Application of bacteriophages
Expert Round Table Participants N , Rustam Aminov A , Jonathan Caplin B , Nina Chanishvili C , Aidan Coffey D , Ian Cooper E , Daniel De Vos F , Jiří Doškař G , Ville-Petri Friman H , İpek Kurtbӧke I , Roman Pantucek J , Jean-Paul Pirnay F , Grégory Resch K , Christine Rohde L , Wilbert Sybesma M and Johannes Wittmann LA School of Medicine and Dentistry, University of Aberdeen, UK
B School of Environment and Technology, University of Brighton, UK
C Eliava Institute of Bacteriophage, Microbiology and Virology, Tbilisi, Georgia
D Cork Institute of Technology, Department of Biological Sciences, Ireland
E School of Pharmacy and Biomolecular Sciences, University of Brighton, UK
F Laboratory for Molecular and Cellular Technology, Queen Astrid Military Hospital, Brussels, Belgium
G Masaryk University, Faculty of Science, Department of Experimental Biology, Brno, Czech Republic
H University of York, Department of Biology, UK
I University of the Sunshine Coast, GeneCology Research Centre and the Faculty of Science, Health, Education and Engineering, Qld, Australia
J Masaryk University, Faculty of Science, Department of Experimental Biology, Brno, Czech Republic
K University of Lausanne, Department of Fundamental Microbiology, Switzerland
L Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany
M Nestec Ltd – Nestlé Research Center, Lausanne, Switzerland
N Email: Phage_therapy@pha.ge
Microbiology Australia 38(2) 63-66 https://doi.org/10.1071/MA17029
Published: 11 April 2017
The emergence of antibiotic-resistant bacteria and decrease in the discovery rate of novel antibiotics takes mankind back to the ‘pre-antibiotic era’ and search for alternative treatments. Bacteriophages have been one of promising alternative agents which can be utilised for medicinal and biological control purposes in agriculture and related fields. The idea to treat bacterial infections with phages came out of the pioneering work of Félix d‘Hérelle but this was overshadowed by the success of antibiotics. Recent renewed interest in phage therapy is dictated by its advantages most importantly by their specificity against the bacterial targets. This prevents complications such as antibiotic-induced dysbiosis and secondary infections. This article is compiled by the participants of the Expert Round Table conference ‘Bacteriophages as tools for therapy, prophylaxis and diagnostics’ (19–21 October 2015) at the Eliava Institute of Bacteriophage, Microbiology and Virology, Tbilisi, Georgia. The first paper from the Round Table was published in the Biotechnology Journal1. This In Focus article expands from this paper and includes recent developments reported since then by the Expert Round Table participants, including the implementation of the Nagoya Protocol for the applications of bacteriophages.
Antimicrobials are one of the most successful forms of therapy but their broad and often indiscriminate use resulted in a widespread antimicrobial resistance2. The annual death toll due to multidrug-resistant bacterial infections is estimated at 23 000 in the US and 25 000 in Europe3,4. Complementary strategies are urgently needed, and bacteriophage therapy offers:
-
Specificity, and target-directed removal of pathogens via narrow spectrum, which do not affect beneficial commensals;
-
Multiplication at infection sites, thus amplifying the local antimicrobial effects;
-
Minimum, if any, side-effects;
-
Resistance can be managed by introduction of new bacteriophages, which is faster and cheaper compared to new antibiotics;
-
Bacteriophages are active against multidrug-resistant and biofilm-forming bacteria;
-
Lytic bacteriophages may limit the evolution and spread of antimicrobial resistance5;
-
Bacteriophages act in synergy with antibiotics;
-
Phage CRISPR-Cas systems provide a new way to target antibiotic-resistant pathogens6.
