Introducing CRC SAAFE: a new Cooperative Research Centre (CRC) focused on managing AMR in agriculture and the environment
E. Donner A B * , R. J. Soares Magalhães
A B * , R. J. Soares Magalhães  A C , A. C. Barnes
A C , A. C. Barnes  A D , A. Jex
A D , A. Jex  A E F , V. Jarocki
A E F , V. Jarocki  A G , B. Drigo
A G , B. Drigo  A B , S. Djordjevic
A B , S. Djordjevic  A G and N. J. Ashbolt
A G and N. J. Ashbolt  A B
A B
A
B
C
D
E
F
G

Prof. Erica Donner is Research Director of Australia’s Cooperative Research Centre (CRC) for Solving Antimicrobial Resistance in Agribusiness, Food and Environments (SAAFE), and a Research Professor in the Future Industries Institute at The University of South Australia. An interdisciplinary environmental scientist specialising in systems-based contaminants analysis, risk assessment and management, Erica is a member of the Australian Strategic and Technical Advisory Group on Antimicrobial Resistance (ASTAG). |

Prof. Ricardo Soares Magalhães is Professor of Zoonotic Disease Epidemiology and Biosecurity with The University of Queensland, and Director of the Queensland Alliance for One Health Sciences. He is a spatial epidemiologist with international expertise in One Health surveillance, working on projects with the Food and Agriculture Organization of the United Nations, World Health Organization and other partners. Specialising in data science, industry digital transformation, disease surveillance and management, Ricardo leads CRC SAAFE’s Analytics Program. |

Prof. Andrew Barnes is a medical microbiologist who has specialised in livestock vaccines for 30 years. He leads CRC SAAFE’s Solutions Program. His University of Queensland Aquatic Animal Health Lab is focused on aquatic animal health and immunity and developing vaccines for a broad range of finfish species. Andy worked for the Scottish Office of Agriculture and Fisheries Department, Aqua Health Ltd and Novartis before transitioning to an academic career at The University of Queensland. |

Assoc. Prof. Aaron Jex is CRC SAAFE’s Monitoring Program lead, specialising in waterborne disease and molecular diagnostics. A National Health and Medical Research Council L1 Leadership Fellow, he is a Lab Head in the Population Health and Immunity Division of the Walter and Eliza Hall Institute, and Associate Professor of Parasitology in the Faculty of Veterinary and Agricultural Sciences with The University of Melbourne. |

Dr Veronica Jarocki is a CRC SAAFE Foundational Postdoctoral Fellow, specialising in genomic epidemiology and pathogen genomics. She is a key participant in SAAFE’s Monitoring Foundations Projects. She is based at the Australian Institute for Microbiology and Infection (AIMI), University of Technology—Sydney. |

Dr Barbara Drigo is Enterprise Fellow and Senior Research Fellow, and Senior Lecturer at The University of South Australia STEM with expertise in microbial ecology, AMR, wastewater-based epidemiology, plant–soil–microbial interactions and climate change. Barbara leads the environmental molecular microbial ecology component of several projects on food, soil and water AMR, safety and security in Australia and the Pacific Islands, and is working on horticulture, aquaculture and water industry foundation projects with CRC SAAFE. |

Prof. Steven Djordjevic is Professor of Infectious Disease and Group Leader with AIMI, at the University of Technology—Sydney. His research generates and interrogates genomic and proteomics data to mitigate AMR, understand pathogen evolution and identify and characterise novel antigens for vaccine development. Prof. Djordjevic is a cofounder and a member of the governing board and the scientific management committee of the Australian Centre for Genomic Epidemiological Microbiology (AusGEM), which is a collaborative partnership with the NSW Department of Primary Industries. |

Prof. Nicholas Ashbolt is a globally recognised expert in the behaviour and movement of environmental pathogens in relation to water guidelines. His work has contributed to the risk-based approach adopted for water quality management in Australia as well as the most recent recreation, drinking and reuse guidelines for the World Health Organization and US Environmental Protection Agency. Nicholas holds the Peter Teasdale Chair in Environmental Health Risk Assessment at The University of South Australia. He leads risk assessment and management initiatives across CRC SAAFE’s research programs, with a specific focus on establishing SAAFE’s Living Lab program. |
Abstract
Antimicrobial resistance (AMR) is a complex challenge that poses a critical threat to food and water safety and security as well as to human, animal and environmental health. It is projected to cost the global economy US$100 trillion by 2050. Australia’s new Cooperative Research Centre (CRC) for Solving Antimicrobial Resistance in Agribusiness, Food and Environments (SAAFE) is part of Australia’s One Health approach to mitigating AMR. SAAFE’s 10-year, A$150-million industry-led program will help protect Australia’s food and agribusiness industries, and the environments in which they operate, from the growing threat of AMR. Through its research programs, CRC SAAFE uses a partner-based approach to assist industries to monitor, analyse and mitigate AMR, with projects spanning horticulture, viticulture, aquaculture, animal industries, water and waste.
