Microsphaeropsis arundinis: an emerging cause of phaeohyphomycosis in cats and people
George Reppas A , Thomas Gottlieb B , Mark Krockenberger C , Catriona Halliday D and Richard Malik E FA Vetnostics, 60 Waterloo Road, North Ryde, NSW 2113, Australia
B Concord Repatriation General Hospital, Concord NSW 2139, Australia
C Faculty of Veterinary Science, The University of Sydney, NSW 2006, Australia
D CIDMLS, ICPMR – Pathology West, Westmead Hospital, Westmead, NSW 2145, Australia
E Centre for Veterinary Education, The University of Sydney, NSW 2006, Australia
F Corresponding author. Email: Richard.Malik@sydney.edu.au
Microbiology Australia 36(2) 74-78 https://doi.org/10.1071/MA15025
Published: 17 March 2015
Microsphaeropsis arundinis is an anamorphic dematiaceous fungus ubiquitous in soil and fresh water1–4. It typically inhabits terrestrial plant hosts1–4 and has a well-known association with Aruno donax, a garden escape weed known as ‘giant reed’ or ‘elephant grass’. M. arundinis (fungi imperfecti) is a coelomycete, which encompasses an emerging group of pathogens capable of causing soft tissue infections, mostly in immunocompromised human patients. Such disease typically arises secondary to traumatic inoculation of fungal elements into the subcutis. The infection may spread to contiguous subcutaneous tissues or via the lymphatics in a sporotrichoid manner. The first reports of this organism causing disease occurred just over 10 years ago, and since then an increasing number of cases have been encountered, but so far only in cats and people. In cats, lesions are most consistently encountered on their distal extremities, viz. on or near the toes.
In 2004, Kluger et al. reported the first Microsphaeropsis arundinis infection in a mammalian host1. The patient was a seven-year-old cat living in suburban Sydney. It had a granulomatous lesion within the deep tissues of the distal forelimb. The cat had a concurrent Fusarium chlamydosporum infection affecting another limb. A few months later, Pendle et al. from Royal North Shore Hospital reported the same organism as a cause of disease in two immunocompromised human patients, with limited archival information on a third case, a patient with acute myeloid leukaemia seen 23 years earlier2. Is it a coincidence that the first reports of a new mammalian fungal pathogen occurred at virtually the same time, and in the same city, in both human and veterinary (feline) patients? It may be, but it would neglectful not to look further for factors that may explain why humans and cats were becoming infected by this hitherto non-pathogenic fungus. These events also emphasise the ‘One Medicine – One Health’ approach to infectious disease investigation, with animals representing sentinels for the occurrence of human disease. This is particularly the case for fungal diseases acquired from the environment.
In 2009, the first M. arundinis infection in the USA was reported in a human patient receiving immunosuppressive therapy for a renal transplant3. The man was domiciled in Florida, an area with a subtropical environment likely favourable to this organism. Sydney, while potentially temperate in climate by latitude, is classified as subtropical in rainfall distribution and temperature, with summer distribution of rainfall and mild winter temperatures.
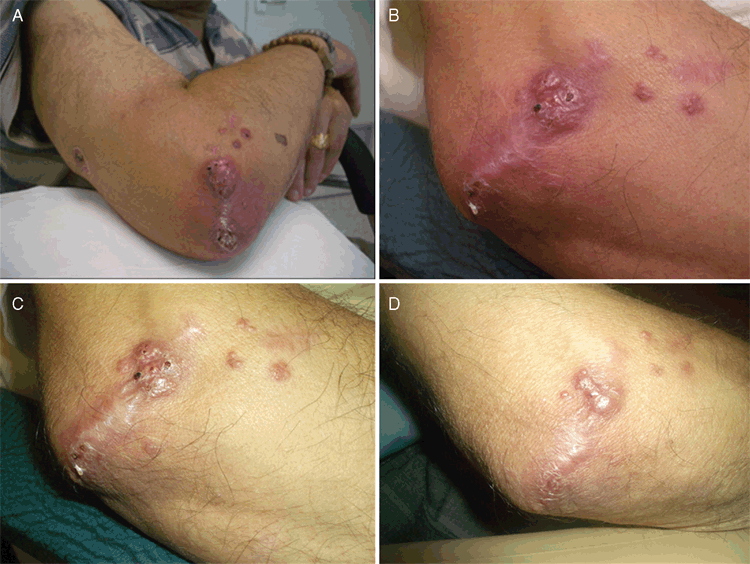
In 2010, our group again reported disease caused by M. arundinis infection affecting the distal extremity of a cat (Figure 1), although this animal also had a lesion on its face4. Halliday molecularly characterised the internal transcribed spacer (ITS1), 5.8S and ITS2 regions and the D1/D2 region of the 28S rDNA gene cluster of the available human (four) and feline (two) isolates4,5. These included a case (contributed by Tom Gottlieb) of refractory dermal plaques in a renal transplant recipient (Figure 2; Table 1). The isolates were deposited in various culture collections and the merged ITS and LSU sequences of the six isolates were deposited in the GenBank database (www.ncbi.nlm.nih.gov/genbank/).