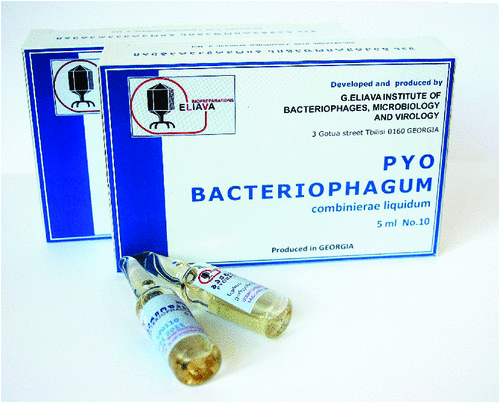
Bacteriophage therapy was pioneered at the Eliava Institute in Tbilisi, Georgia (Figure 1), and the reader is referred to the Historical Review article by Chanishvili and Sharp (2008)7 published in Microbiology Australia.
|
Therapeutic application of bacteriophages and resistance: the case of Phagoburn
Large burn wounds lead to immunosuppression, making burn patients susceptible to infections. Although medical advances have resulted in increased survival of burn victims, most deaths are due to the wound sepsis or sepsis secondary to pneumonia. Animal studies showed that bacteriophages could rescue mice and guinea pigs with infected burn wounds or bacteraemia. Ongoing studies conducted following standard clinical trial guidelines and practices by ‘PhagoBurn’ (www.phagoburn.eu) will contribute towards generation of clinical level information related to the applications of phages. This phase I/II multi-centric, randomised, controlled and single-blinded clinical trial involves 15 burn units in France, Switzerland and Belgium and targets burn wounds infected by Escherichia coli or Pseudomonas aeruginosa. Manufacturing the investigational products that compline Good Manufacturing Practices (GMP) took 20 months and encountered poly-infection issues hampered the recruitment of patients8. However, the Phagoburn study has established new phage manufacturing approach that will encourage regulators to review their policies related to phage therapy8.
Antagonistic bacterium-phage co-evolution is a dynamic process in which phage-resistant bacteria and infective bacteriophages are selected in turn. While emergence of bacteria resistant against challenging bacteriophages is a part of this coevolution, it could be problematic in therapy and it should be prevented. Interestingly, while phage-resistant P. aeruginosa were readily selected in vitro when challenged by the anti-P. aeruginosa phages used in Phagoburn, such selection was not observed in a rat model of experimental endocarditis9. Accordingly, two resistant variants recovered in vitro showed >70% and >40% decreased infectivity, explaining the failure to recover them from in vivo biopsies. These variants had lost lipopolysaccharide (LPS) and impaired pili, respectively, both structures being known as phage receptors10. This study illustrated that phage resistance can emerge at a very high cost in terms of virulence, possibly leading to in vivo survival for the bacterium. This observation, which is not new11, has clinical relevance and the phage resistance should be carefully evaluated in future clinical trials.
Bacteriophages for food hygiene and safety and environmental applications
Bacteriophages have been used since the 1980s to control and eliminate bacterial contaminants from food surfaces, food-borne spoilage bacteria and bacteria causing gastrointestinal diseases12 as well as to decontaminate raw food. Due to their specificity, bacteriophages are attractive for sanitisation of ready-to-eat foods (RTE) such as milk, vegetables and meat products13. In 2007, the US Department of Agriculture (USDA) approved bacteriophage products targeting Salmonella species and E. coli O157:H7. They are designed as spray sanitisers to disinfect cattle hides prior to slaughter to reduce pathogen contamination of meat14. In parallel, the commercial product Agriphage™ was developed to control black spot disease on tomato and pepper plants caused by Xanthomonas campestris and Pseudomonas syringae15.
Similarly, bacteriophages are also potentially useful as surface and environment decontaminants. Listeria phages (3.5 × 108 PFU/mL), for instance, were as effective as a 20-ppm solution of a quaternary ammonium compound (QAC) disinfectant for stainless steel decontamination. Interestingly, synergism between different bacteriophages and phages-QAC was reported with bacteriophages being unaffected by QAC at 50 ppm and up to 4 hours of contact time16.
Agricultural applications of bacteriophages
Bacteriophage effects on target pathogens depend on the ecological and environmental context such as abiotic environmental factors or surrounding microbial community. For example, phage-mediated killing of pathogenic bacteria can be amplified in the presence of non-pathogenic bacteria that impose strong resource competition with the pathogen. More recently, it was shown that the presence of antimicrobial producing Bacillus amyloliquefaciens could shape the effect of bacteriophage selection on the plant pathogen Ralstonia solanacearum17. In this case, the effect was driven by evolutionary trade-off where evolving resistance to a phage led to increased susceptibility to antimicrobials produced by B. amyloliquefaciens. Similar evolutionary trade-offs can also lead to lowered expression of multiple important R. solanacearum virulence factors and reduced virulence in tomato in vivo18. Identifying bacteriophages that impair pathogen virulence by binding to various surface structures (flagella, pili and LPS), could be important for selecting therapeutic bacteriophages19.
