Public health aspects of Dengue virus infection relevant to Australia
Trine Gulholm A B and William D Rawlinson A B C D EA Serology and Virology Division, Department of Microbiology NSW Health Pathology, Prince of Wales Hospital, Sydney, NSW, Australia
B School of Medical Sciences, University of New South Wales, Sydney, NSW, Australia
C School of Women’s and Children’s Health, University of New South Wales, Sydney, NSW, Australia
D School of Biotechnology and Biomolecular Sciences, University of New South Wales, Sydney, NSW, Australia
E Tel: +61 2 9382 9113, Fax: +61 2 9382 9098, Email: w.rawlinson@unsw.edu.au
Microbiology Australia 38(4) 191-193 https://doi.org/10.1071/MA17066
Published: 31 October 2017
Dengue is endemic in over 100 countries. The disease is not endemic in Australia currently, although the mosquito vector and imported cases cause sporadic outbreaks, predominantly in Queensland. The illness dengue fever causes a spectrum of disease from asymptomatic or a minor febrile illness through to a fatal disease caused by shock from plasma leakage or haemorrhage. There is currently no specific treatment for dengue. Dengue is mainly diagnosed using serology, antigen detection and PCR. Serological diagnosis of dengue can be difficult because of cross reactions with other flaviviruses. A vaccine is available and registered in Australia, however the overall efficacy is just over 50%. Surveillance, disease recognition, outbreak control and prevention of exposure are strategies used to combat dengue in Australia.
Epidemiology
Dengue is a viral, mosquito borne disease. It is caused by one of four serotypes of the Dengue virus (DENV), with a fifth type described from the forests of Malaysia in 2013 thought to be mainly enzootic1. It is a member of the Flavivirus genus along with Zika virus (ZIKV), yellow fever and Japanese encephalitis virus (JEV). DENV is transmitted through bites from infected Aedes aegypti and Aedes albopictus mosquitos2 (Figure 1)3. There is no documented direct person to person transmission for DENV, however transmission can occur through organ transplant, blood transfusions and vertically from mother to fetus4. In contrast, direct transmission has been recorded for ZIKV through sexual contact.5
Dengue is widespread throughout the tropics. The disease is endemic in over 100 countries, with the Americas, South-East Asia and Western Pacific regions the most seriously affected. It is estimated that 50–100 million people develop disease due to Dengue every year, including 500 000 severe infections, and 22 000 deaths, mostly among children4. Risk factors for epidemics include: (1) environmental factors such as rainfall, temperature and humidity; (2) increased urbanisation as Aedes aegypti, the main mosquito vector, favours the urban setting6; and (3) changes in exposure with land clearing. This year has seen several outbreaks of dengue. Sri Lanka reported 80732 dengue cases, including 215 deaths, from January to July 2017. This is 4.3-fold higher than normal rate7. Vietnam has seen 90 626 cases in 2017 as of August, including 24 deaths. This is an increase by 67% compared to 20168. Dengue is on the World Health Organization’s (WHO) list of Neglected Tropical Diseases (NTDs), although the appropriateness of this has been questioned by some. The characteristics of NTDs include being poverty related and lacking in public health attention. Dengue does not only affect poor and marginalised groups, and has become a high-profile disease with significant research funding in some countries9.
Clinical features and diagnostic issues
Typically, the illness Dengue fever manifests with fever, headache, retro-orbital pain, myalgia and rash after an incubation period ranging from 3–14 days (usually 5–7 days). In 2009, WHO reclassified dengue into levels of severity. The disease ranges from a mild febrile illness (most common) to severe dengue, the latter being more common in children and in secondary dengue infection. Severe dengue includes dengue haemorrhagic fever and dengue shock syndrome consisting of severe bleeding, shock and/or organ failure10. Dengue disease consists of three phases. The first phase is the febrile illness, typically lasting 2–7 days. Next comes the critical phase where the fever settles and transudation occurs from leaky capillaries. Finally, there is the recovery phase where transudation stops and reabsorption starts. The patients’ vital signs stabilise and the characteristic convalescent rash appears6. The overall mortality rate of dengue is less than 1%. Severe dengue, when left untreated, has a mortality rate up to 50%4. There is no specific treatment for dengue, only supportive care.
