenesis during growth in host cells but not in vitro10. In addition, loss of pakB results in the inappropriate production of yeast cells at 25°C. The GTPases rasA, cflA (encoding a Cdc42 orthologue) and cflB (encoding a Rac orthologue) have both overlapping and unique functions. For example, RasA functions upstream of CflA during germination of conidia, hyphal cell polarised growth and yeast cell morphogenesis, whereas CflB is important for conidiophore morphogenesis and hyphal cell branching. Importantly, CflA is upstream of PakA during the transition from conidia to yeast cells at 37°C highlighting a distinct temperature regulated yeast morphogenesis pathway. In many other fungal pathogens orthologous factors to rasA, cflA, cflB and pakA have also been shown to affect morphogenesis (for example, Almeida et al.11).
More recently, upstream factors, important for sensing temperature and the host cell environment, as well as transcriptional processes triggered to effect morphogenesis, have been characterised. A derivative of prokaryotic two-component systems, the hybrid histidine kinases (HKK) are a major class of sensor systems used by fungi to transmit information from the external environment12. Two HKKs of T. marneffei, encoded by drkA and slnA, are required for different aspects of yeast morphogenesis in macrophages: SlnA is important for germination and DrkA for the transition to yeast cells. These HKKs also have additional roles including stress adaptation, asexual development, hyphal morphogenesis and cell wall integrity showing that they are key factors in the ability of the various cell types to respond to the external environment and trigger the correct cellular response. In both B. dermatitidis and H. capsulatum, the DrkA orthologue is essential for the hyphal to yeast transition and mutants are severely compromised in their virulence13.
At the other end of the spectrum, very few transcription factors have been identified as major regulators of vegetative cell type morphogenesis as it relates to pathogenicity. The velvet family of factors play an important role in H. capsulatum yeast cell morphogenesis14 but this is not conserved in T. marneffei and these factors have diverse roles in other fungi. In contrast, the hgrA gene, encoding a C2H2 zinc finger transcription factor, plays a central role in hyphal cell morphogenesis and its activity must be downregulated in order to generate the pathogenic yeast cell type, either in vitro or in macrophages15. Loss of HgrA also leads to cell wall defects and increased sensitivity to cell wall, oxidative, but not osmotic stress agents. Based on these studies and those in other fungi, it is clear that the HgrA family of transcription factors are conserved regulators.
Future directions
Despite the efforts of many groups around the world, working on a range of dimorphic fungal pathogens, studies into the mechanisms that control this morphogenetic transition, which is central to pathogenicity, are in their infancy. A handful of key factors have been identified and these are excellent entry points into uncovering the network of genes that regulate this process. With the newly developed high-throughput genomic tools such as ChIP-seq that are now available and established in these various dimorphic pathogens, our understanding of these systems is primed to uncover new and exciting avenues for the control and treatment of these infections.
References
[1] Sil, A. and Andrianopoulos, A. (2014) Thermally dimorphic human fungal pathogens-polyphyletic pathogens with a convergent pathogenicity trait. Cold Spring Harbor perspectives in medicine, in press.[2] Segretain, G. (1959) Penicillium marneffei n.sp., agent of a mycosis of the reticuloendothelial system. Mycopathologia 11, 327–353.
| 1:STN:280:DyaF3c7pslehtQ%3D%3D&md5=1928292bc913dc18cd0b9044b8e7ea0bCAS | 14444578PubMed |
[3] Vanittanakom, N. et al. (2006) Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin. Microbiol. Rev. 19, 95–110.
| Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhsFGjtbs%3D&md5=fd5abbb0b0a7b2c17eda6942972adfc3CAS | 16418525PubMed |
[4] Henk, D.A. et al. (2012) Clonality despite sex: the evolution of host-associated sexual neighborhoods in the pathogenic fungus Penicillium marneffei. PLoS Pathog. 8, e1002851.
| 23055919PubMed |
[5] Andrianopoulos, A. (2002) Control of morphogenesis in the human fungal pathogen Penicillium marneffei. Int. J. Med. Microbiol. 292, 331–347.
| Control of morphogenesis in the human fungal pathogen Penicillium marneffei.Crossref | GoogleScholarGoogle Scholar | 12452280PubMed |
[6] Samson, R.A. et al. (2011) Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 70, 159–183.
| Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC383gsFKntA%3D%3D&md5=5cc61a51fff1acc4275ea9e2999fc273CAS | 22308048PubMed |
[7] Boyce, K.J. and Andrianopoulos, A. (2011) Ste20-related kinases: effectors of signaling and morphogenesis in fungi. Trends Microbiol. 19, 400–410.
| Ste20-related kinases: effectors of signaling and morphogenesis in fungi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXps1entbw%3D&md5=5b09dd27fe2a3df1b201188c3ed5ad27CAS | 21640592PubMed |
[8] Boyce, K.J. and Andrianopoulos, A. (2013) Morphogenetic circuitry regulating growth and development in the dimorphic pathogen Penicillium marneffei. Eukaryot. Cell 12, 154–160.
| Morphogenetic circuitry regulating growth and development in the dimorphic pathogen Penicillium marneffei.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitlGjsbg%3D&md5=e984ec49cbc343448d151bbd6bfecf0eCAS | 23204189PubMed |
[9] Boyce, K.J. and Andrianopoulos, A. (2007) A p21-activated kinase is required for conidial germination in Penicillium marneffei. PLoS Pathog. 3, e162.
| A p21-activated kinase is required for conidial germination in Penicillium marneffei.Crossref | GoogleScholarGoogle Scholar | 17983267PubMed |
[10] Boyce, K.J. et al. (2009) In vivo yeast cell morphogenesis is regulated by a p21-activated kinase in the human pathogen Penicillium marneffei. PLoS Pathog. 5, e1000678.
| In vivo yeast cell morphogenesis is regulated by a p21-activated kinase in the human pathogen Penicillium marneffei.Crossref | GoogleScholarGoogle Scholar | 19956672PubMed |
[11] Almeida, A.J. et al. (2009) Cdc42p controls yeast-cell shape and virulence of Paracoccidioides brasiliensis. Fungal Genet. Biol. 46, 919–926.
| Cdc42p controls yeast-cell shape and virulence of Paracoccidioides brasiliensis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlCntb3E&md5=c5519e0a2b32e701a0d5602a65c54f18CAS | 19686860PubMed |
[12] Boyce, K.J. et al. (2011) The two-component histidine kinases DrkA and SlnA are required for in vivo growth in the human pathogen Penicillium marneffei. Mol. Microbiol. 82, 1164–1184.
| The two-component histidine kinases DrkA and SlnA are required for in vivo growth in the human pathogen Penicillium marneffei.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhs1antrjL&md5=4f06a1ee8a5046272e51e9f2d550bf38CAS | 22059885PubMed |
[13] Nemecek, J.C. et al. (2006) Global control of dimorphism and virulence in fungi. Science 312, 583–588.
| Global control of dimorphism and virulence in fungi.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjvVGktbs%3D&md5=ab13d0965a978b8f4e1ad932942016e8CAS | 16645097PubMed |
[14] Beyhan, S. et al. (2013) A temperature-responsive network links cell shape and virulence traits in a primary fungal pathogen. PLoS Biol. 11, e1001614.
| 1:CAS:528:DC%2BC3sXht1ygu7vE&md5=67f0a43fe965b28e7226a2e35d1f6034CAS | 23935449PubMed |
[15] Bugeja, H.E. et al. (2013) HgrA is necessary and sufficient to drive hyphal growth in the dimorphic pathogen Penicillium marneffei. Mol. Microbiol. 88, 998–1014.
| HgrA is necessary and sufficient to drive hyphal growth in the dimorphic pathogen Penicillium marneffei.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXot1arur4%3D&md5=d81470550044eb3f422de6abbb6915fbCAS | 23656348PubMed |
Biographies
Hayley Bugeja is a post-doctoral research fellow in the School of BioSciences at The University of Melbourne. Her research focus is to understand how gene expression is regulated during growth, development and the establishment of cellular identity using fungi as model systems. This has been fostered through her research dissecting the molecular mechanisms controlling the dimorphic transition, and its contribution to virulence, in the human pathogen Talaromyces marneffei.
Alex Andrianopoulos is a Reader in Genetics in the Genetics, Genomics and Development cluster in the School of BioSciences at The University of Melbourne. His research is focused on understanding the fundamental molecular mechanisms that control cellular morphogenesis and development using a number of model fungi and how these mechanisms underpin virulence and pathogenicity in pathogenic fungi.