Challenges, progress and strategies in the search for a cure for HIV
Christina C Chang A B and Sharon R Lewin A B CA Department of Infectious Diseases
Alfred Hospital and Monash University
Melbourne, Vic. 3000, Australia
B Centre for Biomedical Research
Burnet Institute
Melbourne, Vic. 3000, Australia
C Tel: +61 3 9076 8491
Fax: +61 3 9076 2431
Email: sharon.lewin@monash.edu
Microbiology Australia 35(2) 72-78 https://doi.org/10.1071/MA14023
Published: 5 May 2014
The past three decades has seen a major transformation in the understanding and management of HIV infection. Effective combination antiretroviral therapy (cART) has transformed HIV from a universal death sentence to a chronic manageable disease. Current cART regimens are simpler - often only a single daily (combination) pill, but treatment must be taken life-long. Life expectancy of an HIV-infected person who receives effective cART, is now similar to a person without HIV1. The cost of long-term treatment is significant. There is now intense scientific interest in finding a cure for HIV infection or a way to allow patients to safely stop cART and remain healthy with the virus under control. A cure for HIV could either be a ‘sterilising cure’ – where there is no evidence of persistent HIV infection2,3 or a ‘functional cure’ where HIV is still present at low levels and health is maintained in the absence of cART2.
Why is curing HIV so difficult?
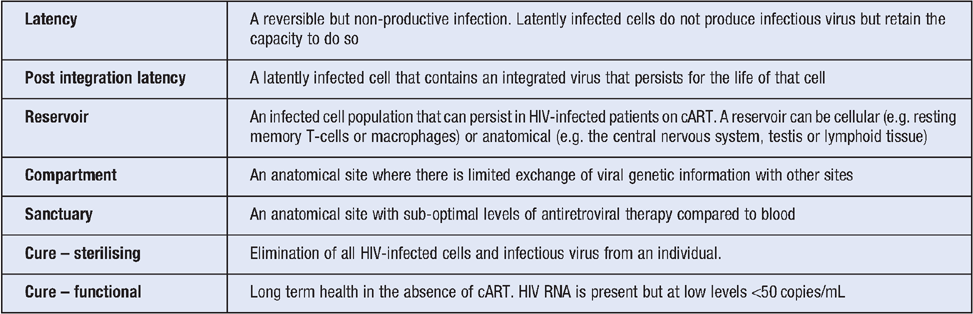
HIV replicates at high levels in activated CD4+ T-cells. In the absence of treatment, there is a slow and gradual loss of CD4+ T-cells from blood and tissue and associated immune dysfunction. cART effectively stops active virus replication and within just weeks of starting treatment, there is a rapid decline in HIV RNA in blood to undetectable levels (<20 copies/ml). However, in nearly all patients, HIV RNA rebounds rapidly when treatment is stopped, usually within 2–3 weeks4. HIV can persist in patients on suppressive ART as a result of long lived latently infected cells, low level virus replication in some patients and anatomical reservoirs. Definitions of each of these terms are shown in Table 1 (adapted from Eisele and Siliciano3).
|
HIV latency and factors that regulate virus expression
HIV, similar to other retroviruses is able to establish latent infection in resting CD4+ T-cells5,6. Latency occurs when HIV enters a cell and integrates into the host cellular DNA but there is absence of virus production. However, when given the appropriate stimuli, infectious virus can re-emerge7–10. Latency primarily occurs in long-lived central memory and transitional memory T-cells and less frequently in naïve T-cells6,11 and cells of other lineages including monocytes, macrophages12,13 and astrocytes14–16. Latently infected cells can also be maintained through homeostatic proliferation11 and recently, latency has also been demonstrated in memory stem cells which although infrequent, have the capacity to undergo self-renewal and expand over time on ART17. Latently infected resting CD4+ T-cells in patients on cART are estimated to occur at a frequency of 60 per million CD4+ T-cells18 and there is likely to be some inter-patient variability. Unfortunately there are currently no phenotypic markers of a latently infected cell in vivo and this is a top priority for research currently.
Latent HIV infection in CD4+ T-cells is established early in HIV infection and is seen even in patients who commenced cART within the first week of HIV infection19,20. Latency can be established in CD4+ T-cells via two pathways: first, when the virus infects an activated CD4+ T-cell and the infected cell survives and reverts to a resting memory T-cell carrying an integrated provirus3,9,21. This is referred to as post-activation latency22. Second, via direct infection of resting CD4+ T-cells, which in vitro requires additional stimuli such as chemokines23, dendritic cell (DC) contact24 or a high level of infecting virus and spinoculation25, termed pre-activation latency22. The relative contribution of pre- and post-activation latency in vivo is unknown.