Keywords: agriculture, antimicrobial resistance, environment, food, One Health, risk assessment, solutions, stewardship.
Introduction
Australia’s Cooperative Research Centres (CRCs) program is an important part of the national innovation and research agenda, helping to put science at the centre of industry policy and strategy. The Program has been supported by successive Australian Governments for over 30 years, funding medium to long-term industry-led collaborative research initiatives to improve the competitiveness, productivity and sustainability of Australian industries.
The CRC for Solving Antimicrobial Resistance in Agribusiness, Food and Environments (SAAFE), funded under round 23 of the CRC program, is one of Australia’s newest CRCs. Established in 2023, SAAFE is a not-for-profit, partner-based organisation, governed by an independent board and is a key part of Australia’s effort to mitigate antimicrobial resistance (AMR). SAAFE has been granted A$34.5 million from the CRC program to help protect Australia’s food and agribusiness industries, and the environments in which they operate, from the growing threat of AMR. This Commonwealth investment is in line with Australia’s commitment to mitigate AMR, as documented in the National Antimicrobial Resistance Strategy – 2020 and Beyond1 and the One Health Master Action Plan for Australia’s National Antimicrobial Resistance Strategy – 2020 and beyond.2 SAAFE will help implement these policies by bringing together industry, research and government agencies to tackle AMR using a coordinated and cross-sectoral approach. Working with partner organisations from industries such as viticulture, aquaculture, horticulture, water, organic waste, stockfeed and animal industries, SAAFE aims to tackle AMR across a diverse and complex range of systems and environments. Over the next decade, SAAFE will invest in end-user supported (co-funded) collaborative research to understand and mitigate AMR, helping partners to develop, share and implement solutions, and adopt best practice AMR stewardship.
Managing AMR as a multi-sectoral One Health issue
Antimicrobials are essential assets for a wide range of primary industries (Fig. 1), hence the impacts of AMR are far-reaching. They range from treatment failure, morbidity, mortality and production losses to regulatory pressure and trade impacts. Effective engagement and support across a multitude of different industries and regulators is imperative to generate and prioritise action, because AMR management is complicated by pervasive uncertainties, the multi-sectoral nature of the problem, and the complex interrelatedness of AMR risks and drivers. Clear communication is needed to counter the propensity for AMR and its mitigation to be perceived as abstruse, too costly or even just somebody else’s problem.
Antimicrobials are used in many different contexts, but the more we use them, the more we encourage the development and spread of AMR. A ‘One Health’ approach to managing AMR recognises that populations, species, facilities, and industries co-exist within environments, and are connected by the movement of people, animals, goods and materials. The drivers of AMR, as well as the resistant organisms and their genes (all represented here by white dots), may likewise be connected across populations, species and environments. CRC SAAFE is using systems-based approaches to identify AMR interconnections and dependencies, while supporting partners with AMR risk assessment and management controls. Image © SAAFE.
