Endemic Australian arboviruses of human health significance
David W SmithDepartment of Microbiology, PathWest Laboratory Medicine WA, Hospital Avenue, Nedlands, WA 6009, Australia
Faculty of Health and Medical and Sciences, University of Western Australia, Nedlands, WA 6009, Australia
Tel: +61 8 6383 4438, Email: david.smith@health.wa.gov.au
Microbiology Australia 39(2) 88-90 https://doi.org/10.1071/MA18024
Published: 13 April 2018
Each year many thousands of cases of human arbovirus infection are notified within Australia, acquired either within Australia or when travelling overseas1. These cause diseases varying from fever and aches, to debilitating joint disease, to encephalitis and death. The arboviruses endemic to Australia are all maintained in a cycle between mosquitoes (and rarely midges) and a bird or mammalian host2. As such, the virus activity is dependent on rainfall and temperature conditions that are conducive to mosquito breeding, and to virus replication and amplification (Figure 1). Those conditions being met, there have to be suitable amplifying animal hosts nearby, and their absence is one of the factors that protects most of the larger urban populations in Australia. Then, of course, humans have to be exposed to the infected mosquitoes to get disease.
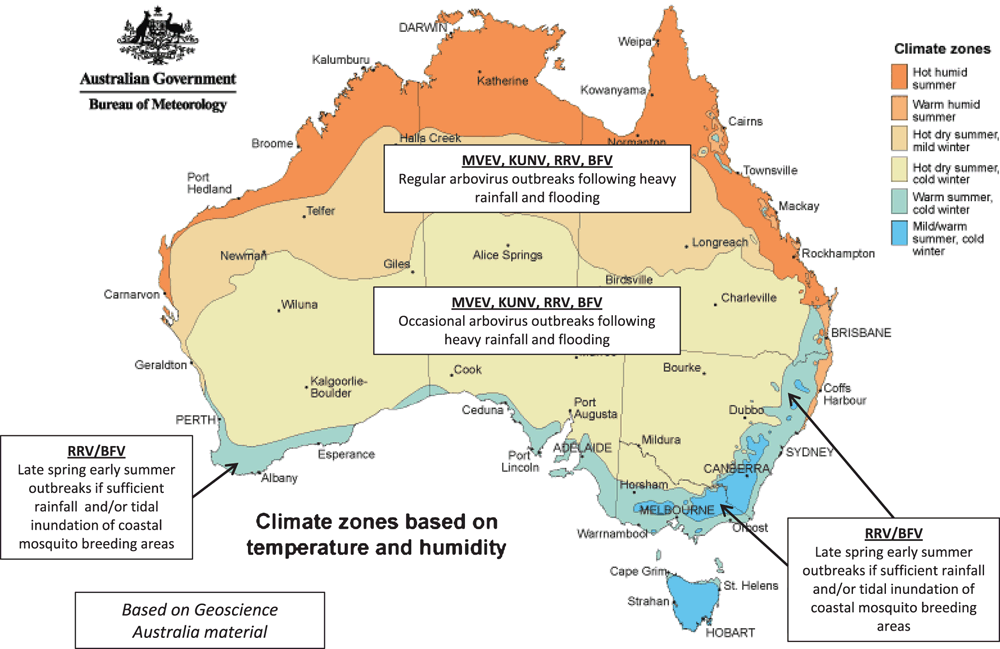
|
The most common arbovirus infections in Australia are due to the alphaviruses, almost entirely Ross River virus (RRV) and Barmah Forest virus (BFV). Serologically proven RRV disease was first described in 1928 in south-eastern Australia3, but has been postulated to have been responsible for an earlier outbreak in Victoria in 18684. It is transmitted by a range of mosquito species (especially Culex annulirostris, Aedes vigilax and Aedes camptorhynchus) and the primary amplifying hosts are the macropods (kangaroos, wallabies and euros)2,5,6. While the virus has been found in all Australian states and territories, the largest numbers of cases occur in south-eastern Queensland, coastal NSW and in the south-west of WA. However, the highest individual risk of infection is in the less populous area of northern Queensland, northern WA and the NT. Infection is asymptomatic in 25–99% of infected individuals. The acute clinical illness is unpleasant and typically lasts up to 4 weeks but persists much longer in some. Joint pains, muscle pains, and lethargy may continue for months or years, especially if the illness is superimposed on pre-existing joint disease5,7,8. This is a common feature of the arthritogenic alphavirus infections worldwide including chikungunya and the Sindbis viruses5,9, the clinical illness resulting from a complex interaction between the virus and host responses that we are just beginning to understand5,7,9. Treatment remains symptomatic5.
BFV ecology and geographic distribution are similar to those of RRV, but human disease is less common2,6. Localised epidemics may occur as it enters new areas, after which it settles down to a pattern of low level endemic activity.