Once the virus integrates into the host genome, latency is maintained via multiple molecular mechanisms9,22. These include transcriptional interference due to the site and orientation of the provirus in the cell chromosome; epigenetic silencing by post-transcriptional modifications of the histone tails thereby modulating the chromatin structure; absence of nuclear host transcription activators required for HIV expression (e.g. Nuclear factor-kB (NF-kB)); presence of nuclear transcription repressors; inefficient elongation of HIV transcripts related to absence of Tat protein; nuclear retention of multiply spliced RNA; and impaired translation of virus transcripts due to short-interfering RNAs (siRNA) and microRNAs (reviewed in Coiras et al.26).
Residual virus replication
Whether there is residual virus replication (i.e. new rounds of HIV infection) in patients on cART remains controversial. Traditional methods of measuring sequence evolution over time suggest there is minimal change in plasma virus or cell-associated HIV DNA in patients on suppressive cART arguing that there is minimal virus replication27. In addition, development of drug resistance is not observed in patients on cART arguing against any replication28. However, recent evidence suggest that drug penetration in tissue sites such as the gastrointestinal tract and lymphoid tissue is sub-optimal in patients on cART which could potentially favour some residual replication in these tissue sites29. Two randomised studies of patients on suppressive cART who intensified their cART regimen with the addition of an integrase inhibitor, raltegravir demonstrated that residual replication occurred in roughly 30% of patients30,31. The persistent detection of low level viraemia at 1–3 copies/mL in nearly all patients on cART32 may represent either release of virus from long-lived latently infected cells or residual virus replication. The former is more likely given the relative homogeneity of genetic sequences seen in low-level viraemia over time33.
Anatomical reservoirs
HIV can also persist in distinct anatomical sites – due to long-lived latently infected cells or residual virus replication. Best examples of this are studies of the central nervous system (CNS). Patients well-suppressed on cART may rarely have detectable HIV RNA in the cerebrospinal fluid (CSF)34,35 and the genetic resistance patterns in these compartments may also differ36. Within the CNS, long-lived latently infected cells such as microglial cells and astrocytes have also been detected14–16,37. Other anatomical reservoirs such as gastrointestinal tracts and lymphoid tissue are likely to also be important.
Is a cure possible?
Over the last few years, several cases of HIV cure have been reported. The only case of a sterilising cure is Timothy Brown, also known as the ‘Berlin patient’38. Timothy Brown received two allogeneic stem cell transplants for acute myeloid leukaemia from a donor with a homozygous CCR5 delta32 deletion38. CCR5 is a co-receptor for HIV and is required for HIV to enter a cell. In the absence of CCR5, a T-cell is resistant to infection with CCR5-using strains of HIV39–41. Remarkably, Mr Brown remains cured of HIV six years after cessation of cART with no detectable infectious virus in blood or tissue38,42,43.
The other cases of HIV cure are all cases of functional cure – or long-term control in the absence of cART. These occur largely in the setting of early cART treatment – perhaps early enough to limit the number of latently infected cells and or to preserve an adequate HIV-specific T-cell response20,44. Indeed, a group of 14 post-treatment controllers (called the VISCONTI cohort) demonstrated controlled viraemia for a median of 89 months after cART interruption, which was initiated during primary infection45. Other studies of treatment interruption following initiation of cART in acute infection have found that post-treatment control varies from 2–15%46–48. The variation is most likely related to timing of initiation of cART, duration of cART and other still unknown factors.
The ‘Mississippi baby’ born to an HIV-infected woman who did not receive pre-natal care was commenced on cART at 30 hours of birth, but discontinued cART at 18 months. Twenty-four months after cessation of ART, neither HIV RNA nor HIV antibodies have been detected49. Residual HIV DNA has since been detected in both CD4+ T-cells and monocytes but at very low levels50. Identifying patients who are acutely infected with HIV is difficult in everyday practice and commencing immediate cART has its challenges. A new study, Eramune-03 (ULTRA-STOP) NCT01876862 will scrutinise factors that predict safe treatment interruption in patients treated during chronic infection with favorable profiles that are consistent with a small reservoir51.
Current research strategies towards a cure
Haematological stem cell transplantation (HSCT)
It remains unclear what led to Timothy Brown’s cure and whether transplantation plays a role in the cure agenda. Was it the HIV-resistant CCR5 delta32 deletion donor morrow? Or was it the conditioning prior to transplant? Was it, in fact, graft-versus-host-disease (GVHD) that eliminated residual infected cells? The recent report of two patients from Boston who received a CCR5 wild-type HSCT and who had a prolonged period of undetectable HIV DNA following transplantation (2 and 4 years), unfortunately experienced viral rebound 12 and 32 weeks after cessation of cART52,53. The time to viral rebound may be an indirect measure of the number of residual infected cells. Mathematical modelling suggests that if only one HIV-infected cell remains, the chance of cure is high. In comparison, viral rebound is still expected in 3 and 10 years, respectively, if 100 or 10 infected cells remain54.
