Management of antibiotic resistance in the intensive care unit setting from an international perspective
Stefano Di Bella A B and Nicola Petrosillo A CA 2nd Division
National Institute for Infectious Diseases ‘L. Spallanzani’
via Portuense 292
Rome 00149, Italy
Tel: +3 906 5517 0294
Fax: +3 906 5517 0486
B Email: stefano932@gmail.com
C Email: nicola.petrosillo@inmi.it
Microbiology Australia 35(1) 63-65 https://doi.org/10.1071/MA14018
Published: 4 February 2014
Infections represent one of the most threatening complications for intensive care unit (ICU) patients. Approximately 50% of all ICU patients are treated for infection or suspected infection during their ICU stay, of which approximately half are acquired during the ICU stay. Multidrug-resistant (MDR) organisms are often the etiologic agents with a dramatic impact in morbidity and mortality rates. The emergence of carbapenemase-producing bacteria, in particular the emerging K. pneumoniae strains harboring the plasmid-encoded KPC-type carbapenemase and the New Delhi metallo-beta-lactamase 1 (NDM-1), in many countries is an example of the continuous evolution and spread of bacterial resistance. Infection prevention and control and antimicrobial stewardship programs in the ICU setting are demonstrating good results and need continuous implementation.
During the past decade, the consumption of antimicrobial agents has continued to increase among ICU patients worldwide, with more than 60% of these patients treated with antimicrobials1. In the past decade, the frequency of administration of different classes of antimicrobials has changed, with increases in the use of carbapenems and ureidopenicillins and a decrease in that of cephalosporins2. Moreover, antibiotics such as colistin, fosfomycin, daptomycin, tigecycline and linezolid have been used more commonly for the treatment of MDR pathogens. Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus species, extended-spectrum β-lactamase or metallo β-lactamase-producing Enterobacteriaceae, as well as MDR Pseudomonas aeruginosa and Acinetobacter spp., represent the most common pathogens in the ICU setting.
In the United States between1999 and 2007 increases of 104% and 182% in third-generation cephalosporin resistance for K. pneumoniae and E. coli, respectively, were observed. From the same study an increase of 54% in carbapenem resistance for P. aeruginosa was registered3. The last threat is represented by carbapenemase-producing Enterobactericeae, including emerging strains harboring the plasmid-encoded KPC-type carbapenemase and the New Delhi metallo-beta-lactamase 1 (NDM-1), among others. The NDM enzymes, first reported in 2008 from a Swedish patient of Indian origin are now found worldwide4 and have also been transmitted to E. coli, Salmonella and P. aeruginosa5–7. This is a clear example of the ease and velocity of the global diffusion of resistance, in both developed and poorly resourced countries. An example of the impact of this phenomenon is found in Greece, where the proportion of imipenem-resistant K. pneumoniae increased from less than 1% in 2001 to 20% in isolates from hospital wards and 50% in isolates from ICUs in 20068.
Clostridium difficile is another globally ‘emerging’ bacterium in ICUs. The incidence of C. difficile in the ICU setting is around 4%, double that of all hospitalised patients3. A recent study showed that approximately 20% of patients admitted to the ICU with C. difficile infection had been receiving antibiotics without any obvious evidence of infection, with an accompanying 28% in-hospital mortality9.
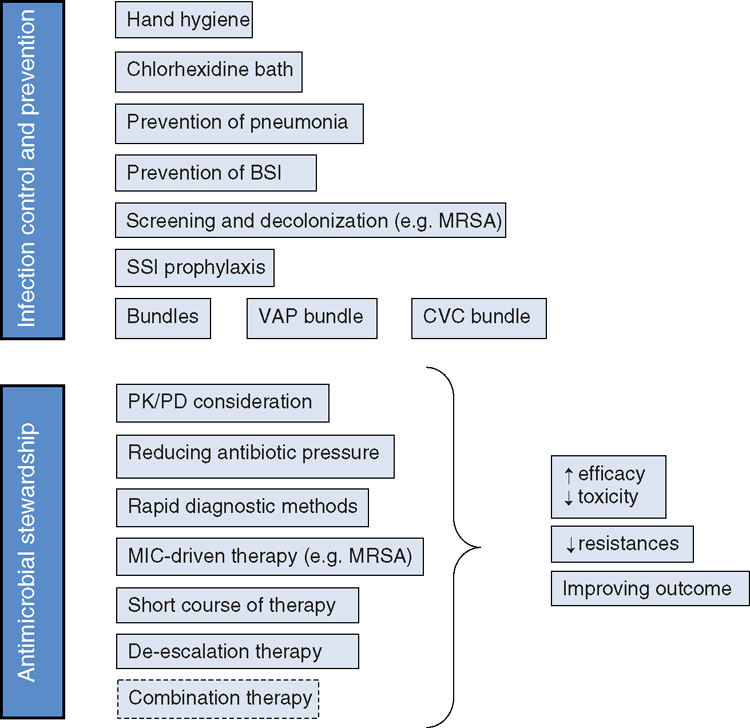
To date, several strategies have been demonstrated to be effective in reducing the trend of antimicrobial resistance (Figure 1). Two major strategies have been delineated by the Infectious Diseases Society of America (IDSA): (1) infection prevention and control; and (2) antimicrobial stewardship. Infection prevention and control measures mainly include hand hygiene, chlorexidine body washes, selected screening for MDR bacterial carriage and decolonization (e.g. for MRSA).