Dengue is diagnosed using serology, antigen detection and nucleic acid testing, predominantly PCR. All flaviviruses are serologically related. Flavivirus serology is complicated by cross reactivity within the genus and infection with a second flavivirus (ZIKV, JEV, Yellow fever virus) can cause a misleading rise in antibody titre of the previously encountered flavivirus (immune recall or original antigenic sin). This is caused by cross-reactive epitopes on the flavivirus E protein11. IgG is of little value in the diagnosis of recent infection unless paired sera are tested. A positive IgG can mean a previous infection with a flavivirus or previous vaccination against a flavivirus (YFV or JEV). Testing of convalescent serum is recommended to determine which species caused the infection. However, dengue IgM in a patient with classical dengue symptoms who have been to an endemic area in the last 10 days is sufficient for diagnosis12. IgM appears within a few days in primary infection and lasts for weeks to months but can be absent in secondary infection. Like IgG, IgM can cross react between the flaviviruses. Neutralisation assays are more discriminating (but cross reactions still occur) and measures the ability of the patient’s serum to prevent growth of different flaviviruses. The neutralisation titre to the infecting virus should be at least 4-fold higher than to the other flaviviruses.
RT-PCR can also be used with several commercial assays now available. The advantages are rapid turnaround time and rapid and reliable serotyping from the onset of the illness. RT-PCR can be used for both primary and secondary disease, but the viraemia is shorter and viral load lower in secondary infection compared to primary13. The sensitivity of RT-PCR varies with time from onset of illness, with 75% on day 1, 92.9% on day 3 and falling to 50% by day 514.
The antigen test for dengue detects the dengue non-structural protein 1 (NS1) that is secreted from infected cells. It is detectable early in the disease in both primary (from disease onset until day 9 or longer) and secondary infection (but antigenaemia is shorter in secondary infection and sensitivity is lower). It does not distinguish between the different serotypes15. The commercial development of the NS1antigen detection has improved dengue diagnosis. The assay allows early diagnosis, is simple and quick and has high sensitivity (90% during febrile phase in primary disease, 60–80% in secondary infection)16 and specificity (over 98%)17. Differentiation between ZIKV and DENV virus is important both for epidemiological mapping and to counsel individual patients. This is especially problematic in Brazil since 2015, where ZIKV infections occur on a background of endemic DENV. It is recommended to do serology plus NS1 antigen to improve diagnostic accuracy, and reduce misdiagnosis of ZIKV and DENV11.
Dengue in Australia
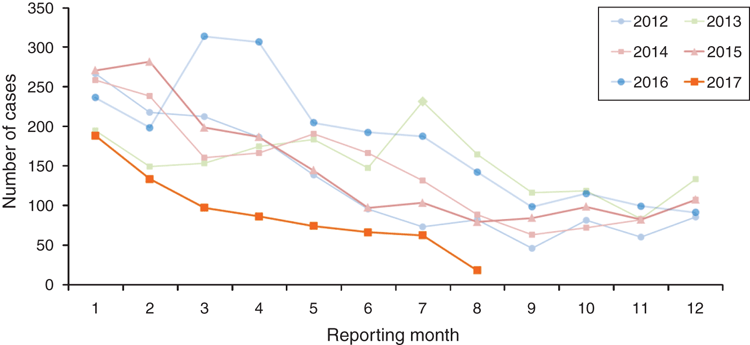
The Aedes aegypti mosquito is present in North Queensland and most commonly found around Townsville and Cairns. DENV is not endemic in Queensland, although problems may arise when a traveller arrives viraemic with DENV from an endemic area. The local mosquitos can bite the affected person and transfer to other people resulting in local transmission which can result in limited local spread, and periodic epidemics. In the past 12 years, outbreaks due to all four serotypes have been documented in North Queensland. Dengue is notifiable to Public Health Units (PHU) in Australia. When a laboratory confirms dengue infection, they inform their local PHU. The priority is urgent if the patient is in a dengue receptive area where there is potential for ongoing transmission, and routine (within 48 hours) in other areas18. The PHU investigates to establish if the patient acquired the virus overseas or locally, with PHU directed active case finding. In 2016 there were 2129 cases of dengue confirmed in Australia, 553 in WA, Victoria 461 and NSW 45019. As of 29 August there were 723 laboratory confirmed cases reported in Australia this year (see Figure 2)8. Since 2005, the most common countries of acquisition for imported DENV to Australia were Indonesia (35%), Thailand (17%), the Philippines (6%) and Papua New Guinea (6%)20.
|
The national surveillance of dengue in Australia is primarily through disease notification by medical practitioners and laboratories, and vector surveillance programs at major seaports and airports with intensive vector control of outbreaks in dengue receptive areas (Queensland Dengue Management Plan). The response includes mosquito elimination, as these may have fed on the viraemic patient while the DENV is still incubating in the mosquito20. The World Mosquito Program (formerly known as The Eliminate Dengue (ED) Program) is using Wolbachia-infected mosquitos to reduce dengue transmission via rendering them resistant to DENV infection. Field trials have been done in several countries including Australia, showing that the Wolbachia infected mosquitos can be successfully introduced into wild mosquito populations21.