Clearly the toxicity, safety and ethics of HSCTs preclude this as a practical HIV cure for all. Further, finding a CCR5 delta32 deletion donor is extremely difficult as this is exceedingly rare globally55. Stem cell donor registries do not currently routinely test for CCR5 status56. A database of CCR5 delta32 negative cord blood exists and dual transplantation (cord blood and HSCT) is being considered56,57.
Gene therapy
A variety of stem-cell based gene therapy are being explored – targeting cellular genes necessary for viral replication (such as CCR5 co-receptor), directly against HIV gene expression (such as tat and rev protein) and those that introduce genes to interfere with HIV replication (such as host restriction factors or fusion inhibitors)58. Zinc finger proteins are sequence-specific DNA binding proteins that can be coupled to a DNA endonuclease (zinc finger nuclease (ZFN)) to cut DNA at specific sites58. CCR5 ZFNs aims to eliminate CCR5 expression thus gene-modifying the cell to be resistant to HIV. This is currently performed using an adenovirus delivery vector to T-cells ex vivo and future studies will use a similar approach for stem cells51. This approach was shown to be safe in a recent study of 12 patients who received CCR5-modified cells59. Indeed, CCR5-modified CD4+ T-cells peaked one week after engraftment and constituted a median frequency of 8.8% of peripheral blood mononuclear cells and 13.9% of total circulating CD4+ T-cells59. The modified cells declined but persisted for up to 48-weeks and were also detectable in the rectal mucosa59. Even though HIV viral load rebounded following cART treatment interruption, the decline in CCR5-modified CD4+ T-cells was less than in the unmodified CD4+ T-cell population suggesting some survival advantage59.
The other approach is to target the virus itself. A lentiviral vector to introduce a gene encoding a short hairpin RNA (sh5) against CCR5 is being combined with a HIV fusion inhibitor (C46)56. This LVsh5/C46 vector is being trialled as a Phase I/II clinical trial (CAL-USA-11, NCT01734850) in HIV-infected patients51. A phase II gene therapy trial of a tat-vpr specific anti-HIV ribozyme (OZ1) showed no difference in plasma HIV viral load but the OZ1 treated group demonstrated higher CD4+ T-cell counts compared to the placebo group60.
Newer site-specific gene editing techniques include homing endonucleases, transcription activator-like effectors nucleases (TALENs) which offer more DNA recognition specificity, and the more versatile, cluster regularly interspaced palindromic repeats (CRISPR) locus and their surrounding cohort of CRISPR-associated (Cas) genes, termed CRISPR/Cas9 system (reviewed in Manjunath61 and Choudhary and Margolis62). A new CRISPR/Cas9 system targeting the HIV LTR could potentially be used to remove integrated virus from long-lived latently infected cells63.
Activating latent HIV
Activating transcription from latently infected cells is being investigated as part of a ‘kick and kill’ strategy, which aims to induce production of virus from latency (the kick), thus making the recently activated latently infected cell susceptible to virus-induced cytolysis or HIV-specific immunity (the kill)64. Some approaches include histone deacetylase inhibitors (HDACi), disulfiram, methylation inhibitors, cytokines and immune modulators (reviewed in Wightman et al.65).
Histone deacetylase inhibitors (HDACi)
Histone acetyltransferase leads to a hyperacetylated euchromatin state which is a transcriptionally-active, open state while histone deactylase (HDAC) leads to a closed, transcriptionally-repressed, heterochromatin state66. HDACis can activate latent HIV in vitro (reviewed in Wightman et al.65) in latently infected T-cell lines, primary models of latency and resting CD4+ T-cells from HIV-infected patients on cART67,68. Some recent work using CD4+ T-cells from HIV-infected patients on ART demonstrated minimal release of virus into supernatant following stimulation with an HDACi alone69,70. Furthermore, using an in vitro model of latency, stimulation with an HDACi did not lead to cell death69. In HIV-infected patients on cART, administration of the HDACi vorinostat clearly induced HIV transcription, as measured by an increase in cell-associated unspliced HIV RNA71–73. However, neither the single dose nor the repeated dose strategy induced a significant increase in plasma RNA, nor were the number of latently infected cells reduced71–73.
Recently, a more potent pan-HDACi, panobinostat was administered 3-times a week, fortnightly for 8 weeks in a Danish trial of HIV-infected adults on suppressive cART. An increase in HIV transcription and a possible increase in transient viraemia was seen74. A single-dose, placebo-controlled, dose-escalation study of another HDACi romidepsin NCT01933594 in HIV-infected patients is being planned51. Other HDACis in early clinical development but with likely potential activity against latent HIV include belinostat, givinostat and entinostat22. Whether HDACi alone will lead to a significant reduction in latently infected cells remains unknown and further trials are needed to address this.
Disulfiram
Disulfiram is a member of the dithiocarbamate family, a broad class of metal-chelators, used previously to deter alcohol abuse75. Disulfiram activated viral production from latent HIV in vitro, in some but not all laboratory models of latency76 and it was recently demonstrated that activation was secondary to modification of the PTEN (phosphatase and tensin homologue) pathway77. An open label study of daily disulfiram given for 14 days recorded a transient rise in HIV RNA in a subset of patients with high disulfiram levels without a reduction in the number of latently-infected cells78. A dose-escalation study is currently ongoing (NCT01944371)51.