Antimicrobial stewardship is defined as the optimal selection, dosage and duration of antimicrobial agents for the appropriate indication, which results in maximum benefit and minimum adverse events and development of antimicrobial resistance. Antimicrobial stewardship in the ICU assumes not only an understanding of bacterial susceptibility patterns, but also the knowledge of several other complementary factors such as the infection site and the patient’s pathophysiology10.
In critically ill patients there are several reasons for an inadequate pharmacokinetic–pharmacodynamic (PK/PD) ratio leading to insufficient antibiotic concentration to inhibit causative pathogens at the site of infection10,11. Plasma clearance of antimicrobials may be increased in critically ill patients as a result of enhanced renal excretion (burn patients, sepsis, inotropic drugs), changes in renal clearance or glomerular hyperfiltration, increased drug metabolism induced by concomitant administration of other drugs or increased drug extraction by the use of different extrarenal depuration techniques12. Hydrophilic drugs such as β-lactams experience the higher pharmacokinetic changes. In fact, β-lactams generally demonstrate a low volume of distribution and, in the critical care setting, strategies such as loading doses and extended or continuous infusions are used to achieve PK/PD targets that are not possible with conventional dosing strategies13. In these patients a timed urinary creatinine clearance (e.g. collection over 8 h) seems to be the most useful measure of renal function and is useful to guide antimicrobial dosage14.
In conclusion, a better understanding of PK/PD relationships and intrinsic PD characteristics of drugs (e.g. lipophilic vs hydrophilic), as well as modifying antibiotic dosage and selecting the proper agent on the basis of the site of infection are determinants of outcome.
Until now, only a few studies have evaluated the impact of antimicrobial stewardship in ICU patients. A recent study conducted in a medical ICU in North Carolina evaluated an intervention period when an infectious disease specialist discussed antimicrobial use with the ICU team. The authors found significant reductions in extended-spectrum penicillin, carbapenem, vancomycin and metronidazole consumption and an increase in the use of narrow-spectrum penicillins after the intervention. Moreover, use was registered. There also was a reduction in mechanical ventilation days, length of stay and hospital mortality, thus demonstrating a clinical impact15. Another study conducted in Canada evaluated the effect of introducing an antimicrobial stewardship program (ASP) in a medical-surgical ICU16. The authors found a significant increase in the treatment of sterile-site cultures after ASP and a reduction in inappropriate treatment of non-sterile-site cultures. Moreover, an overall reduction in cost and mean defined daily doses were observed.
Another recent prospective study in the ICU of a public hospital in Atlanta on critically ill adults receiving empiric imipenem or piperacillin-tazobactam compared three periods: the first was baseline; the second with partial participation of an infectious diseases physician; and the third with a direct participation in interdisciplinary rounds with the medical ICU team. Almost 700 patients were evaluated and the results showed that the second and third periods were associated with appropriate antimicrobial selection and with lower rates of resistance17.
These results demonstrate that active communication with an infectious disease practitioner significantly reduces ICU antibiotic overuse through early modification or cessation of antibiotics with no increase in mortality. Moreover, the introduction of an ASP in the ICU is associated with improved microbiologically targeted therapy based on sterile-site cultures and improved documentation of antimicrobial use in the medical record. Audit and feedback had an influence on antimicrobial prescription patterns in the ICU with a favorable impact in the emergence of resistance.
Antimicrobial stewardship should be multidisciplinary, taking advantage of expertise from intensivists, infectious disease specialists, microbiologists and pharmacists. Infection control and antimicrobial stewardship represent the last attempt to ‘defend the fort’ while waiting for new antimicrobials and innovative strategies of international surveillance and antimicrobial policy.
References
[1] Vincent, J.L. et al. (2009) International study of the prevalence and outcomes of infection in intensive care units. JAMA 302, 2323–2329.| International study of the prevalence and outcomes of infection in intensive care units.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFCjsr3L&md5=6a94a72829af6f138c0479bf31f8b19eCAS | 19952319PubMed |
[2] Sociedad española de medicina intensiva crítica y unidades coronaries (SEMICYCUC) grupo de trabajo de enfermedades infecciosas (2012) Informe. http://hws.vhebron.net/envin-helics/Help/Informe%20ENVIN-UCI%202012.pdf (accessed on 24 November 2013).