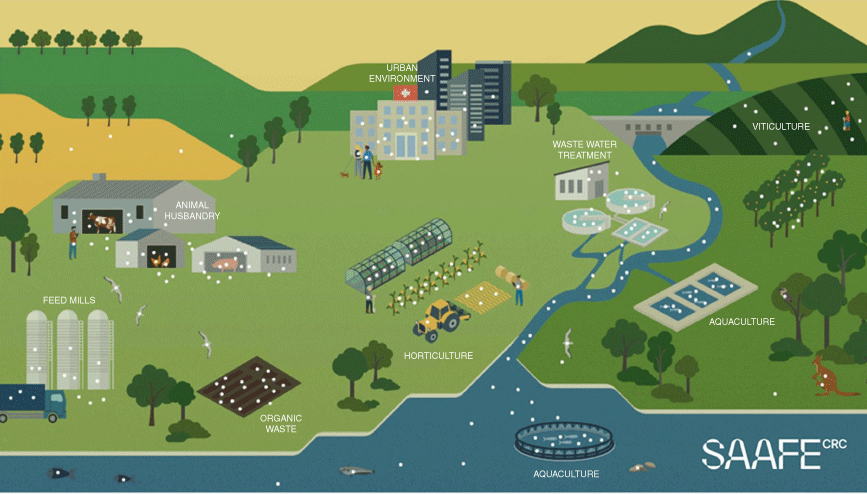
Collaboration is critical as we seek to support industries, communities and government partners to tackle AMR, because, just as no single industry is solely responsible for AMR, no single industry can solve it alone. This is why SAAFE is uniquely cross-sectoral in its partnerships, bringing together stakeholders from broad range of industries (Fig. 2). This networked approach to research and innovation is key to addressing AMR because food and agribusiness industries are linked by environmental systems and supply chains, and interactions and interdependencies are thus central to understanding the drivers and impacts of AMR. SAAFE’s work is underpinned by a One Health approach, which recognises the complex interconnections between people, animals, plants and their shared environments.3 A One Health approach to managing AMR requires both sector-specific and coordinated action across all sectors where antimicrobials are used or are present. SAAFE places the environmental dimensions of AMR at the heart of the solution, in recognition of the key connecting role played by environmental systems and matrices (i.e. water, wastewater, organic waste, air, soil), and the vast richness and diversity of environmental microbiomes, which are the original source of many antimicrobials and part of the next generation of solutions.
From presence to risk attribution to management
A burgeoning literature provides evidence of the presence of antimicrobial resistant organisms and genes in various environments and products, but it is important to recognise that not all resistance is of equal concern, and that AMR presence is not synonymous with risk.4 AMR is a wicked problem that challenges current risk assessment approaches, so this is an area where SAAFE’s foundational projects are heavily focused. When considering reports about the detection of antimicrobial residues and AMR-containing microorganisms and genes, industry and regulators need to be able to answer the questions, ‘so what does it mean’ and ‘what concentrations are important’. Currently, updates on international antibiotic residue limits are occurring,5–8 but it is still unclear how these limits may be used in food or feed trade for Australia, let alone how to interpret pathogen or antimicrobial resistance gene detections other than by using some recent generic principles.9 The list of key AMR pathogens is also expanding from largely a focus on bacteria10 to recent inclusion of fungi.10,11 SAAFE’s Monitoring, Analytics & Solutions Programs (Box 1) will develop improved understanding of the interconnectivities across the environment–food–health nexus and associated risks, and provide information on the financial implications of different management options and decisions. As a major issue for plant industries, antifungal resistance research is firmly in scope for SAAFE, with initial projects including Wine Australia’s antifungal resistance program (with the South Australian Research and Development Institute and Curtin University) and the Western Australian Department of Primary Industries and Regional Development’s fungicide resistance in apple scab project.