IL-7
IL-7 has recently been investigated as an agent that could enhance T-cell proliferation79 and potentially activate HIV transcription from latency, but recent studies suggested that IL-7 causes proliferation of both infected and uninfected cells and an expansion of the HIV reservoir80 and an increase in HIV DNA (Eramune 01, NCT01019551)51.
Reducing virus replication and/or immune activation
Persistent immune activation and inflammation in HIV-infected patients well-suppressed on cART has been associated with HIV persistence81. Whether this means that virus persistence drove immune activation or whether immune activation drove virus persistence is unknown. Previous attempts of adding additional antiretroviral agents to a standard cART regimen – ‘cART intensification’ – have failed to reduce the number of latently infected cells when measured as either low level viraemia or cell-associated HIV RNA or HIV DNA30,31,82. However, some intensification studies, specifically with raltegravir have demonstrated a reduction in markers of T-cell activation30 and d-dimer31 in a subset of patient. Anti-inflammatory agents such as statins, chloroquine and hydroxycholoroquine, selective COX-2 inhibitors, and leflunomide are being trialled to reduce immune activation but the effect of these agents on virus persistence is unknown (reviewed in Deeks et al.83).
References
[1] van Sighem, A.I. et al. (2010) de WF. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 24, 1527–1535.| de WF. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals.Crossref | GoogleScholarGoogle Scholar | 20467289PubMed |
[2] Dieffenbach, C.W. and Fauci, A.S. (2011) Thirty years of HIV and AIDS: future challenges and opportunities. Ann. Intern. Med. 154, 766–771.
| Thirty years of HIV and AIDS: future challenges and opportunities.Crossref | GoogleScholarGoogle Scholar | 21628350PubMed |
[3] Eisele, E. and Siliciano, R.F. (2012) Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 37, 377–388.
| Redefining the viral reservoirs that prevent HIV-1 eradication.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtlyltLvN&md5=203b86d4a892ad55d54cb3bfccc14e86CAS | 22999944PubMed |
[4] Chun, T.W. et al. (2010) Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS 24, 2803–2808.
| Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication.Crossref | GoogleScholarGoogle Scholar | 20962613PubMed |
[5] Chun, T.W. et al. (1995) In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat. Med. 1, 1284–1290.
| In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXpslGhtbg%3D&md5=e9b500822e232e21226cc6073a15bba3CAS | 7489410PubMed |
[6] Chun, T.W. et al. (1997) Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183–188.
| Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjtF2gtr8%3D&md5=be48a764652ead0d1a93515dfdcaaffaCAS | 9144289PubMed |
[7] Finzi, D. et al. (1997) Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300.
| Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXntlOmsro%3D&md5=2b8a543615a83d2b649dc13afd7805d2CAS | 9360927PubMed |
[8] Finzi, D. et al. (1999) Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5, 512–517.
| Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXivVKksro%3D&md5=141b790dd80d5b783af61b60a39242d7CAS | 10229227PubMed |
[9] Choudhary, S.K. and Margolis, D.M. (2011) Curing HIV: pharmacologic approaches to target HIV-1 latency. Annu. Rev. Pharmacol. Toxicol. 51, 397–418.
| Curing HIV: pharmacologic approaches to target HIV-1 latency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisFOju7k%3D&md5=979f6cc7ad3759eac5c751ece7f67a78CAS | 21210747PubMed |
[10] Pace, M.J. et al. (2012) Directly infected resting CD4+T cells can produce HIV Gag without spreading infection in a model of HIV latency. PLoS Pathog. 8, e1002818.
| Directly infected resting CD4+T cells can produce HIV Gag without spreading infection in a model of HIV latency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFeisrfP&md5=aee55001a148d2530dd243250df2f7d4CAS | 22911005PubMed |
[11] Chomont, N. et al. (2009) HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15, 893–900.
| HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnsFSit7Y%3D&md5=155b7bc6e9229946056523a0b7b85fc2CAS | 19543283PubMed |
[12] Ellery, P.J. et al. (2007) The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J. Immunol. 178, 6581–6589.
| 1:CAS:528:DC%2BD2sXksl2ms7k%3D&md5=a09ced280a1bcae603554804e5a34222CAS | 17475889PubMed |
[13] Sonza, S. et al. (2001) Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. AIDS 15, 17–22.
| Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M3htFChtw%3D%3D&md5=50f890a24776f3ae98218e837749c8a6CAS | 11192864PubMed |
[14] Churchill, M.J. et al. (2006) Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J. Neurovirol. 12, 146–152.
| Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues.Crossref | GoogleScholarGoogle Scholar | 16798676PubMed |
[15] Gorry, P.R. et al. (1999) Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of gag, env, and nef mRNAs despite efficient expression of Tat and Rev. J. Virol. 73, 352–361.