Sindbis virus in Africa and Europe causes an RRV-like illness, but the lineage found within Australia rarely causes illness2,5,6. Chikungunya virus has never been detected within Australia despite having circulated within our region over many decades and the presence of a vector, Ae. aegypti, in north Queensland.
The diagnosis of the alphavirus infections is mainly serological as any detectable viraemia is generally gone by the time patients see a doctor. For both RRV and BFV10,11, detection of IgM has proven unreliable as an indicator of recent infection in itself, due to false positive results and the long-term persistence of IgM. Therefore, wherever possible, confirmation of recent infection should be sought by testing of paired acute and convalescent serum samples to show either IgG seroconversion (usually using the enzyme immunoassay tests) or a significant rise in IgG levels (usually using the haemagglutination inhibition titres)12.
While less common as a cause of illness in Australia, the flaviviruses have the potential to cause much more serious disease6,13. Most of the serious disease is due to Murray Valley encephalitis virus (MVEV), with occasional cases due to the Kunjin strain of West Nile virus (KUNV).
MVEV is closely related to Japanese encephalitis virus and is maintained primarily in a mosquito (Culex annulirostris)-water bird cycle2,6,13. Encephalitis develops in only 1 : 200–1 : 1000 infected people, carrying with it a mortality of 20–25% and persisting neurological deficits in approximately 50% of survivors. Epidemics occurred on the eastern coast of Australia in the early 20th century, then in 1951 and 1974 in the Murray Valley region of the southeast. Since then activity has been almost completely confined to north western and central Australia14. This enzootic focus results in a small number of human cases each year in north western Australia. It was also believed to be the source of all of the occasional larger outbreaks that spread beyond these areas, resulting from carriage of virus by infected migratory waterbirds into areas of flooding. The last major outbreak in 2011 changed that perception and suggested more than one enzootic focus in Australia. It included 16 confirmed human cases and many equine cases, in two separate but simultaneous outbreaks (one in south-eastern Australia and the other in north-western Australia) between March and May13. The former was the first substantial activity in the south-east since 1974, while the latter represented a severe but not exceptional season for the north-west. It further demonstrated the significant changes in the epidemiology of MVEV disease since 2000. While the burden of disease and death continues in Aboriginal communities, especially in young children, there has been a relative increase in cases and deaths in non-Aboriginal adults. This change is believed to be due to the spread of the virus into wider areas of WA, increased work and tourist travel in the risk areas, and growth in the resident populations in those areas13.
Japanese encephalitis virus (JEV) is found widely throughout Asia, including our immediate northern neighbours2. It is maintained in a cycle between pigs and mosquitoes, principally Ae. aegypti and Ae. albopictus. In 1995, JEV entered and established in the Torres Strait Islands (TSI) and has transiently entered the Cape York Peninsula. No human cases have occurred within mainland or territorial Australia since 1998. However, the virus itself is still present in TSI and the threat of introduction into northern Queensland remains, as both Ae aegypti and large populations of feral pigs are present.
Dengue virus (DENV) was endemic across northern Australia and some more southerly areas up until the 1940s15. Control of its major vector species (Ae. aegypti) led to DENV elimination from all of Australia, though the mosquito has remained in parts of northern Queensland, and occasional limited outbreaks occur following introduction of the virus into local mosquito populations by viraemic travellers16. Control of the outbreaks relies on control of the vector mosquitoes and avoidance of exposure, including novel biological control programs17.
The other endemic flaviviruses of Australia that have been shown to infect humans include Kokobera (KOKV), Stratford (STRV), Edge Hill and Alfuy viruses6. Human infection is uncommon and diagnosed clinical illness is rare; KOKV and STRV having been described as causing fever and polyarthralgia.
However, it is clear that there are many other arboviruses present within our mosquito populations18. Some of these have been shown to infect humans and to cause disease in animal models19, but further work is needed to properly assess the significance of these arboviruses and those yet to be discovered.
References
[1] Knope, K.E. et al. (2016) Arboviral diseases and malaria in Australia, 2013–14: annual report of the National Arbovirus and Malaria Advisory Committee. Commun. Dis. Intell. 40, E401–E436.[2] Mackenzie, J.S. et al. (1994) Arboviruses causing human disease in the Australasian zoogeographic region. Arch. Virol. 136, 447–467.
| Arboviruses causing human disease in the Australasian zoogeographic region.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2czgsVentg%3D%3D&md5=db269e12dad607d5ac367c4a708dd87fCAS |
[3] Appuhamy, R.D. et al. (2010) Toponymous diseases of Australia. Med. J. Aust. 193, 642–646.
[4] Kelly-Hope, L.A. et al. (2004) Ross River virus disease in Australia, 1886–1998, with analysis of risk factors associated with outbreaks. J. Med. Entomol. 41, 133–150.
| Ross River virus disease in Australia, 1886–1998, with analysis of risk factors associated with outbreaks.Crossref | GoogleScholarGoogle Scholar |
[5] Smith, D.W. et al. (2017) The alphaviruses. In Clinical Virology, 4th edn. (Richman, D.D. et al., eds), pp. 1347–1380, ASM Press.
