Mammalian cell cultures
George LovreczBioManufacturing, CSIRO
343 Royal Parade
Parkville, Vic. 3052, Australia
Tel: +61 3 9662 7348, or +61 3 9545 7862 (Clayton)
Email: george.lovrecz@csiro.au
Microbiology Australia 38(2) 67-69 https://doi.org/10.1071/MA17030
Published: 22 March 2017
There is increasing demand worldwide for high-quality complex proteins for treating diseases and for clinical and pre-clinical studies involving recombinant proteins, vaccines, and monoclonal antibodies, many of which are the products of mammalian cell cultures. Biologics or protein-based drugs had a global market over US$200 billion in 2016, with eight of the top 10 selling drugs with a combined global sales over US$55 billion produced using mammalian cell cultures1,2. Recombinant proteins are also significant global economic drivers of both vaccine and biomarker development. In addition, it is estimated that more than half of the Australian biotechnology companies are utilising mammalian cell culture or their products3.
The general principles and methods to handle and genetically manipulate mammalian cells are closely related to microbial systems, for example scale-up, equipment and vector design, so a brief comparison of their attributes to traditional microbial systems is warranted.
Brief history
While mammalian cell culturing, often referred to as tissue culture, has over a 70-year history, it moved from exploratory research to a well-established technology only four decades ago. Advances in molecular and cell biology, combined with gene technology and bioengineering, transformed laboratory-scale mammalian cell cultures to an economical industrial process. A few examples to illustrate this transformation include the first large-scale suspension cultures in 1963, the production of monoclonal antibodies using hybridoma technology starting in 1975 and the industrial scale culture of genetically engineered cells from the early 1990s4. Currently mammalian cultures are offering almost limitless application from the manufacturing of various cell products (monoclonal antibodies, cytokines, signalling proteins, stem cells for cell therapy, engineered viruses) to pharmacology research (including toxicology, in vitro models, screening for new drugs and understanding their action).
General features of mammalian cell cultures
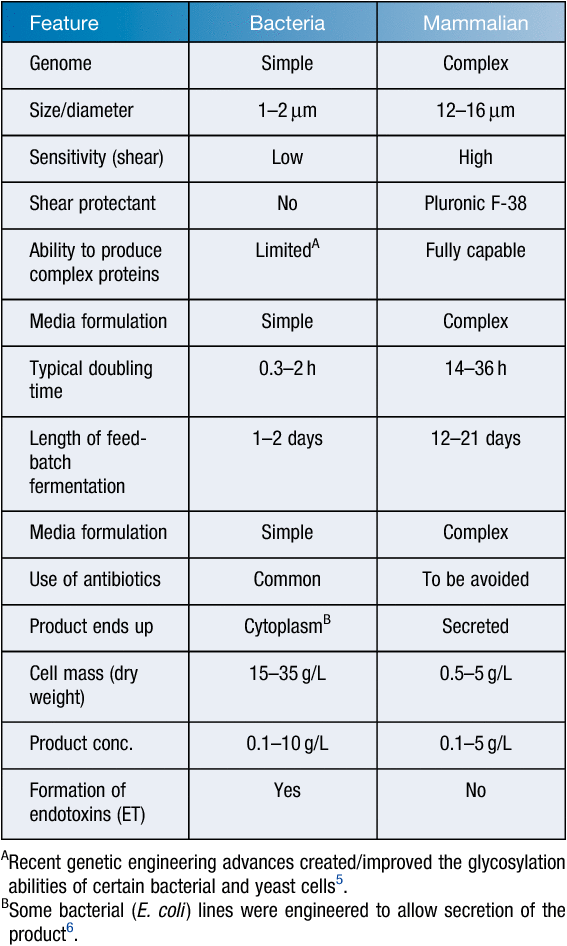
Traditionally large-scale mammalian cell cultures were difficult to handle (contamination issues, complex nutrients requirements and shear sensitivity and therefore the need for specialised equipment and processes resulting in high cost) especially when compared to microbial cultures (refer to Table 1 to compare mammalian and bacterial cells). This, combined with relatively slow growth (typical doubling times are in the range of 14–32 h) and the requirements for increased environmental control (cleanliness of workplace, reagents, etc.) makes them less attractive when one wants to establish an industrial process. However, their ability to produce complex recombinant glycoproteins identical to their wild-type counterpartsa makes them the number one choice over bacterial, yeast or insect cultures7,8.