|

|
Since the first human report by Pendle et al.2, there have been at least six additional M. arundinis infections reported in human patients, individual cases being seen at St George Hospital, Wollongong Hospital, Concord Hospital (Figure 2), Westmead Hospital and Prince of Wales Hospital, and a further case from Florida in the USA (Table 1). The additional five Australian cases have been collated and submitted for peer review, including the case in Table 1 and Figure 2.
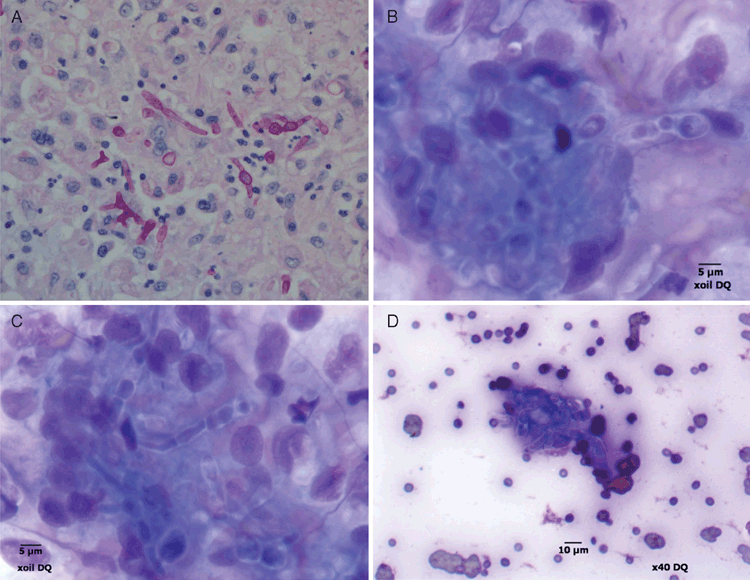
In the veterinary arena, we continue to see M. arundinis infections in cats along the East coast of Australia. It is now probably the most common cause of feline subcutaneous phaeohyphomycosis in this region, with five additional cases between 2009 and 2012 (Table 2). There does not appear to be any age predisposition in cats (range 5–20 years) and no gender preponderance. Geographically, two cats were domiciled in Sydney, two cats resided in the Central Coast of NSW, one was from Newcastle, another from Wollongong, while the last cat was from Brisbane. While there is the potential of geographical bias due to the catchment area of our pathology services, all these cats lived in coastal environments, which are becoming increasingly warmer and more humid. Lesions were invariably present on distal extremities, with either forelimbs or hind limbs being affected. Microscopy of needle aspirates or crush preparations from lesions were suggestive of phaeohyphomycosis, with pigmented bulbous septate hyphae or pseudo-hyphae of variable length, and occasional yeast-like forms evident in the tissues (Figure 3B–D). Histopathological specimens showed pyogranulomatous inflammation (Figure 3A), with occasional multi-nucleate giant cell formation. The organism grows well on routine fungal media such as Sabouraud dextrose agar (containing antibiotics) and microscopically shows irregularly-shaped pigmented septate hyphae, but no conidia. Since species identification is made difficult by the inability to induce sporulation, PCR amplification and sequence analysis was typically used to establish a specific diagnosis (as outlined above). This could be done using not only colonial material or fresh biopsy specimens from representative lesions but also paraffin-embedded formalin-fixed tissues5. Unlike the situation in people where most patients appear to be immunosuppressed by comorbid disease (renal failure, diabetes), corticosteroids or immunomodulatory drugs, most cats appear immune-competent. Cats with co-infections with other fungi (e.g. Fusarium spp.) are postulated to have had penetrating injuries contaminated by multiple fungi normally residing in soil.
