Assessing enteric helminths in refugees, asylum seekers and new migrants
Sarah Hanieh A D , Norbert Ryan B and Beverley-Ann Biggs B CA Victorian Infectious Diseases Reference Laboratory, Peter Doherty Institute for Immunity and Infection, Parkville, Vic. 3050, Australia
B Department of Medicine, The University of Melbourne, Peter Doherty Institute for Immunity and Infection, Parkville, Vic. 3050, Australia
C The Victorian Infectious Diseases Service, Royal Melbourne Hospital, Parkville, Vic. 3050, Australia
D Corresponding author. Department of Medicine, The University of Melbourne, Doherty Institute, Parkville, Vic. 3050, Australia, Tel: +61 3 8344 3257, Fax: +61 3 9347 1863, Email: shanieh@unimelb.edu.au
Microbiology Australia 37(1) 15-19 https://doi.org/10.1071/MA16006
Published: 29 February 2016
Currently there are 59.5 million people forcibly displaced worldwide as a result of conflict, human rights violations, generalised violence or persecution. Of these, 19.5 million are refugees and 1.8 million are asylum seekers. Each year Australia accepts 13 750 refugees through the offshore Humanitarian program, and in 2016 that number will almost double with the addition of 12 000 refugees from Syria and Iraq. Many refugees have complex medical needs and have reached Australia after a difficult journey, often involving time in refugee camps and exposure to traumatic events including physical hardship and illness. Refugees often come from parts of the world where parasitic and tropical infectious diseases are prevalent and untreated. This article provides a review of enteric helminth infections in refugees, including asylum seekers and those from a refugee-like background.
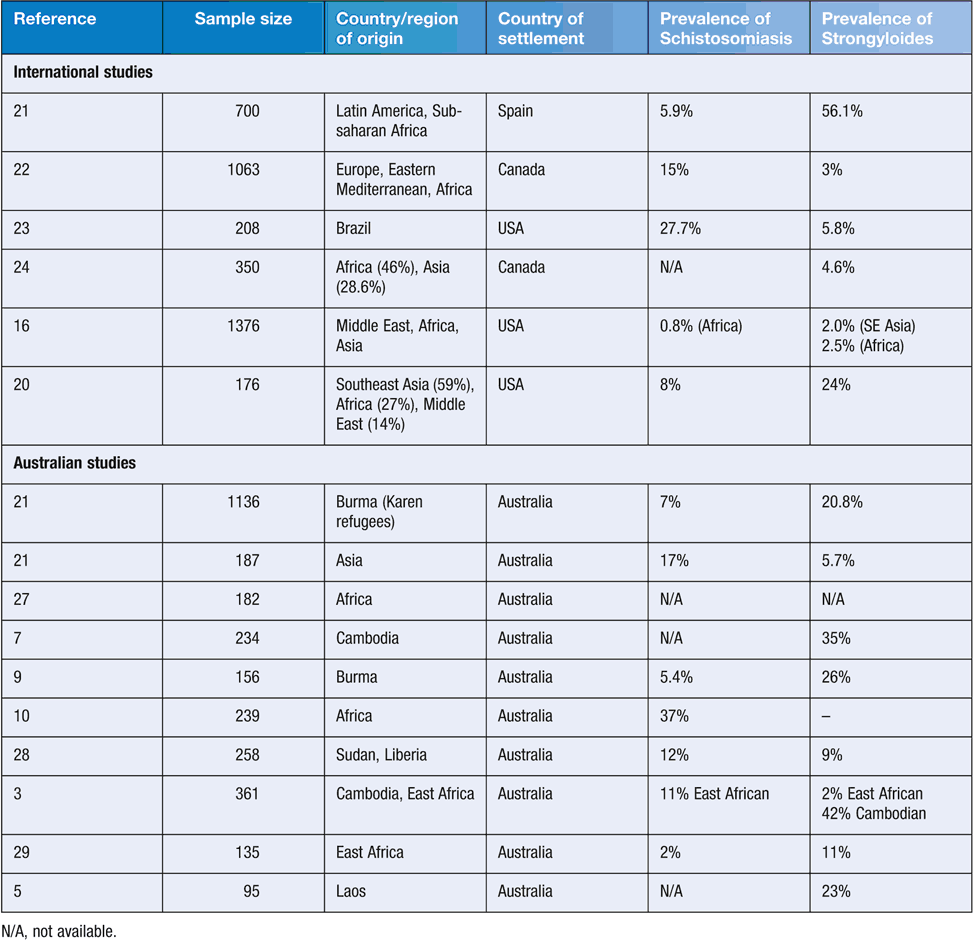
Parasitic infections in refugees and new migrants reflect the underlying epidemiology of parasites in areas where refugees may have been exposed, including the country of origin, migration journey to Australia, and place of detention. Factors such as poverty, disruption of basic services, poor sanitation/hygiene (e.g. quality of drinking water, access to running water, access to footwear) and insufficient access to adequate health care and treatment may significantly increase the risk of exposure to intestinal parasitic disease in the refugee population1. Other practices such as the use of night-soil as fertiliser, dietary habits, and past occupational exposures are likely to play an important role in increasing the burden of disease. Soil transmitted helminth (STH) infections are very common in those living in resource constrained settings1. Treatment of refugees and asylum seekers is often empirical, in refugee camps, before departure as part of the pre-departure health check, or after arrival in Australia. More serious infections, such as strongyloidiasis, schistosomiasis, opisthorchus and Taenia solium, require diagnosis and specific treatment. Table 1 summarises the findings of recent prevalence studies of strongyloidisis and schistosomiasis in refugee groups from Australia and overseas.
|
Strongyloidiasis