| 1:CAS:528:DyaK1cXotFSks7g%3D&md5=367c9b407f33c5e469611e1851dd7f65CAS | 9847339PubMed |
[16] Narasipura, S.D. et al. (2014) Epigenetic regulation of HIV-1 latency in astrocytes. J. Virol. 88, 3031–3038.
| Epigenetic regulation of HIV-1 latency in astrocytes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmsVOkurg%3D&md5=3071f38d4bdb5df34fc074264c5197fcCAS | 24352441PubMed |
[17] Buzon, M.J. et al. (2014) HIV-1 persistence in CD4(+) T cells with stem cell-like properties. Nat. Med. 20, 139–142.
| HIV-1 persistence in CD4(+) T cells with stem cell-like properties.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmtVeqsg%3D%3D&md5=0befd00e4b5cbdf9e0a331144df71343CAS | 24412925PubMed |
[18] Ho, Y.C. et al. (2013) Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551.
| Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1yrurrI&md5=7cc6d4a02ff1a5519e6400dcb84aee00CAS | 24243014PubMed |
[19] Chun, T.W. et al. (1998) Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 95, 8869–8873.
| Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXkvFalt78%3D&md5=0cb24881d15a92704c9424072c8f0312CAS | 9671771PubMed |
[20] Ananworanich, J. et al. (2014) Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS 28, 1015–20.
| Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXlsF2htL8%3D&md5=c9a7bb76205ff77a9824555bef9d7fc1CAS | 24384692PubMed |
[21] Siliciano, R.F. and Greene, W.C. (2011) HIV latency. Cold Spring Harb Perspect Med 1, a007096.
| HIV latency.Crossref | GoogleScholarGoogle Scholar | 22229121PubMed |
[22] Katlama, C. et al. (2013) Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet 381, 2109–2117.
| Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltVGhtL0%3D&md5=c2f5defc72602e1e959c575568828d09CAS | 23541541PubMed |
[23] Saleh, S. et al. (2007) CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood 110, 4161–4164.
| CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXotFyj&md5=255ced4d1a24ae31af49ed52a429beaaCAS | 17881634PubMed |
[24] Evans, V.A. et al. (2013) Myeloid dendritic cells induce HIV-1 latency in non-proliferating CD4+ T cells. PLoS Pathog. 9, e1003799.
| Myeloid dendritic cells induce HIV-1 latency in non-proliferating CD4+ T cells.Crossref | GoogleScholarGoogle Scholar | 24339779PubMed |
[25] O’Doherty, U. et al. (2000) Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74, 10 074–10 080.
| Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXnsFOltLg%3D&md5=20efd2ecb4186a5462aaa0d06b2c0776CAS |
[26] Coiras, M. et al. (2009) Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat. Rev. Microbiol. 7, 798–812.
| Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1Kqsb7J&md5=99c15a4f37e75564ec19e0ae814acf7cCAS | 19834480PubMed |
[27] Josefsson, L et al. (2013) The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc. Natl. Acad. Sci. USA 110, E4987–E4996.
| The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXnsV2nsA%3D%3D&md5=0b53eacd08048b2b4e19114efb74bf74CAS | 24277811PubMed |
[28] Hermankova, M. et al. (2001) HIV-1 drug resistance profiles in children and adults with viral load of <50 copies/ml receiving combination therapy. JAMA 286, 196–207.
| HIV-1 drug resistance profiles in children and adults with viral load of <50 copies/ml receiving combination therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlsVymtr4%3D&md5=8f8cb0e3dc3bb74dd4f58461f0de5107CAS | 11448283PubMed |
[29] Fletcher, C.V. et al. (2014) Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl. Acad. Sci. USA 111, 2307–2312.
| Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXisFOksb4%3D&md5=6f8cd1b8c4cfa4144f6239bae5e01e2cCAS | 24469825PubMed |
[30] Buzón, M.J. et al. (2010) HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat. Med. 16, 460–465.
| HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects.Crossref | GoogleScholarGoogle Scholar | 20228817PubMed |
[31] Hatano, H. et al. (2013) Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: a randomized, placebo-controlled trial. J. Infect. Dis. 208, 1436–1442.
| Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: a randomized, placebo-controlled trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1SisLnP&md5=3dffd5497761f470dec51d0cfff17519CAS | 23975885PubMed |
[32] Palmer, S. et al. (2008) Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. USA 105, 3879–3884.
| Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjslSiur0%3D&md5=56655235a79d904e6d63219fe99f17ebCAS | 18332425PubMed |
[33] Bailey, J.R. et al. (2006) Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J. Virol. 80, 6441–6457.
| Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XntFWisrc%3D&md5=8e4e9a62733324e119f8c147ea744117CAS | 16775332PubMed |
[34] Canestri, A. et al. (2010) Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin. Infect. Dis. 50, 773–778.
| Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 20100092PubMed |
[35] Garvey, L.J. et al. (2009) Detectable cerebrospinal fluid HIV RNA with associated neurological deficits, despite suppression of HIV replication in the plasma compartment. AIDS 23, 1443–1444.
| Detectable cerebrospinal fluid HIV RNA with associated neurological deficits, despite suppression of HIV replication in the plasma compartment.Crossref | GoogleScholarGoogle Scholar | 19564723PubMed |
[36] Strain, M.C. et al. (2005) Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J. Virol. 79, 1772–1788.
| Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFCjurg%3D&md5=5141bfa991643ad6a9f1fc0535745b8bCAS | 15650202PubMed |
[37] Thompson, K.A. et al. (2011) Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am. J. Pathol. 179, 1623–1629.
| Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVSqtb3J&md5=0a4acfdd4c2c51b409aaaa5cceeb70a8CAS | 21871429PubMed |
[38] Hütter, G. et al. (2009) Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 360, 692–698.
| Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation.Crossref | GoogleScholarGoogle Scholar | 19213682PubMed |
[39] Michael, N.L. et al. (1997) The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat. Med. 3, 338–340.
| The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhsFGrur8%3D&md5=a99585f08e7d99f1161fe7abb36afd53CAS | 9055864PubMed |
[40] Huang, Y. et al. (1996) The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 2, 1240–1243.
| The role of a mutant CCR5 allele in HIV-1 transmission and disease progression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xms1WqurY%3D&md5=92adaa8243f9f750e2b9b7e7e096993fCAS | 8898752PubMed |
[41] Dean, M. et al. (1996) Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273, 1856–1862.
| Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlvFCrsLY%3D&md5=253c2b27a591c2149b0a0563d934a450CAS | 8791590PubMed |
[42] Yukl, S.A. et al. (2013) Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 9, e1003347.
| Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpslOhur8%3D&md5=c426590eb1221a2ec362a96e9587c529CAS | 23671416PubMed |
[43] Allers, K. et al. (2011) Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 117, 2791–2799.
| Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjslOgtL8%3D&md5=941464681c9993467d166cd39f0e29ceCAS | 21148083PubMed |
[44] Hocqueloux, L. et al. (2013) Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts. J. Antimicrob. Chemother. 68, 1169–1178.
| Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlvFWgsro%3D&md5=45c1436457d477495ebd55b7c7d50793CAS | 23335199PubMed |
[45] Sáez-Cirión, A. et al. (2013) Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 9, e1003211.
| Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study.Crossref | GoogleScholarGoogle Scholar | 23516360PubMed |
[46] Goujard, C. et al. (2007) Interruption of antiretroviral therapy initiated during primary HIV-1 infection: impact of a therapeutic vaccination strategy combined with interleukin (IL)-2 compared with IL-2 alone in the ANRS 095 Randomized Study. AIDS Res. Hum. Retroviruses 23, 1105–1113.
| Interruption of antiretroviral therapy initiated during primary HIV-1 infection: impact of a therapeutic vaccination strategy combined with interleukin (IL)-2 compared with IL-2 alone in the ANRS 095 Randomized Study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFarsbrJ&md5=daf31c5d2c7ea3a197d1919d60eb00daCAS | 17919105PubMed |
[47] Lodi, S. et al. (2012) Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion. Arch. Intern. Med. 172, 1252–1255.
| Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion.Crossref | GoogleScholarGoogle Scholar | 22826124PubMed |
[48] Stohr, W. et al. (2013) Duration of HIV-1 viral suppression on cessation of antiretroviral therapy in primary infection correlates with time on therapy. PLoS ONE 8, e78287.
| Duration of HIV-1 viral suppression on cessation of antiretroviral therapy in primary infection correlates with time on therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslWksL7K&md5=04e9670003e9fb87a53bb464c725d2dbCAS | 24205183PubMed |
[49] Persaud, D. et al. (2013) Absence of detectable HIV-1 viremia after treatment cessation in an infant. N. Engl. J. Med. 369, 1828–1835.
| Absence of detectable HIV-1 viremia after treatment cessation in an infant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslGhtb7M&md5=f408ae1b1a976a0a257f6e76b57e15f2CAS | 24152233PubMed |
[50] Persaud, D. et al. (2014) Virologic control by 1 year of age significantly reduces HIV-1 reservoirs in perinatal infection. 2014 Mar 5.
[51] ClinicalTrials.gov (2014) U S National Institutes of Health. http://clinicaltrials.gov/ (accessed 8 February 2014).
[52] Henrich, T.J. et al. (2013) Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J. Infect. Dis. 207, 1694–1702.
| Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXntVOrsLw%3D&md5=67625edaa71e0adf4312c2f66fee79e8CAS | 23460751PubMed |
[53] Henrich, T.J. et al. (2013) Mathematical modeling of HIV-1 latent reservoir dynamics following hematopoietic stem cell transplantation. 2013 Dec 3.