|
Genetic engineering for mammalian cultures
While general molecular and cell biology has similar tools and methods available to create and manipulate mammalian cells when compared to microbial cells, the level of complexity is much higher and thus requires greater efforts. These days the complexities of mammalian gene regulation are well understood, which allows the assembly of sophisticated gene constructs that enable high levels of recombinant protein biosynthesis/expression following delivery into host cells9. Transient transfection refers to a process by which plasmid vectors carrying the gene of interest are introduced into mammalian cells, typically human embryonic kidney (HEK293) and Chinese hamster ovary (CHO) cells, and culture is continued for a relatively short period (9–11 days) to allow the recovery and characterisation of the encoded protein. Stable transfection refers to an approach where drug selectable markers, for example genes encoding the enzymes dihydrofolate reductase (DHFR) or glutamine synthetase (GS), are included on the plasmid vectors; this allows the small percentage of cells that take up and integrate plasmid DNA into a chromosome to be selected for by growth in the presence of toxic drugs, such as methotrexate in the case of DHFR. This approach allows for the isolation of stably transfected, clonal cell lines that produce high levels of target proteins. Furthermore, increasing the concentration of the selective drug in the cell culture can actually amplify the number of copies carried at a particular chromosomal location, resulting in increased productivity. This platform technology, using predominantly CHO cells and, to a lesser extent, the mouse myeloma cell lines NSO and Sp2/010, is the most commonly used approach for the industrial scale production of complex biologics for therapeutic use.
Scale-up requirements for mammalian cultures
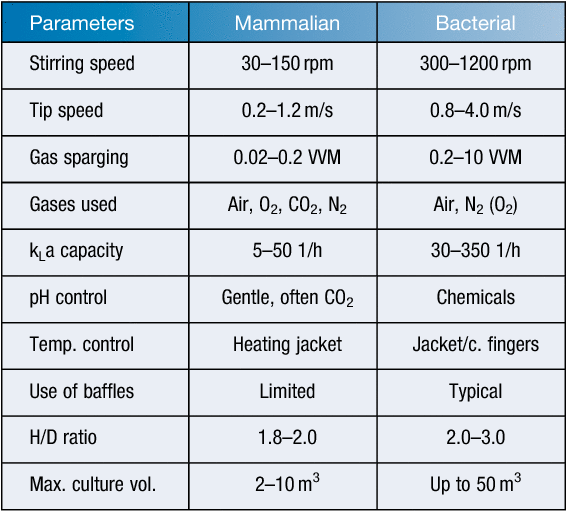
As mentioned above, mammalian cells are highly shear sensitive when compared to bacterial cells as they lack a robust cell wall and are covered by a relatively sensitive phospholipid bilayer membrane. This normally limits not only the operational parameters (stirring speed, gas sparging: oxygenation, pH control, vessel H/D ratio) for large-scale cultures but even the general handling of the cells, including scale-up and storage4. For example, long-term storage of mammalian cells requires liquid nitrogen tanks and optimised procedures including the correct choice of freeze-mix and controlled freezing methods11. Table 2 lists a number of typical operational parameters for consideration.