| Box 1.CRC SAAFE’s collaborative research program is industry-led and solutions-driven. It comprises three cross-cutting and integrated programs. |
| 1. The monitoring program |
| Measuring AMR so it can be managed |
| SAAFE’s Monitoring Program, led by Assoc. Prof. Aaron Jex, is building the expertise, platforms and protocols needed to underpin research and surveillance of AMR genes, drivers and organisms’ distribution, dynamics, and concentrations in Australia’s wastewater, organic wastes and water supplies, and other relevant food and agri-industry matrices and products. This will support AMR risk assessment and risk reduction strategies and interventions, and provide core resources, including new diagnostic tools, Standard Operating Procedures, method decision matrices and other documentation, and expertise. |
| 2. The analytics program |
| Integrating data to empower better decision making |
| AMR data-driven insights and reporting are needed to protect Australia’s reputation as a premium, safe food producer and to secure and grow international market access. SAAFE’s Analytics Program, led by Prof. Ricardo Soares Magalhães, focuses on secure data integration across agriculture, food and water value chains to mitigate AMR risks to businesses and consumers, and provide industry-specific AMR intelligence allowing better decision-making in food production, processing and regulation. SAAFE is working with partners to develop standards for data governance and management, and develop and test integrated IT systems allowing partners to track, manage and mitigate AMR, while supporting interoperability with other national initiatives and systems. The IT systems developed will feed into modern risk-assessment modelling pipelines to identify where management actions are best placed, and to understand the cost ramifications of different management strategies. SAAFE will be using a ‘Living Labs’ approach led by Prof. Nicholas Ashbolt that supports coordinated risk assessments and technology demonstrations and testing in the ‘real world’. |
| 3. The solutions program |
| Stopping the evolution and spread of AMR |
| The Solutions Program, led by Prof. Andrew Barnes, is focused on developing, testing and improving interventions that mitigate AMR in agribusiness, food, water and waste systems. This includes developing new targeted AMR treatments and alternatives, ranging from vaccines and optimised feeds, to engineering solutions to improve water quality and waste treatment, reduce infection and disease, and decrease AMR loads to receiving environments and in value chains. Drawing from the outputs and innovations from the Monitoring and Analytics program, SAAFE’s Solutions Program activities will also include best practice guideline development for industry self-regulation and antimicrobial stewardship, and end-user front-end digital technology for improved management of AMR risks. |
Staying on top of emerging risks
To support proactive action on AMR, progress needs to be made understanding and predicting emerging risks related to pathogen evolution, propagation and cross-sectoral transmission. Attempts for cross-sectoral AMR surveillance have been shrouded by uncertainties on indicator pathogens and organisms, surveillance design (e.g. sampling frames, sample matrices and laboratory methods) and sustainability concerns. One organism proposed for cross-sectoral AMR surveillance is extended-spectrum beta-lactamase Escherichia coli.12,13 E. coli is posited as a useful model organism for One Health surveillance as it demonstrates the complexity of One Health AMR.13 Displaying vast genetic versatility with more than 9000 sequence types residing within eight major phylogenetic groups, E. coli serovars are broadly classified as commensals or pathogens. Pathogenic E. coli serovars are implicated in a wide array of diseases, both intestinal and extraintestinal, and are classified into 14 pathotypes.14 Among these, extraintestinal pathogenic E. coli (ExPEC) is a colonising opportunistic pathogen residing quietly in the gut,15 until certain, often poorly understood, conditions precipitate disease. E. coli transitions from different body temperatures including humans and other warm-blooded vertebrates (37°C), birds (42°C) and reptiles (variable) and diverse host epithelial surfaces, into waste, wastewater, aquatic and soil environments where it is exposed to complex selection pressures including antimicrobial (antibiotics, biocides, metals), chemical (chlorine, ozone) and mutational (UV) stressors. E. coli is resident in the faecal microbiota of mammals, birds, reptiles and insects; however, the description of naturalised environmental strains of E. coli has challenged the prevailing dogma that E. coli is strictly faecally derived.16 In humans, E. coli is shed at 107–108 colony forming units per gram of faeces,17 and similar levels are found in the faeces of pigs, poultry and wildlife. Global annual pig production is estimated at ~1 billion pigs while at any one time there are an estimated 26 billion poultry inhabiting the planet.18 As such, vast numbers of E. coli are present in municipal sewage, animal waste lagoons and other environments, including on artificial surfaces such as roofs, roads, pavements and the plastisphere. Air-borne carriage of E. coli occurs,19 and manuring practices and extreme weather events can redistribute E. coli onto agricultural lands, and into waterways and groundwater.20 The complexity of E. coli ecology highlights the importance of genomic surveillance to understand One Health AMR connections and risks.13
As we strive to understand the relevance of environmental AMR and its connections to human and animal disease risks, we must also look further into microbial networks and ecology. For example, drinking water generally has a disinfectant residual to minimise microbial growth and limit infectious enteric pathogen concentrations in the water distributed to customers. However, the interaction of disinfectant residuals with distribution pipe materials and pipe biofilms is complex,21–23 and premise plumbing gives rise to the largest waterborne health burden in developed countries today (principally in healthcare settings),24,25 due to the growth of opportunistic pathogens, such as Legionella pneumophila and Pseudomonas aeruginosa, in association with pipe biofilms and free-living amoebae.26 With increasing AMR in these environmentally sourced, opportunistic pathogens,27–30 the role of free-living amoebae in facilitating horizontal gene transfer in biofilms is of growing interest.31,32 SAAFE research is exploring quantitative microbial risk assessment approaches33 using novel AMR-pathogen dose-response models,34 to better understand these kinds of risks, which are also relevant across the broader One Health AMR spectrum.