[3] Doyle, J.S. et al. (2011) Epidemiology of infections acquired in intensive care units. Semin. Respir. Crit. Care Med. 32, 115–138.
| Epidemiology of infections acquired in intensive care units.Crossref | GoogleScholarGoogle Scholar | 21506049PubMed |
[4] Laxminarayan, R. et al. (2013) Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 13, 1057–1098.
| Antibiotic resistance-the need for global solutions.Crossref | GoogleScholarGoogle Scholar | 24252483PubMed |
[5] Liu, Z. et al. (2013) Identification and characterization of the first Escherichia coli strain carrying NDM-1 gene in China. PLoS ONE 8, e66666.
| Identification and characterization of the first Escherichia coli strain carrying NDM-1 gene in China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVWksrvJ&md5=fdfde7b3ebc3ef155cc32bc1184df0abCAS | 23762496PubMed |
[6] Savard, P. et al. (2011) First NDM-positive Salmonella sp. strain identified in the United States. Antimicrob. Agents Chemother. 55, 5957–5958.
| First NDM-positive Salmonella sp. strain identified in the United States.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFehsLnI&md5=944c72755f9a349237a6278094dc9ce6CAS | 21968356PubMed |
[7] Carattoli, A. et al. (2013) Isolation of NDM-1-producing Pseudomonas aeruginosa sequence type ST235 from a stem cell transplant patient in Italy, May 2013. Euro Surveill. 18, pii:20633.
[8] Vatopoulos, A. (2008) High rates of metallo-beta-lactamase-producing Klebsiella pneumoniae in Greece – a review of the current evidence. Euro Surveill. 13, pii:8023.
[9] Marra, A.R. et al. (2007) Hospital-acquired Clostridium difficile-associated disease in the intensive care unit setting: epidemiology, clinical course and outcome. BMC Infect. Dis. 7, 42.
| Hospital-acquired Clostridium difficile-associated disease in the intensive care unit setting: epidemiology, clinical course and outcome.Crossref | GoogleScholarGoogle Scholar | 17517130PubMed |
[10] Pea, F. and Viale, P. (2006) The antimicrobial therapy puzzle: could pharmacokinetic-pharmacodynamic relationships be helpful in addressing the issue of appropriate pneumonia treatment in critically ill patients? Clin. Infect. Dis. 42, 1764–1771.
| The antimicrobial therapy puzzle: could pharmacokinetic-pharmacodynamic relationships be helpful in addressing the issue of appropriate pneumonia treatment in critically ill patients?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmsVCgsbo%3D&md5=6ca296dbbd4180d77d5052c8e9b8fde6CAS | 16705585PubMed |
[11] Dellinger, R.P. et al. (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 39, 165–228.
| Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3szjs1Witw%3D%3D&md5=1cebb84289f4d4de9093a167a3b7ba0eCAS | 23361625PubMed |
[12] Udy, A.A. et al. (2011) Implications of augmented renal clearance in critically ill patients. Nat. Rev. Nephrol. 7, 539–543.
| Implications of augmented renal clearance in critically ill patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFWmu7vK&md5=7530fc2da439c595de789383173b5d9fCAS | 21769107PubMed |
[13] Sinnollareddy, M.G. et al. (2012) β-lactam pharmacokinetics and pharmacodynamics in critically ill patients and strategies for dose optimization: a structured review. Clin. Exp. Pharmacol. Physiol. 39, 489–496.
| β-lactam pharmacokinetics and pharmacodynamics in critically ill patients and strategies for dose optimization: a structured review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XotlWktrk%3D&md5=e0f77c5171bd75399b9bd07ebba35f55CAS | 22519600PubMed |
[14] Udy, A.A. et al. (2013) Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 39, 2070–2082.
| Clinical implications of antibiotic pharmacokinetic principles in the critically ill.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVOktL3O&md5=fee7d0b47901da4edf6feb49352e1038CAS | 24045886PubMed |
[15] Rimawi, R.H. et al. (2013) Impact of regular collaboration between infectious diseases and critical care practitioners on antimicrobial utilization and patient outcome. Crit. Care Med. 41, 2099–2107.
| Impact of regular collaboration between infectious diseases and critical care practitioners on antimicrobial utilization and patient outcome.Crossref | GoogleScholarGoogle Scholar | 23873275PubMed |
[16] Katsios, C.M. et al. (2012) An antimicrobial stewardship program improves antimicrobial treatment by culture site and the quality of antimicrobial prescribing in critically ill patients. Crit. Care 16, R216.
| An antimicrobial stewardship program improves antimicrobial treatment by culture site and the quality of antimicrobial prescribing in critically ill patients.Crossref | GoogleScholarGoogle Scholar | 23127353PubMed |
[17] DiazGranados, C.A. (2012) Prospective audit for antimicrobial stewardship in intensive care: impact on resistance and clinical outcomes. Am. J. Infect. Control 40, 526–529.
| Prospective audit for antimicrobial stewardship in intensive care: impact on resistance and clinical outcomes.Crossref | GoogleScholarGoogle Scholar | 21937145PubMed |
Biographies
Dr Stefano Di Bella is an Infectious Diseases registrar attending the National Institute for Infectious Diseases ‘Lazzaro Spallanzani’ in Rome, Italy. His research interests are emerging bacterial infections and multidrug-resistant bacterial infections in critically ill patients.
Professor Nicola Petrosillo is Director of an Infectious Diseases Division at the National Institute for Infectious Diseases ‘Lazzaro Spallanzani’ of Rome, Italy. He is President of the Italian Society for Healthcare-associated Infections (GIMPIOS). His research interests are healthcare-associated infections, including ICU and transplant-related infections.