|
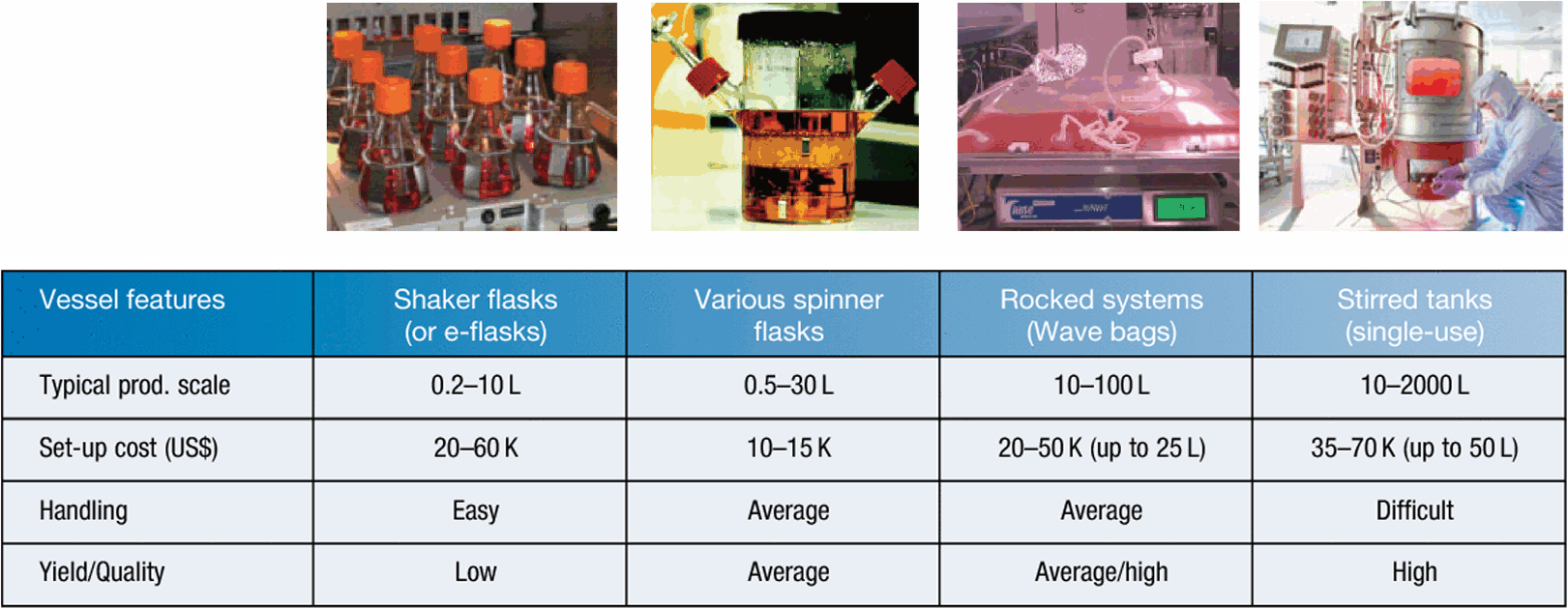
Traditionally scale-up of mammalian cell cultures started with Petri dishes, T-flasks, roller bottles and spinner flasks. Larger-scale (5L and above) cultures were run in glass or stainless-steel bioreactors4. The recent advances in bioprocessing techniques combined with the increased pressure to decrease cost of goods by creating multi-product facilities while minimising potential cross contaminations endorsed the use of single-use systems for both small and large-scale (normally up to 2000 L) cultures12. Table 3 lists the most common vessels used for the scale-up of mammalian cell cultures today. In Australia a number of vendors sell these systems, e.g. Thermo Fisher, Corning, Nalgene, Greiner, Eppendorf, Infors, Millipore, Pall, Sartorius, Applicon and GE Healthcare.
|
Future trends
Nowadays large-scale mammalian cell cultures are well established techniques for the production of biologics in the pharmaceutical industry. During the past two decades processes were streamlined and with the wide use of Process Analytical Techniques (PAT) and Quality By Design (QbD)8 principles, yields and consistency of the production runs were substantially increased. This, combined with the recent introduction of single-use systems, increased both production capacity and flexibility of the pharmaceutical companies. As a consequence the cost of goods were greatly reduced, for example typical monoclonal antibody production cost should be less than US$300/g13.