The highest prevalence of Strongyloides stercoralis infection occurs in refugees from Africa and South-East Asia2. Of those arriving in the last decade, the Burmese groups (e.g. Karen, Chin) have the highest prevalence (26.0%). Earlier data show an even higher prevalence in Lao and Cambodian refugees3–5. Infections may persist for decades after leaving an endemic area due to a continual cycle of auto-infection. Although many patients are asymptomatic or have minimal clinical symptoms, they remain at risk for subsequent hyperinfection if immunosuppressed6. Strongyloides antibodies decline after effective treatment7.
Schistosomiasis
The highest prevalence of schistosomiasis is found in Africa, accounting for an estimated 95% of global cases. Species involved are S. mansoni, S. haematobium and S. intercalatum. S. japonicum is present along the Yangtze River in China and the Philippines. S. mekongi is found in the Mekong river valley. Schistosoma infection may also be encountered in other areas such as parts of Indonesia, the Caribbean, the Arabian Peninsula, Madagascar, the Middle east and Turkey8. Prevalence of schistosomiasis infection in refugees in Australia, as determined by serology, has been shown to range from 5.4% in Burmese9 to 37% in Africans10.
Schistosoma antibodies are thought to persist in those from endemic areas despite prior treatment. A long-term study of schistosomiasis serology post-treatment showed an immediate increase in titre and then a fourfold decline in most travellers after 6–12 months. However, for immigrants, serology remained elevated even three years after effective treatment in a proportion of patients11.
Soil-transmitted helminths (STH)
Many refugees will have received empirical albendazole as part of the predeparture medical assessment conducted by the International Organisation of Migration on behalf of the Australian Government. This has significantly altered the prevalence and patterns of intestinal helminths in refugees12. However, albendazole has limited effectiveness against Trichuris trichiura, and is not an effective treatment for some less common helminths found in refugees and asylum seekers (see below).
Less common helminth infections
The majority of Asian refugees currently entering Australia are from Myanmar, from camps on the Thai border. Within Thailand, especially the north east, Opisthorchis viverrini is highly prevalent. Infection results from consumption of raw, uncooked or fermented fish containing metacerciae. Long-term infection may cause cholangitis, obstructive jaundice, cholecystitis, periductal fibrosis and bile duct cancer, contributing to a liver cancer rate in excess of 70 per 100 000 in NE Thailand. There is a paucity of data on faecal microscopy findings for refugees from Myanmar. However, the presence of vector cyprinoid fish and substantial wetlands suggests that infection with Opisthorchis viverrini is also likely.
Other serious infections in refugees from Asia include Taenia solium (pork tapeworm) with the potential risk of cysticercosis for both patient and household members. In Thailand faecal tests for helminth eggs have revealed a prevalence of Taenia spp of 2.3–3.7% in communities with high migrant populations along the Thai border. In the remote western border area of Kanchanaburi three species of tapeworm T. saginata, T. solium and T. asiatica co-exist in the human population13.