Vaccination and future direction
Dengvaxia (CYD-TDV, Sanofi Pasteur) is a live attenuated tetravalent dengue vaccine. It was first registered in Mexico in 2015 for use in 9–45 year olds living in endemic areas22. It is now registered in 18 countries including Australia (licenced by the TGA in July 2017)23. A systematic review of the vaccine showed overall efficacy was 54% with serotype specific efficacy ranging from 77% for DENV4 to 34% for DENV2. It is given as a 3-dose series on a 0/6/12 month schedule. It is currently licenced for people living in dengue endemic areas, and not for short term travellers to these regions24. Other vaccines under development include Takeda’s tetravalent dengue vaccine which are in phase II clinical trial aimed at children from 2–17 years of age25. The Dengvaxia is available, but it is not yet as effective as one would hope. There are some very early steps towards treatment, but supportive care is the mainstay of current care. A new and exciting development is that Sofosbuvir, a drug active against the RNA hepacivirus Hepatitis C Virus, is showing some activity against DENV in vitro26. Surveillance, disease recognition, and outbreak control will remain key elements to combatting dengue in Australia, along with increased public awareness on avoiding exposure.
References
[1] Mustafa, M.S. et al. (2015) Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med. J. Armed Forces India 71, 67–70.| Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2MvovFSruw%3D%3D&md5=2ba6f82436a53e882feebb9c9ac48e19CAS |
[2] Burrell, C.J. et al. (2017) Fenner and White’s Medical Virology. 5th edn. Academic Press.
[3] CDC Public Health Image Library (2017). https://phil.cdc.gov/phil/details_linked.asp?pid=9261 (accessed September 2017).
[4] Centers for Disease Control and Prevention (2012) Dengue homepage. http://www.cdc.gov/dengue (accessed August 2017).
[5] Atkinson, B. et al. (2016) Detection of Zika Virus in semen. Emerg. Infect. Dis. 22, 940.
| Detection of Zika Virus in semen.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXjt12js7g%3D&md5=9a16e7bf067a68cd792ade5512878fd4CAS |
[6] Tai, A.Y.C. et al. (2017) Management of dengue in Australian travelers: a retrospective multicenter analysis. Med. J. Aust. 206, 295–300.
| Management of dengue in Australian travelers: a retrospective multicenter analysis.Crossref | GoogleScholarGoogle Scholar |
[7] WHO (2017) Emergencies preparedness, response. www.who.int/csr/don/19-july-2017-dengue-sri-lanka/en/ (accessed September 2017).
[8] WHO (2017) Dengue situation update Number 524. www.wpro.who.int/emerging_diseases/dengue_biweekly_20170829.pdf?ua=1 (accessed September 2017).
[9] Horstick, O. et al. (2015) Reviewing dengue: Still a neglected tropical disease? PLoS Negl. Trop. Dis. 9, e0003632.
| Reviewing dengue: Still a neglected tropical disease?Crossref | GoogleScholarGoogle Scholar |
[10] World Health Organization (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. WHO Press.
[11] Muller, D.A. et al. (2017) Clinical and laboratory diagnosis of dengue virus infection. J. Infect. Dis. 215, S89–S95.
| Clinical and laboratory diagnosis of dengue virus infection.Crossref | GoogleScholarGoogle Scholar |
[12] Australian Government, Department of Health (2016) Flavivirus laboratory case definition. http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-phlncd-flavivirus.htm (accessed 28 August 2017).
[13] Najioullah, F. et al. (2014) Evaluation of four commercial real-time RT-PCR kits for the detection of dengue viruses in clinical samples. Virol. J. 11, 164.
| Evaluation of four commercial real-time RT-PCR kits for the detection of dengue viruses in clinical samples.Crossref | GoogleScholarGoogle Scholar |
[14] Ahmed, N.H. and Broor, S. (2014) Comparison of NS1 antigen detection ELISA, real time RT-PCR and virus isolation for rapid diagnosis of dengue infection in acute phase. J. Vector Borne Dis. 51, 194–199.