As governments introduce more cost-cutting measures in healthcare, pharma companies are under constant pressure to further reduce prices. This, combined with the expiry of several patents covering blockbuster therapeutic antibodies such as Humira™, Remicade™, Rituxin™ and Herceptin™, has accelerated the development of biosimilars (or follow-on biologics), which in turn will increase competition and the prospect of increased process optimisation for mammalian cell cultures14.
Acknowledgements
Many thanks to Tim Adams (CSIRO) for his general advice and correcting the Genetic Engineering section.
References
[1] EvaluatePharma® World Preview (2016) Outlook to 2022. 9th edition.[2] https://www.drugs.com/slideshow/looking-ahead-pharma-projections-for-2016-and-beyond-1230
[3] Australasian Biotechnology (2016) AusBioSTOCK. The Journal of AusBiotech 26, 86–90.
[4] Freshney, R.I. (2016) Culture of Animal Cells. 7th edition. Wiley Blackwell.
[5] Nielsen, J. et al. (2013) Production of biopharmaceutical proteins by yeast. Advances through metabolic engineering. Bioengineered 4, 207–211.
| Production of biopharmaceutical proteins by yeast. Advances through metabolic engineering.Crossref | GoogleScholarGoogle Scholar |
[6] Kotzsch, A. et al. (2011) A secretory system for bacterial production of high-profile protein targets. Protein Sci. 20, 597–609.
| A secretory system for bacterial production of high-profile protein targets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvFWnu7c%3D&md5=075fa272030d65596145b2d6822239bfCAS |
[7] Wurm, F.M. (2004) Review: production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 22, 1393–1398.
| Review: production of recombinant protein therapeutics in cultivated mammalian cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXptlSls7k%3D&md5=b58e21014be5dbd067cb7a97f02ecaa7CAS |
[8] Mammalian Cell Cultures for Biologics Manufacturing (2014) Advances in Biochemical Engineering/Biotechnology 139 (Zhou, W. and Kantardjieff, A. eds)
[9] Khan, K.H. (2013) Gene expression in mammalian cells and its applications. Adv. Pharm. Bull. 3, 257–263.
[10] Walsh, G. (2014) Biopharmaceutical benchmarks. Nat. Biotechnol. 32, 992–1000.
| Biopharmaceutical benchmarks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhslaqs7nP&md5=600467f2ed561354e6e50a2c33c0be5eCAS |
[11] Ryan, J. (2004) General Guide for Cryogenically Storing Animal Cell Cultures. Corning Life Sciences Technical Bulletin: https://www.corning.com/media/worldwide/cls/documents/t_cryoanimalcc.pdf
[12] Shukla, A.A. and Gottschalk, U. (2013) Single-use disposable technologies for biopharmaceutical manufacturing. Trends Biotechnol. 31, 147–154.
| Single-use disposable technologies for biopharmaceutical manufacturing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslWqtb7N&md5=f70d999cfbd2d5c6377998a316214cbbCAS |
[13] Kelley, B. (2009) Industrialization of mAB production technology: the bioprocessing industry at a crossroads. MAbs 1, 443–452.
| Industrialization of mAB production technology: the bioprocessing industry at a crossroads.Crossref | GoogleScholarGoogle Scholar |
[14] http://gabi-journal.net/
Biography
George Lovrecz is a Senior Principal Research Scientist at CSIRO BioManufacturing (Parkville and Clayton), involved in the scale-up and optimisation of mammalian and insect cell cultures. George’s team has extensive client engagement across the academic and commercial sectors to translate discoveries to industrial processes and to support pharmaceutical research to allow preclinical, animal and Phase-I trials. George is also engaged in training and education as an adjunct professor of RMIT and Monash University.
a Correct post-translational modifications including the formation of di-sulphide bridges for proper folding and glycosylation, are essential for biological activity.