|
In veterinary practice, frustratingly, repeat samples for culture and antifungal susceptibility are often difficult to obtain. This is usually because serial specimen collection generally requires sedation or anaesthesia with concomitant cost and morbidity. To overcome this potential limitation, we have found that there is sufficient fungal nucleic acid preserved in methanol-fixed, DiffQuik®-stained smears to permit successful DNA purification, panfungal PCR and sequence analysis using material scraped from the cytological specimens (Table 2). The method6, adapted from a similar technique used for diagnosis of veterinary mycobacteria specimens7, was successful in 4/4 feline M. arundinis cytology slides in which it was attempted (Table 2).
Antifungal minimum inhibitory concentration breakpoints have not been determined for this organism, although broth microdilution assays suggest most isolates are susceptible to broad spectrum azoles (itraconazole, voriconazole and posaconazole), amphotericin B and terbinafine, with variable susceptibility to fluconazole and resistance to echinocandins and flucytosine. Clinically, itraconazole, posaconazole, ketoconazole and terbinafine all appear to have good in vivo activity.
As voriconazole causes neurotoxicity in many feline patients8, we currently recommend posaconazole to treat M. arundinis infections in cats. Although the drug is expensive, it is palatable, available in a liquid form, has minimal hepatotoxicity (unlike itraconazole) and possesses favourable and reliable pharmacokinetics in this species, where once daily administration with meals is convenient for owners4. Terbinafine is probably also a good option for cats, with established pharmacokinetics and inexpensive generic formulations available, but we have no experience to date. Despite long courses of therapy, and in some cases cytoreductive surgery (typically toe amputation), there is a tendency for infections in cats to recur months or years after apparently successful therapy. This does not appear to be the case in human patients. Despite this propensity to recur, the organism seems inherently of low virulence, producing indolent infections without dissemination. This is possibly due to the organism favouring lower temperatures for growth, like many fungal saprophytes. The few well documented human cases reported to date have been cured with monotherapy using a triazole or terbinafine, sometimes combined with debridement or amputation of infected tissues2,3.
We speculate that this infection might be becoming more common due to expansion in the range of ‘elephant grass’ or expansion of other plant hosts capable of supporting its environmental growth. The location of lesions on the toes of cats suggests penetrating injury of distal extremities during digging may play a role in disease pathogenesis. For the same reason, it might be expected that cat scratch injuries (typically to the face) might also be contaminated by this organism9,10, as in the case reported by Krockenberger et al.4. Similar predisposing factors may be operating in people who garden without protective measures (gloves and long sleeved apparel) and therefore may be at risk for development of M. arundinis infections through contamination of skin wounds, especially if they have diabetes or are receiving immunosuppressive therapy. It is interesting that cats have an apparent predisposition, as to date there have been no reported infections in non-feline veterinary patients, i.e. dogs, horses, cows, etc. The reason for this species predilection requires investigation. Time may reveal the answers to these questions as we actively search for more cases!
Acknowledgements
The authors thank Sharon Chen for information on the additional Australian human Microsphaeropsis arundinis infections from a manuscript currently under review.