[15] Hermann, L.L. et al. (2014) Evaluation of a dengue NS1 antigen detection assay sensitivity and specificity for the diagnosis of acute dengue virus infection. PLoS Negl. Trop. Dis. 8, e3193.
| Evaluation of a dengue NS1 antigen detection assay sensitivity and specificity for the diagnosis of acute dengue virus infection.Crossref | GoogleScholarGoogle Scholar |
[16] Simmons, C.P. et al. (2012) Dengue. N. Engl. J. Med. 366, 1423–1432.
| Dengue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlslOrsbw%3D&md5=1776c6f38b36e398e68596511761e583CAS |
[17] Wang, S.M. and Sekaran, S.D. (2010) Evaluation of a commercial SD dengue viris NS1 antigen capture enzyme-linked immunosorbent assay kit for early diagnosis of dengue virus infection. J. Clin. Microbiol. 48, 2793–2797.
| Evaluation of a commercial SD dengue viris NS1 antigen capture enzyme-linked immunosorbent assay kit for early diagnosis of dengue virus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1Omu7vM&md5=27c97369540686f110d0cf57ff3f7682CAS |
[18] NSW Health (2015) Dengue control guideline. http://www.health.nsw.gov.au/Infectious/controlguideline/Pages/dengue.aspx (accessed 28 August 2017).
[19] Sydney Morning Herald (2017) Dengue fever cases hit 20-year high in Australia. http://www.smh.com.au/national/health/dengue-fever-cases-hit-20year-high-in-australia-20170112-gtqq5b.html (accessed September 2017).
[20] Queensland Health (2015) Queensland Dengue management plan 2015–2020.
[21] Eliminate Dengue Australia (2017) Eliminate Dengue. http://www.eliminatedengue.com/australia (accessed August 2017).
[22] WHO (2017) Immunization, vaccines and biologicals. http://www.who.int/immunization/research/development/dengue_vaccines/en/ (accessed August 2017).
[23] Sanofi Pasteur (2017) Dengue vaccine registered in 18 countries. http://dengue.info/dengue-vaccine-registered-in-18-countries/ (accessed September 2017).
[24] Malisheni, M. et al. (2017) Clinical efficacy, safety, and immunogenicity of a live attenuated tetravalent dengue vaccine (CYD-TDV) in children: a systematic review with meta-analysis. Front. Immunol. 8, 863.
| Clinical efficacy, safety, and immunogenicity of a live attenuated tetravalent dengue vaccine (CYD-TDV) in children: a systematic review with meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[25] Sáez-Llorens, X. et al. (2017) Safety and immunogenicity of one versus two doses of Takeda’s tetravalent dengue vaccine in children in Asia and Latin America: interim result from a phase 2, randomized, placebo-controlled study. Lancet Infect. Dis. 17, 615–625.
| Safety and immunogenicity of one versus two doses of Takeda’s tetravalent dengue vaccine in children in Asia and Latin America: interim result from a phase 2, randomized, placebo-controlled study.Crossref | GoogleScholarGoogle Scholar |
[26] Gan, C.S. et al. (2017) Sofusbovir as treatment against dengue? Chem. Biol. Drug Des. , .
| Sofusbovir as treatment against dengue?Crossref | GoogleScholarGoogle Scholar |
Biographies
Trine Gulhom is a dual trainee in Microbiology and Infectious Diseases with a special interest in Tropical Diseases.
Professor William Rawlinson is a Medical Virologist and is Director of the Division of Serology and Virology (SAViD) and a NSW State Reference Laboratory in HIV, in the Department of Microbiology SEALS. He is a consultant position to the Department of Infectious Diseases, Prince of Wales and Sydney Children’s Hospital. He holds a conjoint academic position as Professor in the School of Medical Science and the School of Biotechnology and Biomolecular Sciences at The University of New South Wales, currently supervising PhD, Masters and Honours students. His major research interest is in human cytomegalovirus (CMV) infection of mothers and babies, particularly mechanisms of transplacental virus transmission. The research group that he heads studies congenital infections, enteroviruses, hepatitis viruses, respiratory viruses, novel antivirals and antiviral resistance.