Considerations in the Syrian refugee population
In September 2015, it was announced that 12 000 Syrian and Iraqi refugees would be accepted to Australia as part of the Humanitarian Program during 2016–17. The recent prevalence of enteric parasite infections is not well documented in Syria. However, schistosomiasis is considered to have low endemnicity in Iraq (0.1%) and Syria (<10% prevalence in 2010)14. There is no information available on the prevalence of S. stercoralis in Syria; however, a hospital-based survey in Iraq reported a prevalence of 24.2%15. Chang et al. reported a low prevalence of other parasitic infections in refugees from Iran and Iraq16.
Diagnosis
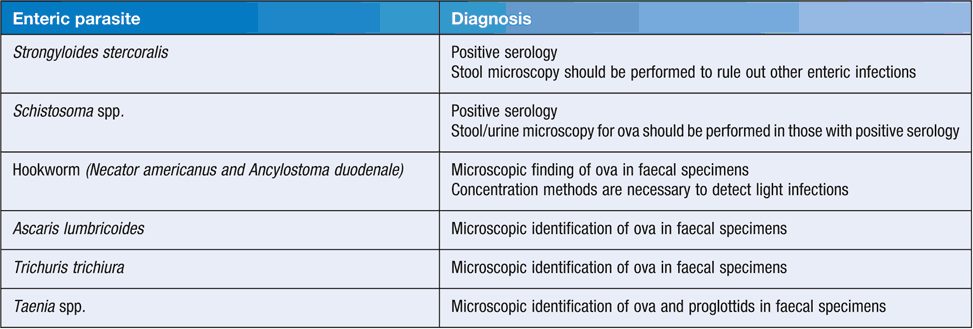
In Australia, the number of faecal microscopy tests performed has fallen in some States (e.g. NSW), with more emphasis being placed on empirical treatment of STH and the use of serology for the diagnosis of S. stercoralis or Schistosoma spp. Current recommended diagnostic tests for enteric parasites are shown in Table 2.
|
Challenges and limitations of testing for schistosoma and strongyloides infections in a refugee population
Serology may overestimate the prevalence of disease due to cross-reactivity with other nematode infections and there is difficulty distinguishing recent from past (and cured) infections. Serological titres (OD values) in the equivocal and low positive ranges are difficult to interpret. Follow-up serology should preferably be done in the same laboratory and in parallel with previous specimens where available. The interpretation of Schistosoma serology in Australia presents several challenges as test methodology varies between laboratories. VIDRL in Melbourne, use the Fumouze indirect haemagglutination test (IHA). This is based on an antigen derived from adult worms of S. mansoni. Estimates in one comparison study show that the sensitivity of this test is 76.2% for Schistosoma mansoni, and slightly lower for S. haematobium, with a specificity of 99%17. However, in NSW at ICPMR, an ‘in house’ ELISA assay is the preferred assay used, based on S. mansoni egg antigen. Two commercial assays based on the use of a similar antigen showed 71.4–85.7% sensitivity but reduced specificity of 76.9–88.4%. Both ELISA assays showed cross-reactivity with cestode, nematode and trematode infections17. The sensitivity of these assays for other species of Schistosoma, such as S. haematobium, S. intercalatum, S. mekongi and S. japonicum, is not specified; however, it is likely to be reduced.
The sensitivity and specificity of Strongyloides stercoralis serology is reported to be up to 94.6% and 99.6% respectively, depending on the assay used18. However, as there is no gold standard test for comparison, these are estimations only. Serological titres decline with effective treatment over a 12 month period7,18.
Persistence of parasitic infections in refugee populations
Several studies have demonstrated that serious intestinal parasitic infections may persist for many years after arrival in Australia. A 2002 study of Laotian refugees who had arrived in Australia during 1974–91 showed that strongyloides serology was positive in 24% and 3 carried Opisthorchis, compared to respective prevalences of 19.2% and 41%, on initial screening by faecal microscopy4. As liver flukes survive for approximately 7–10 years, the three cases of Opisthorchis identified may well represent reinfection on subsequent visits to Laos.