Education, training and extension
Through an extensive program of training and education, SAAFE will equip the next generation of practitioners and professionals with the tools, technology and know-how to manage AMR. The SAAFE Foundation Postdoctoral Fellows and the Scholars (Higher Degree by Research, HDR) program, form an industry-focused education and training program to build capability and capacity in AMR monitoring, analytics and solutions. The Scholars program features opportunities for individualised and cohort-based training and development experiences to support timely completion, develop One Health AMR literacy, and amplify industry readiness. Project selection for the first PhD cohort is in progress, with ~20 PhD candidates anticipated to commence by mid-2025. A range of other HDR and academic training opportunities will be offered including internships and summer scholarships, as well as vocational training, short courses and bespoke workshops.
Looking forward
Currently, Australian food and agriculture products have a global reputation for premium quality, safety and sustainability. Coordinated, evidence-based guidance facilitating best practice AMR stewardship in the agribusiness, food and environmental sectors will help ensure this reputation into the future. SAAFE is supporting partners to develop and commercialise the tools needed to tackle AMR across value chains, to de-risk circular economies, and increase production and quality-based competitiveness. Action against AMR will ensure that partners are better positioned to secure, expand, and diversify export markets, strengthen biosecurity and outbreak response, and reach productivity targets. It will also protect human and environmental health.
After 1 year of establishment, recruitment and planning, 2024 sees SAAFE commencing its first tranche of foundation projects, while fostering collaboration between projects, sectors and other AMR initiatives. For more information, follow SAAFE on LinkedIn, register for upcoming newsletters, or, from June 2024, visit SAAFE’s new website or tune in to the ‘AMR Conversations with SAAFE’ webinar series and podcast. SAAFE’s second annual AMR Solutions Summit will be held at the National Wine Centre in Adelaide over 17–18 September 2024.
Data availability
Data sharing is not applica ble as no new data were generated or analysed during this study.
Declaration of funding
CRC SAAFE receives funding from the Australian Commonwealth government through the CRC program.
References
1 Department of Health and Aged Care and Department of Agriculture, Fisheries and Forestry (2020) Australia’s National Antimicrobial Resistance Strategy – 2020 and beyond. Commonwealth of Australia. https://www.amr.gov.au/resources/australias-national-antimicrobial-resistance-strategy-2020-and-beyond
2 Department of Health and Aged Care and Department of Agriculture, Fisheries and Forestry (2021) One Health Master Action Plan for Australia’s National Antimicrobial Resistance Strategy – 2020 and beyond. Commonwealth of Australia. https://www.amr.gov.au/resources/one-health-master-action-plan-australias-national-antimicrobial-resistance-strategy-2020-and-beyond
3 Mettenleiter TC et al. (2023) The One Health High-Level Expert Panel (OHHLEP). One Health Outlook 5, 18.
| Crossref | Google Scholar | PubMed |
4 Zhang A-N et al. (2021) An omics-based framework for assessing the health risk of antimicrobial resistance genes. Nat Commun 12, 4765.
| Crossref | Google Scholar |
5 Tell J et al. (2019) Science‐based targets for antibiotics in receiving waters from pharmaceutical manufacturing operations. IEAM 15(3), 312-319.
| Crossref | Google Scholar |
6 European Commission Directorate-General for Environment (2022) Update on progress and implementation – European Union strategic approach to pharmaceuticals in the environment. Publications Office of the European Union. 10.2779/037747
7 European Commission (2022) Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL concerning urban wastewater treatment (recast). Document 52022PC0541. OM(2022) 541 final, 2022/0345 (COD). European Commission, Brussels, Belgium. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52022PC0541
8 EFSA Panel on Biological Hazards (BIOHAZ) et al. (2021) Maximum levels of cross-contamination for 24 antimicrobial active substances in non-target feed. Part 12: tetracyclines: tetracycline, chlortetracycline, oxytetracycline, and doxycycline. EFSA J 19(10), e06864.
| Crossref | Google Scholar |
9 Ågerstrand M et al. (2023) Opportunities to tackle antibiotic resistance development in the aquatic environment through the Water Framework Directive. Ambio 52, 941-951.