[54] Henrich, T.J. et al. (2014) HIV-1 rebound following allogeneic stem cell transplantation and treatment interruption . 2014 Mar 6.
[55] Carrington, M. et al. (1999) Genetics of HIV-1 infection: chemokine receptor CCR5 polymorphism and its consequences. Hum. Mol. Genet. 8, 1939–1945.
| Genetics of HIV-1 infection: chemokine receptor CCR5 polymorphism and its consequences.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmslelsro%3D&md5=e010ccd867f9e4c1c2709b4a6e50f81dCAS | 10469847PubMed |
[56] Burke, B.P. et al. (2014) CCR5 as a natural and modulated target for inhibition of HIV. Viruses 6, 54–68.
| CCR5 as a natural and modulated target for inhibition of HIV.Crossref | GoogleScholarGoogle Scholar |
[57] Zou, S. et al. (2013) Hematopoietic cell transplantation and HIV cure: where we are and what next? Blood 122, 3111–3115.
| Hematopoietic cell transplantation and HIV cure: where we are and what next?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslGhtbfO&md5=2813614a5c4d05a023fbfcf8af5aab49CAS | 24009230PubMed |
[58] Zhen, A. and Kitchen, S. (2014) Stem-cell-based gene therapy for HIV infection. Viruses 6, 1–12.
| Stem-cell-based gene therapy for HIV infection.Crossref | GoogleScholarGoogle Scholar |
[59] Tebas, P. et al. (2014) Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 370, 901–910.
| Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXkt1ehsLk%3D&md5=865da0a8443a419a8a47c21a54180574CAS | 24597865PubMed |
[60] Mitsuyasu, R.T. et al. (2009) Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat. Med. 15, 285–292.
| Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhvFKjt74%3D&md5=5432a2421a9228dd0f3db7f331178ae6CAS | 19219022PubMed |
[61] Manjunath, N. et al. (2013) Newer gene editing technologies toward HIV gene therapy. Viruses 5, 2748–2766.
| Newer gene editing technologies toward HIV gene therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXmsVyjsLY%3D&md5=8937069c100586645445bb63acaffeedCAS | 24284874PubMed |
[62] Choudhary, S.K. and Margolis, D.M. (2011) Curing HIV: pharmacologic approaches to target HIV-1 latency. Annu. Rev. Pharmacol. Toxicol. 51, 397–418.
| Curing HIV: pharmacologic approaches to target HIV-1 latency.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXisFOju7k%3D&md5=979f6cc7ad3759eac5c751ece7f67a78CAS | 21210747PubMed |
[63] Ebina, H. et al. (2013) Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep 3, 2510.
| Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus.Crossref | GoogleScholarGoogle Scholar | 23974631PubMed |
[64] Barton, K.M. et al. (2013) Prospects for treatment of latent HIV. Clin. Pharmacol. Ther. 93, 46–56.
| Prospects for treatment of latent HIV.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVGms7rP&md5=4de724e160d06e35d845aeb6bdd5120cCAS | 23212106PubMed |
[65] Wightman, F. et al. (2012) HDAC inhibitors in HIV. Immunol. Cell Biol. 90, 47–54.
| HDAC inhibitors in HIV.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xkslylug%3D%3D&md5=3ed07032967e0d31d51ddd1943557da3CAS | 22083528PubMed |
[66] Fass, D.M. et al. (2012) Histone acetylation and deacetylation. In Encyclopedia of Molecular Cell Biology and Molecular Medicine (Second edn). (Meyers, R.A., ed.), pp. 1-47, Wiley-VCH Verlag GmbH & Co. KGaA.
[67] Archin, N.M. et al. (2009) Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res. Hum. Retroviruses 25, 207–212.
| Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisVSnurk%3D&md5=23de052928194b2cb3c637be63135293CAS | 19239360PubMed |
[68] Contreras, X. et al. (2009) Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J. Biol. Chem. 284, 6782–6789.
| Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXislWrtbc%3D&md5=7ca05915d9a4dee3c06d1d2375e055ecCAS | 19136668PubMed |
[69] Shan, L. et al. (2012) Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36, 491–501.
| Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjsFGiu7s%3D&md5=ca9f864a3f1fc0a66510734cbad6bf13CAS | 22406268PubMed |
[70] Bullen, C.K. et al. (2014) New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat. Med. 20, 425–429.
| New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXks12msLg%3D&md5=dc201a07c5214eb1a9438d04187b54b2CAS | 24658076PubMed |
[71] Archin, N.M. et al. (2012) Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487, 482–485.
| Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFWjtrfP&md5=3232ff25692c00851930a16274cebb9eCAS | 22837004PubMed |
[72] Elliott, J.H. et al. (2013) The safety and effect of multiple doses of vorinostat on HIV transcription in HIV+ patients receiving cART. 2013 Mar 4.