In a second study of East African refugees, who arrived in Australia in the late 90s, screening for strongyloides and schistosoma serology was positive in 11% and 15%, respectively, of patients some 16 years later in 20063. In a further aspect of the same study, 42% of 234 Cambodians who had arrived in the late 80s still tested positive for strongyloides serology3. This compared with 7.8% of Cambodians who were found to be positive for strongyloides by microscopy at initial health screening4.
Post arrival health assessment for refugees and asylum seekers
Community Health Centres and GP services in suburbs with high rates of migrant and refugee settlement are now responsible for much of the post-arrival refugee health screening, with support from specialist Refugee Health Services. Guidelines for post-arrival assessment for people of refugee-like background were published by the Australian Society for Infectious Diseases in 2009 and are currently being revised19. If possible, a full health assessment of new arrivals is ideally conducted within one month of arrival in Australia, including serology for Strongyloides for all, and Schistosoma in those who have lived or travelled through an endemic area. For many of the clinics in suburbs with high migrant populations, the special pathology requirements are met by private pathology providers.
Summary
Intestinal helminth infections in refugees are common and should remain a high priority for health workers. These populations often have specific needs that should be considered in diagnosis and management of these infections. Burden of disease is likely to reflect the country of origin, journey of migration to Australia, pre-departure treatment and place of detention. Other socio-economic and cultural factors are also likely to play a significant role in exposure risk. Helminth infections may be chronic and persist in humans for more than four decades resulting in serious morbidity and mortality and highlighting the need for early diagnosis.
Acknowledgements
We thank Ms Christalla Hajisava for technical assistance.
References
[1] Freeman, M.C. et al. (2015) Associations between school- and household-level water, sanitation and hygiene conditions and soil-transmitted helminth infection among Kenyan school children. Parasit. Vectors 8, 412.| Associations between school- and household-level water, sanitation and hygiene conditions and soil-transmitted helminth infection among Kenyan school children.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC287gslSltQ%3D%3D&md5=8cb6e22008d5d00f438603e9579df185CAS | 26248869PubMed |
[2] Puthiyakunnon, S. et al. (2014) Strongyloidiasis--an insight into its global prevalence and management. PLoS Negl. Trop. Dis. 8, e3018.
| Strongyloidiasis--an insight into its global prevalence and management.Crossref | GoogleScholarGoogle Scholar | 25121962PubMed |
[3] Caruana, S.R. et al. (2006) Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J. Travel Med. 13, 233–239.
| Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia.Crossref | GoogleScholarGoogle Scholar | 16884406PubMed |
[4] Ryan, N. et al. (1988) Parasitic infections of refugees. Med. J. Aust. 148, 491–494.
| 1:STN:280:DyaL1c3hs1egtQ%3D%3D&md5=a3693ca7eb98a6ac8e3cbe6fe303b667CAS | 3367816PubMed |
[5] de Silva, S. et al. (2002) Chronic Strongyloides stercoralis infection in Laotian immigrants and refugees 7-20 years after resettlement in Australia. Epidemiol. Infect. 128, 439–444.
| Chronic Strongyloides stercoralis infection in Laotian immigrants and refugees 7-20 years after resettlement in Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38zmvVynsw%3D%3D&md5=ad20dea0bfd52c7e413723b56799c643CAS | 12113488PubMed |
[6] Olsen, A. et al. (2009) Strongyloidiasis--the most neglected of the neglected tropical diseases? Trans. R. Soc. Trop. Med. Hyg. 103, 967–972.
| Strongyloidiasis--the most neglected of the neglected tropical diseases?Crossref | GoogleScholarGoogle Scholar | 19328508PubMed |
[7] Biggs, B.A. et al. (2009) Management of chronic strongyloidiasis in immigrants and refugees: is serologic testing useful? Am. J. Trop. Med. Hyg. 80, 788–791.
| 19407125PubMed |
[8] Steinmann, P. et al. (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6, 411–425.
| Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk.Crossref | GoogleScholarGoogle Scholar | 16790382PubMed |
[9] Chaves, N.J. et al. (2009) Screening practices for infectious diseases among Burmese refugees in Australia. Emerg. Infect. Dis. 15, 1769–1772.
| Screening practices for infectious diseases among Burmese refugees in Australia.Crossref | GoogleScholarGoogle Scholar | 19891864PubMed |
[10] Sheikh, M. et al. (2009) The epidemiology of health conditions of newly arrived refugee children: a review of patients attending a specialist health clinic in Sydney. J. Paediatr. Child Health 45, 509–513.