References
[1] Kluger, E.K. et al. (2004) Concurrent Fusarium chlamydosporium and Microsphaeropsis arundinis infections in a cat. J. Feline Med. Surg. 6, 271–277.| Concurrent Fusarium chlamydosporium and Microsphaeropsis arundinis infections in a cat.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2czmt1Gqtg%3D%3D&md5=fe3922ae3655e065dd8a61d4ff4c408aCAS | 15265482PubMed |
[2] Pendle, S. et al. (2004) Phaehyphomycotic soft tissue infections caused by the Coelomycetous fungus Microsphaeropsis arundinis. J. Clin. Microbiol. 42, 5315–5319.
| Phaehyphomycotic soft tissue infections caused by the Coelomycetous fungus Microsphaeropsis arundinis.Crossref | GoogleScholarGoogle Scholar | 15528731PubMed |
[3] Hall, M.R. et al. (2013) Cutaneous Microsphaeropsis arundinis infection initially interpreted as squamous cell carcinoma. Int. J. Dermatol. 52, 84–86.
| Cutaneous Microsphaeropsis arundinis infection initially interpreted as squamous cell carcinoma.Crossref | GoogleScholarGoogle Scholar | 23278613PubMed |
[4] Krockenberger, M.B. et al. (2010) Localised Microsphaeropsis arundinis infection of the subcutis of a cat. J. Feline Med. Surg. 12, 231–236.
| Localised Microsphaeropsis arundinis infection of the subcutis of a cat.Crossref | GoogleScholarGoogle Scholar | 20193914PubMed |
[5] Lau, A. et al. (2007) Development and clinical application of a panfungal PCR assay to detect and identify fungal DNA in tissue specimens. J. Clin. Microbiol. 45, 380–385.
| Development and clinical application of a panfungal PCR assay to detect and identify fungal DNA in tissue specimens.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjtFGnsL4%3D&md5=cda2d024aa01389cd360fe53e9793457CAS | 17122000PubMed |
[6] Reppas, G. et al. (2014) Preliminary investigations utilizing a panfungal PCR to determine the identity of fungal infections in cytological specimens from animals. J. Comp. Path. 150, 1.
[7] Reppas, G. et al. (2013) Detection and identification of mycobacteria in fixed stained smears and formalin-fixed paraffin-embedded tissues using PCR. J. Small Anim. Pract. 54, 638–646.
| Detection and identification of mycobacteria in fixed stained smears and formalin-fixed paraffin-embedded tissues using PCR.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2c7gtlajsA%3D%3D&md5=0ca1215f8dffa626dd2d5a858bc9bec2CAS | 24164562PubMed |
[8] Quimby, J.M. et al. (2010) Adverse neurologic events associated with voriconazole use in 3 cats. J. Vet. Intern. Med. 24, 647–649.
| Adverse neurologic events associated with voriconazole use in 3 cats.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3crivFGrsw%3D%3D&md5=fa0f301df4859eedcb390ed2d9685355CAS | 20384957PubMed |
[9] Malik, R. et al. (2004) Infections and some other conditions affecting the skin and subcutis of the naso-ocular region of cats – clinical experience 1987–2003. J. Feline Med. Surg. 6, 383–390.
| Infections and some other conditions affecting the skin and subcutis of the naso-ocular region of cats – clinical experience 1987–2003.Crossref | GoogleScholarGoogle Scholar | 15546771PubMed |
[10] Malik, R. et al. (2006) Wound cat. J. Feline Med. Surg. 8, 135–140.
| Wound cat.Crossref | GoogleScholarGoogle Scholar | 16368255PubMed |
Biographies
The biography for George Reppas is on page 82.
Associate Professor Gottlieb is head of Infectious Diseases at Concord Hospital, and a clinical senior lecturer at the University of Sydney. He is the current president of the Australian Society for Antimicrobials (ASA) and immediate past president of the Australasian Society for Infectious Diseases (ASID). He is on the executive of the Australian Group on Antimicrobial Resistance (AGAR). He represents ASA on the Antimicrobial Resistance Standing Committee reporting to the Australian Government. He has been chair of advisory committees supervising training in Infectious Diseases and Microbiology, and has participated in the writing groups for the Australian Infection Control Guidelines and Therapeutic Guidelines for Antibiotic. His interests include the diagnosis and management of clinical infectious diseases, hospital infection control and antimicrobial resistance.
The biography for Dr Mark Krockenberger is on page 82.
The biography for Catriona Halliday is on page 82.
The biography for Richard Malik is on page 82.