[73] Archin, N.M. et al. (2014) HIV-1 expression within resting CD4 T-cells following multiple doses of vorinostat. J. Infect. Dis. , .
| HIV-1 expression within resting CD4 T-cells following multiple doses of vorinostat.Crossref | GoogleScholarGoogle Scholar | 24620025PubMed |
[74] Rasmussen, T. et al. (2013) Cyclic dosing of panobinostat to reverse HIV-latency: findings from a clinical trial. 2013 Dec.
[75] Conticello, C. et al. (2012) Disulfiram, an old drug with new potential therapeutic uses for human hematological malignancies. Int. J. Cancer 131, 2197–2203.
| Disulfiram, an old drug with new potential therapeutic uses for human hematological malignancies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XksV2hu7w%3D&md5=52dcfe8a21cd7a3ffdcafd90958e79e7CAS | 22322883PubMed |
[76] Xing, S. et al. (2011) Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J. Virol. 85, 6060–6064.
| Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntFKqs7k%3D&md5=05c6f0eef314c63f487babfe5db3b502CAS | 21471244PubMed |
[77] Doyon, G. et al. (2013) Disulfiram reactivates latent HIV-1 expression through depletion of the phosphatase and tensin homolog. AIDS 27, F7–F11.
| Disulfiram reactivates latent HIV-1 expression through depletion of the phosphatase and tensin homolog.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVClsL3I&md5=732d3a192f57161fbbda71b8850f410cCAS | 22739395PubMed |
[78] Spivak, A.M. et al. (2014) A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin. Infect. Dis. 58, 883–890.
| A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXjs1Cks7w%3D&md5=dbf54e660d933dd21825125fb97507e0CAS | 24336828PubMed |
[79] Levy, Y. et al. (2012) Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin. Infect. Dis. 55, 291–300.
| Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpsVahs7o%3D&md5=fea4d0acd98b2d797812c4c4e6ebd663CAS | 22550117PubMed |
[80] Vandergeeten, C. et al. (2013) Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 121, 4321–4329.
| Interleukin-7 promotes HIV persistence during antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXovFKnt7Y%3D&md5=7563d877bbd755c24d07f1c24ebd1ed3CAS | 23589672PubMed |
[81] Douek, D.C. (2013) Immune activation, HIV persistence, and the cure. Top Antivir Med 21, 128–132.
| 24225078PubMed |
[82] Puertas, M.C. et al. (2014) Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection. AIDS 28, 325–334.
| Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXis1Wks7c%3D&md5=e4f219026054e8fcbebdf7eb9080f758CAS | 24185044PubMed |
[83] Deeks, S.G. et al. (2013) The end of AIDS: HIV infection as a chronic disease. Lancet 382, 1525–1533.
| The end of AIDS: HIV infection as a chronic disease.Crossref | GoogleScholarGoogle Scholar | 24152939PubMed |
[84] Garcia, F. et al. (2013) A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci. Transl. Med. 5, 166ra2.
| A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication.Crossref | GoogleScholarGoogle Scholar | 23283367PubMed |
[85] Hansen, S.G. et al. (2011) Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473, 523–527.
| Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmslSrtr0%3D&md5=9bba16c739a85e5e6b461aadd4db63d1CAS | 21562493PubMed |
[86] Hansen, S.G. et al. (2013) Immune clearance of highly pathogenic SIV infection. Nature 502, 100–104.
| Immune clearance of highly pathogenic SIV infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVWqt7%2FO&md5=b3a658a0d6669799c0b0319d3dd7b1e7CAS | 24025770PubMed |
[87] Eriksson, S. et al. (2013) Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 9, e1003174.
| Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXktlWjtrY%3D&md5=f91b712413178e12d171fbae0ae2cbf4CAS | 23459007PubMed |
[88] Rouzioux, C. and Richman, D. (2013) How to best measure HIV reservoirs? Curr Opin HIV AIDS 8, 170–175.
| How to best measure HIV reservoirs?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXlvVWjsLo%3D&md5=f6af919d6c761d199493a2e7c4a4d766CAS | 23564004PubMed |
[89] Spina, C.A. et al. (2013) An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 9, e1003834.
| An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients.Crossref | GoogleScholarGoogle Scholar | 24385908PubMed |
Biographies
Professor Sharon Lewin, FRACP, PhD is an infectious diseases physician and basic scientist. She is Director of the Department of Infectious Diseases at The Alfred Hospital and Monash University and co-head of the Centre for Biomedical Research, Burnet Institute, Melbourne, Australia and an Australian National Health and Medical Research Council (NHMRC) Practitioner Fellow. Her laboratory focuses on strategies to cure HIV infection. Together with Nobel Laureate Francoise Barre Sinoussi she will co-chair the XXth International AIDS Conference (AIDS2014), which will be held in Melbourne July 2014.
Christina C Chang, MBBS, FRACP is an infectious diseases physician based at The Alfred Hospital, Melbourne. She recently completed her PhD on cryptococcosis-associated immune reconstitution inflammatory syndrome in patients living with HIV and will now undertake postdoctoral research in HIV cure.