| The epidemiology of health conditions of newly arrived refugee children: a review of patients attending a specialist health clinic in Sydney.Crossref | GoogleScholarGoogle Scholar | 19702607PubMed |
[11] Yong, M.K. et al. (2010) Long-term follow-up of schistosomiasis serology post-treatment in Australian travelers and immigrants. J. Travel Med. 17, 89–93.
| Long-term follow-up of schistosomiasis serology post-treatment in Australian travelers and immigrants.Crossref | GoogleScholarGoogle Scholar | 20412174PubMed |
[12] Swanson, S.J. et al. (2012) Albendazole therapy and enteric parasites in United States-bound refugees. N. Engl. J. Med. 366, 1498–1507.
| Albendazole therapy and enteric parasites in United States-bound refugees.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmtFejur8%3D&md5=5ec13b87226ab616ca1aff4c3b8e76bbCAS | 22512482PubMed |
[13] Anantaphruti, M.T. et al. (2010) Molecular and serological survey on taeniasis and cysticercosis in Kanchanaburi Province, Thailand. Parasitol. Int. 59, 326–330.
| Molecular and serological survey on taeniasis and cysticercosis in Kanchanaburi Province, Thailand.Crossref | GoogleScholarGoogle Scholar | 20380891PubMed |
[14] Hotez, P.J. et al. (2012) Neglected tropical diseases of the Middle East and North Africa: review of their prevalence, distribution, and opportunities for control. PLoS Negl. Trop. Dis. 6, e1475.
| Neglected tropical diseases of the Middle East and North Africa: review of their prevalence, distribution, and opportunities for control.Crossref | GoogleScholarGoogle Scholar | 22389729PubMed |
[15] Schär, F. et al. (2013) Strongyloides stercoralis: global distribution and risk factors. PLoS Negl. Trop. Dis. 7, e2288.
| Strongyloides stercoralis: global distribution and risk factors.Crossref | GoogleScholarGoogle Scholar | 23875033PubMed |
[16] Chang, A.H. et al. (2013) Decreasing intestinal parasites in recent Northern California refugees. Am. J. Trop. Med. Hyg. 88, 191–197.
| Decreasing intestinal parasites in recent Northern California refugees.Crossref | GoogleScholarGoogle Scholar | 23149583PubMed |
[17] Kinkel, H.F. et al. (2012) Evaluation of eight serological tests for diagnosis of imported schistosomiasis. Clin. Vaccine Immunol. 19, 948–953.
| Evaluation of eight serological tests for diagnosis of imported schistosomiasis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XotlWktr0%3D&md5=09384d8e90082a30c03b6e61a63b9035CAS | 22441394PubMed |
[18] Bisoffi, Z. et al. (2014) Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl. Trop. Dis. 8, e2640.
| Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection.Crossref | GoogleScholarGoogle Scholar | 24427320PubMed |
[19] Australian Society of Infectious Diseases (2009) Diagnosis, management and prevention of infections in recently arrived refugees Sydney, NSW: ASID.
Biographies
Dr Sarah Hanieh is a paediatric infectious diseases physician and NHMRC Early Career Research Fellow in the Immigrant and International Health Group, at the Peter Doherty Institute for Infection and Immunity, The University of Melbourne. She has interests in child nutrition, infectious diseases and refugee health. Her current research aims to understand the nutritional and infectious causes of impaired child growth and development in resource poor settings.
Norbert Ryan is a Senior Scientist in the Bacteriology Laboratory at Victorian Infectious Diseases Reference Laboratory, Doherty Institute, Melbourne. Areas of interest include parasitology, diagnosis of Legionnaires’ disease and sexually transmitted infections. The use of staining methods for diagnosis of protozoal infections including microsporidia has been a specialty field. He was involved in processing of specimens during the systematic health screening of refugee and family reunion migrant groups conducted by the Victorian Department of health during the late 80s-early 90s.
Professor Beverley-Ann Biggs heads the International and Immigrant Health Group in the Department of Medicine, The University of Melbourne and is an Infectious Diseases Physician in the Victorian Infectious Diseases Service at the Royal Melbourne Hospital. She has a special interest in parasitic and other infectious diseases in refugees and immigrants living in Australia, and has published extensively in this area.
* Joint first authors.