When applied topically or orally to animals, bacteriophages will eventually become associated with the skin and wool/hair of animals. Thus, bacteriophages specific for animal pathogens could be isolated from wool20. These bacteriophages can reduce the number of bacteria associated with ’clumping’, and thus represent an option for agricultural practices as opposed to antibiotics. Similarly, bacteriophages have been recovered from the skin of healthy humans21 or when they were successfully incorporated into fibers used for human clothing22.
Current hurdles and regulatory status of bacteriophages
Bacteriophages are not currently classified in medicinal legislation, since they are neither living nor chemical agents. Therefore, it is complicated to regulate and perform clinical trials and commercialisation23. To ensure the efficiency of phage preparations, their effectiveness and host range towards currently circulating pathogenic strains must be monitored. This might explain why the phage preparations approved in the Russian Federation and Georgia are not static but are continuously updated to target newly emerging pathogenic strains24. Legislation to allow these updates is necessary to circumvent repeated registration procedures.
On 5 July 2016, the Belgian Minister of Social Affairs and Public Health has formally acknowledged that it is difficult to define the status of therapeutic phage preparations: should they be considered as industrially-prepared medicinal products (subjected to constraints related to marketing authorisation) or as magistral preparations (prepared in pharmacies’ officina)25. Magistral preparations (compounded prescription drug products in the US) are made by a pharmacist from the constituent ingredients to meet specific patient needs. On 26 October 2016, it was formally agreed that natural bacteriophages and their products, which are not fully compliant with the European Directive requirements for medicinal products for human use and for which there is no monograph in an official pharmacopoeia, can be processed by a pharmacist as raw materials (active ingredients) in magistral preparations, providing compliance to several logical provisions.
Bacteriophage application in the Access and Benefit Sharing (ABS) context: the Nagoya Protocol
To combat antibiotic resistances, there is urgent need to build up large phage collections against the pathogens like ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa and Enterobacteriaceae). However, culture collections holding and offering quality-checked authenticated bacteriophages in the sense of phage banks are confronted with two constraints. First, there are no requirements for authors by journals to deposit bacteriophages with public repositories before publishing, which differs from agreed procedures for their bacterial hosts26. The second issue that should be considered is the current development of rules for legal handling of bioresources that of course includes the bacteriophages. On 12 October 2014, the Nagoya Protocol https://www.cbd.int/abs/ has been implemented in several countries that ratified the Convention on Biological Diversity (CBD) https://www.cbd.int/. These laws deal with sampling, the accession and distribution of all genetic resources including microorganisms regarding the ABS. One of the reasons for the ratification of the protocol is protecting biodiversity under national sovereignty to prevent ‘biopiracy’ and to restrict access. All microbiologists who are sampling or distributing bioresources must be aware of these restrictions and should refer to their respective national regulations. National regulations might differ in each country and failure to comply with might result in legal consequences. For further information please see the DSMZ website at https://www.dsmz.de/deposit/nagoya-protocol.html.
Conclusions and future perspectives
As already stated by Skurnik and Strauch (2006) a decade ago27, the therapeutic use of bacteriophages, possibly combined with antibiotics, is a promising therapy option. Safe and controlled use of bacteriophage therapy will however, require as detailed information as possible on the properties and behaviour of specific phage-bacterium systems, in vitro and especially in vivo. Susceptibility of bacterial pathogens in vivo to bacteriophages is still not completely understood and requires dedicated (pre-)clinical research on more phage-bacterium systems. The requirements for quality and safety in bacteriophage production and application have been defined and communicated28–30.
Natural resources will need to be utilised further to isolate many more bacteriophages to build-up large phage collections to fight the antibiotic crisis. These efforts will then be translated into cooperation across borders and continents that will be regulated by the Nagoya Protocol to some extent. Therefore, facilitative regulations governing therapeutic use of bacteriophages should be implemented to counter antibiotic resistance on a global scale. Bacteriophage application obviously have significant potential to bridge human and veterinary medicine and bring effective solutions to antibiotic resistance problems as pointed out in this article.