| Crossref | Google Scholar | PubMed |
10 World Health Organization (2022) WHO fungal priority pathogens list to guide research, development and public health action. WHO, Geneva, Switzerland. https://www.who.int/publications-detail-redirect/9789240060241 (accessed 12 May 2024)
11 Fisher MC, Denning DW (2023) The WHO fungal priority pathogens list as a game-changer. Nat Rev Microbiol 21, 211-212.
| Crossref | Google Scholar | PubMed |
12 Puspandari N et al. (2021) Extended spectrum beta-lactamase-producing Escherichia coli surveillance in the human, food chain, and environment sectors: Tricycle Project (pilot) in Indonesia. One Health 13, 100331.
| Crossref | Google Scholar | PubMed |
13 Djordjevic SP et al. (2024) Genomic surveillance for antimicrobial resistance — a One Health perspective. Nat Rev Genet 25, 142-157.
| Crossref | Google Scholar | PubMed |
14 Geurtsen J et al. (2022) Genomics and pathotypes of the many faces of Escherichia coli. FEMS Microbiol Rev 46, fuac031.
| Crossref | Google Scholar | PubMed |
15 Price LB et al. (2017) Colonizing opportunistic pathogens (COPs): the beasts in all of us. PLoS Pathogens 13, e1006369.
| Crossref | Google Scholar | PubMed |
18 Bar-On YM et al. (2018) The biomass distribution on Earth. Proc Natl Acad Sci USA 115, 6506-6511.
| Crossref | Google Scholar | PubMed |
19 Yang T et al. (2024) Antibiotic resistance genes associated with size-segregated bioaerosols from wastewater treatment plants: a review. Environ Pollut 343, 123169.
| Crossref | Google Scholar | PubMed |
20 Black Z et al. (2021) The fate of foodborne pathogens in manure treated soil. Front Microbiol 12, 781357.
| Crossref | Google Scholar | PubMed |
21 Pfaller S et al. (2021) Chloramine concentrations within distribution systems and their effect on heterotrophic bacteria, mycobacterial species, and disinfection byproducts. Water Res 205, 117689.
| Crossref | Google Scholar | PubMed |
22 Revetta RP et al. (2016) Changes in bacterial composition of biofilm in a metropolitan drinking water distribution system. J Appl Microbiol 121, 294-305.
| Crossref | Google Scholar | PubMed |
23 Zhang C et al. (2021) Legionella and other opportunistic pathogens in full-scale chloraminated municipal drinking water distribution systems. Water Res 205, 117571.
| Crossref | Google Scholar |
24 Cassini A et al. (2018) Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the Burden of Communicable Diseases in Europe study, European Union and European economic area countries, 2009 to 2013. Eurosurveillance 23, 17.
| Crossref | Google Scholar | PubMed |
25 Collier SA et al. (2021) Estimate of burden and direct healthcare cost of infectious waterborne disease in the United States. Emerg Infect Dis 27, 140-149.
| Crossref | Google Scholar | PubMed |
26 Ashbolt NJ (2015) Environmental (saprozoic) pathogens of engineered water systems: understanding their ecology for risk assessment and management. Pathogens 4, 390-405.
| Crossref | Google Scholar | PubMed |
27 Barker J et al. (1995) Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob Agents Chemother 39, 2684-2688.
| Crossref | Google Scholar | PubMed |
28 Milligan EG et al. (2023) A systematic review of culture-based methods for monitoring antibiotic-resistant Acinetobacter, Aeromonas, and Pseudomonas as environmentally relevant pathogens in wastewater and surface water. Curr Environ Health Rep 10, 154-171.
| Crossref | Google Scholar | PubMed |
29 Sanz-García F et al. (2022) Evolution under low antibiotic concentrations: a risk for the selection of Pseudomonas aeruginosa multidrug-resistant mutants in nature. Environ Microbiol 24, 1279-1293.
| Crossref | Google Scholar | PubMed |
30 Xiong L et al. (2016) Antibiotic susceptibility of Legionella strains isolated from public water sources in Macau and Guangzhou. J Water Health 14, 1041-1046.
| Crossref | Google Scholar | PubMed |
31 Ashbolt NJ (2023) Conceptual model to inform Legionella–amoebae control, including the roles of extracellular vesicles in engineered water system infections. Front Cell Infect Microbiol 13, 1200478.