[6] Smith, D.W. et al. (2011) The viruses of Australia and the risk to tourists. Travel Med. Infect. Dis. 9, 113–125.
| The viruses of Australia and the risk to tourists.Crossref | GoogleScholarGoogle Scholar |
[7] Suhrbier, A. et al. (2012) Arthritogenic alphaviruses – an overview. Nat. Rev. Rhuematol. 8, 420–429.
| Arthritogenic alphaviruses – an overview.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpsVKltbs%3D&md5=c5fffd1387dcb64e3443bf1b8220f7e2CAS |
[8] Harley, D. et al. (2001) Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin. Microbiol. Rev. 14, 909–932.
| Ross River virus transmission, infection, and disease: a cross-disciplinary review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MrjvVymug%3D%3D&md5=4f6e45476adfc18422e7b72351ff44deCAS |
[9] Chen, W. et al. (2015) Arthritogenic alphaviruses: new insights into arthritis and bone pathology. Trends Microbiol. 23, 35–43.
| Arthritogenic alphaviruses: new insights into arthritis and bone pathology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhs1Kls7fL&md5=ed589402d4c5e4bca9922b393f711563CAS |
[10] Cashman, P. et al. (2008) Barmah Forest virus serology; implications for diagnosis and public health action. Commun. Dis. Intell. Q. Rep. 32, 263–266.
[11] Selvey, L.A. et al. (2014) Ross River virus infection surveillance in the Greater Perth Metropolitan area--has there been an increase in cases in the winter months? Commun. Dis. Intell. Q. Rep. 38, E114–E122.
[12] Surveillance case definitions for the Australian National Notifiable Diseases Surveillance System as of 1 January 2017. http://www.health.gov.au/internet/main/publishing.nsf/Content/cdna-casedefinitions.htm/$File/consolidated-case-definitions.pdf (accessed 12 February 2018).
[13] Selvey, L.A. et al. (2014) The changing epidemiology of Murray Velley encephalitis in Australia: the 2011 outbreak and a review of the literature. PLoS Negl. Trop. Dis. 8, e2656.
| The changing epidemiology of Murray Velley encephalitis in Australia: the 2011 outbreak and a review of the literature.Crossref | GoogleScholarGoogle Scholar |
[14] Mackenzie, J.S. et al. (2017) The ecology and epidemiology of Ross River and Murray Valley encephalitis viruses: examples of One Health in action. Trans. R. Soc. Trop. Med. Hyg. 111, 248–254.
| The ecology and epidemiology of Ross River and Murray Valley encephalitis viruses: examples of One Health in action.Crossref | GoogleScholarGoogle Scholar |
[15] Mackenzie, J.S. et al. (1996) Dengue in Australia. J. Med. Microbiol. 45, 159–161.
| Dengue in Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK28vgs1ymsQ%3D%3D&md5=25b5d4a81113e72fe59d7d8971acd926CAS |
[16] Naish, S. et al. (2014) Spatial and temporal patterns of locally-acquired dengue transmission in northern Queensland, Australia, 1993-2012. PLoS One 9, e92524.
| Spatial and temporal patterns of locally-acquired dengue transmission in northern Queensland, Australia, 1993-2012.Crossref | GoogleScholarGoogle Scholar |
[17] Ritchie, S.A. et al. (2018) Mission accomplished? We need a guide to the ‘post relaese’ world of Wolbachia for Aedes-borne disease control. Trends Parasitol. 34, 217–226.
[18] Shi, M. et al. (2017) High-resolution metatranscriptomics reveals the ecological dynamics of mosquito-associated RNA viruses in Western Australia. J. Virol. 91, e00680-e17.
| High-resolution metatranscriptomics reveals the ecological dynamics of mosquito-associated RNA viruses in Western Australia.Crossref | GoogleScholarGoogle Scholar |
[19] Johansen, C.A. et al. (2017) Characterization of Fitzroy River virus and serologic evidence of human and animal infection. Emerg. Infect. Dis. 23, 1289–1299.
| Characterization of Fitzroy River virus and serologic evidence of human and animal infection.Crossref | GoogleScholarGoogle Scholar |
Biography
Clinical Professor David Smith BMedSc, MBBS, FRCPA, FACTM, FASM, FFSc(RCPA) is a graduate in Medicine from the University of Western Australia and trained in Medical Microbiology in Perth. He is a Medical Virologist at PathWest Laboratory Medicine WA at the QE2 Medical Centre in Perth, Australia, where he is a Director of the Arbovirus Research Laboratory. He is also a Clinical Professor in the Faculty of Health and Medical Sciences at the University of Western Australia. Professor Smith serves on a number of state, national and international committees and advisory groups, and is currently Chair of the National Arbovirus and Malaria Advisory Committee. He has a particular interest in public health issues, including mosquito-borne viruses, influenza and other respiratory viruses, and emerging infections.