References
[1] Expert Round Table on Acceptance and Re-implementation of Bacteriophage Therapy (2016) Silk route to the acceptance and re-implementation of bacteriophage therapy. Biotechnology J. 11, 595–600.[2] Aminov, R.I. (2010) A brief history of the antibiotic era: lessons learned and challenges for the future. Front. Microbiol. 1, 134.
| A brief history of the antibiotic era: lessons learned and challenges for the future.Crossref | GoogleScholarGoogle Scholar |
[3] EMA (2015) Antimicrobial resistance. http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general/general_content_000439.jsp
[4] CDC Report (2013) Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf
[5] Zhang, Q.G. and Buckling, A. (2012) Phages limit the evolution of bacterial antibiotic resistance in experimental microcosms. Evol. Appl. 5, 575–582.
| Phages limit the evolution of bacterial antibiotic resistance in experimental microcosms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVGqsrjJ&md5=60d8cfadc6f2b4e90451e0d33574338cCAS |
[6] Yosef, I. et al. (2015) Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria. Proc. Natl. Acad. Sci. USA 112, 7267–7272.
| Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXosVKruro%3D&md5=58e760fc2a470e40a117026c516f67cbCAS |
[7] Chanishvili, N. and Sharp, R. (2008) Bacteriophage therapy: experience from the Eliava Institute, Georgia. Microbiol. Aust. 29, 96–101.
[8] Servick, K. (2016) DRUG DEVELOPMENT. Beleaguered phage therapy trial presses on. Science 352, 1506.
| DRUG DEVELOPMENT. Beleaguered phage therapy trial presses on.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtV2rtrfJ&md5=0ab197dfa5b70392641e96e05977e67cCAS |
[9] Oechslin, F. et al. (2016) Synergistic interaction between phage therapy and antibiotics clears Pseudomonas aeruginosa infection in endocarditis and reduces virulence. J. Infect. Dis. , jiw632.
| Synergistic interaction between phage therapy and antibiotics clears Pseudomonas aeruginosa infection in endocarditis and reduces virulence.Crossref | GoogleScholarGoogle Scholar |
[10] Bertozzi Silva, J. et al. (2016) Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 363, fnw002.
| Host receptors for bacteriophage adsorption.Crossref | GoogleScholarGoogle Scholar |
[11] León, M. and Bastias, R. (2015) Virulence reduction in bacteriophage resistant bacteria. Front. Microbiol. 6, 343.
| Virulence reduction in bacteriophage resistant bacteria.Crossref | GoogleScholarGoogle Scholar |
[12] García, P.B. et al. (2008) Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 47, 479–485.
| Bacteriophages and their application in food safety.Crossref | GoogleScholarGoogle Scholar |
[13] Endersen, L. et al. (2014) Phage therapy in food industry. Annu. Rev. Food Sci. Technol. 5, 327–349.
| Phage therapy in food industry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXpt1Slsbo%3D&md5=4f0f609b666a92ed53b21c213a3d4c26CAS |
[14] Goodridge, L.D. and Abedon, S.T. (2008) Bacteriophage biocontrol: the technology matures. Microbiol. Aust. 29, 48–49.
[15] Monk, A.B. et al. (2010) Bacteriophage applications: where are we now? Lett. Appl. Microbiol. 51, 363–369.
| Bacteriophage applications: where are we now?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cfisVersA%3D%3D&md5=1d3aa42b44dd3bf0540b7a299e40a3aaCAS |
[16] Roy, B. et al. (1993) Biological inactivation of adhering Listeria monocytogenes by listeria bacteriophages and a quaternary ammonium compound. Appl. Environ. Microbiol. 59, 2914–2917.
| 1:CAS:528:DyaK3sXms1Kgsr8%3D&md5=ef13dab8ef5ac45d3d8c9a430fed02fdCAS |
[17] Wang, X. et al. (2017) Parasites and competitors suppress bacterial pathogen synergistically due to evolutionary trade‐offs. Evolution 71, 733–746.