| Crossref | Google Scholar | PubMed |
32 Zanditenas E, Ankri S (2024) Unraveling the interplay between unicellular parasites and bacterial biofilms: implications for disease persistence and antibiotic resistance. Virulence 15, 2289775.
| Crossref | Google Scholar | PubMed |
33 Ashbolt NJ et al. (2013) Human health risk assessment (HHRA) for environmental development and transfer of antibiotic resistance. Environ Health Perspect 121, 993-1001.
| Crossref | Google Scholar | PubMed |
34 Chandrasekaran S, Jiang SC (2019) A dose response model for quantifying the infection risk of antibiotic-resistant bacteria. Sci Rep 9, 17093.
| Crossref | Google Scholar |
 Prof. Erica Donner is Research Director of Australia’s Cooperative Research Centre (CRC) for Solving Antimicrobial Resistance in Agribusiness, Food and Environments (SAAFE), and a Research Professor in the Future Industries Institute at The University of South Australia. An interdisciplinary environmental scientist specialising in systems-based contaminants analysis, risk assessment and management, Erica is a member of the Australian Strategic and Technical Advisory Group on Antimicrobial Resistance (ASTAG). |
 Prof. Ricardo Soares Magalhães is Professor of Zoonotic Disease Epidemiology and Biosecurity with The University of Queensland, and Director of the Queensland Alliance for One Health Sciences. He is a spatial epidemiologist with international expertise in One Health surveillance, working on projects with the Food and Agriculture Organization of the United Nations, World Health Organization and other partners. Specialising in data science, industry digital transformation, disease surveillance and management, Ricardo leads CRC SAAFE’s Analytics Program. |
 Prof. Andrew Barnes is a medical microbiologist who has specialised in livestock vaccines for 30 years. He leads CRC SAAFE’s Solutions Program. His University of Queensland Aquatic Animal Health Lab is focused on aquatic animal health and immunity and developing vaccines for a broad range of finfish species. Andy worked for the Scottish Office of Agriculture and Fisheries Department, Aqua Health Ltd and Novartis before transitioning to an academic career at The University of Queensland. |
 Assoc. Prof. Aaron Jex is CRC SAAFE’s Monitoring Program lead, specialising in waterborne disease and molecular diagnostics. A National Health and Medical Research Council L1 Leadership Fellow, he is a Lab Head in the Population Health and Immunity Division of the Walter and Eliza Hall Institute, and Associate Professor of Parasitology in the Faculty of Veterinary and Agricultural Sciences with The University of Melbourne. |
 Dr Veronica Jarocki is a CRC SAAFE Foundational Postdoctoral Fellow, specialising in genomic epidemiology and pathogen genomics. She is a key participant in SAAFE’s Monitoring Foundations Projects. She is based at the Australian Institute for Microbiology and Infection (AIMI), University of Technology—Sydney. |
 Dr Barbara Drigo is Enterprise Fellow and Senior Research Fellow, and Senior Lecturer at The University of South Australia STEM with expertise in microbial ecology, AMR, wastewater-based epidemiology, plant–soil–microbial interactions and climate change. Barbara leads the environmental molecular microbial ecology component of several projects on food, soil and water AMR, safety and security in Australia and the Pacific Islands, and is working on horticulture, aquaculture and water industry foundation projects with CRC SAAFE. |
 Prof. Steven Djordjevic is Professor of Infectious Disease and Group Leader with AIMI, at the University of Technology—Sydney. His research generates and interrogates genomic and proteomics data to mitigate AMR, understand pathogen evolution and identify and characterise novel antigens for vaccine development. Prof. Djordjevic is a cofounder and a member of the governing board and the scientific management committee of the Australian Centre for Genomic Epidemiological Microbiology (AusGEM), which is a collaborative partnership with the NSW Department of Primary Industries. |
 Prof. Nicholas Ashbolt is a globally recognised expert in the behaviour and movement of environmental pathogens in relation to water guidelines. His work has contributed to the risk-based approach adopted for water quality management in Australia as well as the most recent recreation, drinking and reuse guidelines for the World Health Organization and US Environmental Protection Agency. Nicholas holds the Peter Teasdale Chair in Environmental Health Risk Assessment at The University of South Australia. He leads risk assessment and management initiatives across CRC SAAFE’s research programs, with a specific focus on establishing SAAFE’s Living Lab program. |