| Parasites and competitors suppress bacterial pathogen synergistically due to evolutionary trade‐offs.Crossref | GoogleScholarGoogle Scholar |
[18] Addy, H.S. et al. (2012) Loss of virulence of the phytopathogen Ralstonia solanacearum through infection by φRSM filamentous bacteriophages. Phytopathology 102, 469–477.
| Loss of virulence of the phytopathogen Ralstonia solanacearum through infection by φRSM filamentous bacteriophages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xos1Sktrc%3D&md5=59872ad8a60af1b498dc439d5b29848bCAS |
[19] Buttimer, C. et al. (2017) Bacteriophages and bacterial plant diseases. Front. Microbiol. 8, 34.
| Bacteriophages and bacterial plant diseases.Crossref | GoogleScholarGoogle Scholar |
[20] Patten, K. M. et al. (1995) Isolation of Dermatophilus congolensis phage from the ‘lumpy wool’ of sheep in Western Australia. Lett. Appl. Microbiol. 20, 199–203.
| Isolation of Dermatophilus congolensis phage from the ‘lumpy wool’ of sheep in Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M3ksVSrsA%3D%3D&md5=616870f6033ec6e24ee88674785970e2CAS |
[21] Foulongne, V. et al. (2012) Human skin microbiota: high diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS One 7, e38499.
| Human skin microbiota: high diversity of DNA viruses identified on the human skin by high throughput sequencing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XptlSnt7o%3D&md5=190d8cf92119a925ac60d7f8a5521aa0CAS |
[22] Mao, J. (2009) Genetically engineered phage fibers and coatings for antibacterial applications. MSc Thesis, Massachusetts Institute of Technology, USA.
[23] Fauconnier, A. (2017) Regulating phage therapy: the biological master file concept could help to overcome regulatory challenge of personalized medicines. EMBO Rep. 18, 198–200.
| Regulating phage therapy: the biological master file concept could help to overcome regulatory challenge of personalized medicines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXotlOmsA%3D%3D&md5=7d4ea35252aa4f041a8c9376e4c0d29dCAS |
[24] Kutter, E. et al. (2010) Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 11, 69–86.
| Phage therapy in clinical practice: treatment of human infections.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXkt1ems7k%3D&md5=4ffa5fddd9d9577ce3743fd202a5c742CAS |
[25] Commission de la santé publique, de l’environnement et du renouveau de la société (2016) Questions jointes de Mme Muriel Gerkens et M. Philippe Blanchart à la ministre des Affaires sociales et de la Santé publique sur ‘la phagothérapie’ à la ministre des Affaires sociales et de la Santé publique’ (N 11955 and N 12911). https://www.dekamer.be/doc/CCRA/pdf/54/ac464.pdf
[26] Murray, R.G.E. (1996) Taxonomic note: a rule about the deposition of type strains. Int. J. Syst. Bacteriol. 46, 831.
| Taxonomic note: a rule about the deposition of type strains.Crossref | GoogleScholarGoogle Scholar |
[27] Skurnik, M. and Strauch, E. (2006) Phage therapy: facts and fiction. Int. J. Med. Microbiol. 296, 5–14.
| Phage therapy: facts and fiction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjvV2ktro%3D&md5=dd1a6d58abe9b1003e143379c821f78bCAS |
[28] Pirnay, J.-P. et al. (2015) Quality and safety requirements for sustainable phage therapy products. Pharm. Res. 32, 2173–2179.
| Quality and safety requirements for sustainable phage therapy products.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXnvVSmtw%3D%3D&md5=205158929be1104c98e8591226e9d2c8CAS |
[29] Verbeken, G. et al. (2014) Call for a dedicated European legal framework for bacteriophage therapy. Arch. Immunol. Ther. Exp. (Warsz.) 62, 117–129.
| Call for a dedicated European legal framework for bacteriophage therapy.Crossref | GoogleScholarGoogle Scholar |
[30] Fauconnier, A. (2017) Regulating phage therapy. Sci. Soc. 18, .
| Regulating phage therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXotlOmsA%3D%3D&md5=7d4ea35252aa4f041a8c9376e4c0d29dCAS |


