Free living amoebae and human disease
Evan Bursle A and Jennifer Robson BA Sullivan Nicolaides Pathology
Whitmore Street
Taringa, Qld 4068, Australia
Email: evanbursle@gmail.com
B Sullivan Nicolaides Pathology
Whitmore Street
Taringa, Qld 4068, Australia
Email: jenny_robson@snp.com.au
Microbiology Australia 37(1) 20-24 https://doi.org/10.1071/MA16009
Published: 11 February 2016
Pathogenic FLA are ubiquitous protozoans and despite frequent human contact remain a rare cause of often devastating infection with poor prognosis. Given changes in climate, human encroachment into the environment, increasing immunosuppression, and improving diagnostic capacity, it is likely we will see increased cases in the future. Early diagnosis is challenging but crucial to achieving a favourable outcome. It is best facilitated by improved awareness of FLA disease, appropriate clinical suspicion and early diagnostic testing.
Free living amoebae (FLA) are a cosmopolitan group of protozoan organisms that do not require a host to survive. Despite their ubiquitous nature, these organisms are uncommon human pathogens. However, four genera contain species known to cause invasive disease in humans: Acanthamoeba, Naegleria, Balamuthia and Sappinia. Acanthamoeba infections may present as granulomatous amoebic encephalitis (GAE), disseminated disease (e.g. cutaneous, sinus or pulmonary infection) or keratitis, with Balamuthia mandrillaris causing similar cutaneous infections and GAE. Naegleria fowleri is responsible for the rapidly progressive primary amoebic meningoencephalitis (PAM). In addition, a single case of human central nervous system (CNS) infection with Sappinia pedata has been reported1, along with isolated cases of corneal infection with Vahlkampfia spp., Hartmannella spp. and Paravahlkamfia spp.2. This review aims to provide an overview of human disease caused by the three most common genera involved, Acanthamoeba, Naegleria and Balamuthia, including their laboratory diagnosis.
Epidemiology
Pathogenic FLA are found worldwide and serological studies suggest human exposure is common3–5. Three cases of Acanthamoeba GAE have been described in Australia6–8. Acanthamoeba sp tolerate a wide range of temperature, pH and osmolarity9 and may be found in air, soil and water samples. They are one of the most commonly isolated FLA in the environment10, and the most common in human infection2. The strongest risk factor for GAE or disseminated infection is immunodeficiency, while keratitis most commonly affects immunocompetent contact lens wearers who often have a history of poor lens hygiene. Exposure is thought to occur by inhalation, mucosal contact or direct inoculation10. Acanthamoeba species were previously classified based on morphology, but are now grouped into 17 genotypes based on 18S rRNA sequencing, with the majority of pathogenic species belonging to the T4 genotype9,11.
Naegleria fowleri may be found in rivers, lakes and soil but does not survive in sea water. As thermophiles, their presence in fresh water is related to temperature and they may even be recovered from thermally polluted waters at high latitudes12. Human exposure occurs through contact with intact or disrupted nasal mucosa, commonly through recreational or nasal ablution practices13 and contaminated drinking water has been implicated as a source of infection in some cases14. Australia has featured prominently in the history of Naegleria fowleri infection, with cases described in Queensland, New South Wales and Western Australia following the first description of the disease in 1965 by two South Australian pathologists, Fowler and Carter15,16. This discovery was related to an outbreak of 20 cases, attributed to a contaminated overland water pipeline which reached optimal temperatures for Naegleria proliferation during the summer months. Cases continued from 1947 to 1972 when public health measures including adequate chlorination were applied17. Several cases in Western Australia have also been associated with overland water pipelines, and Australian drinking water guidelines suggest a Naegleria monitoring and response protocol for water supplies that seasonally exceed 30°C, or 25°C continually18. Today, despite significant advancement in knowledge regarding risk reduction measures, the incidence of infection with Naegleria fowleri appears to be increasing worldwide, with factors such as global warming, substandard water management and sanitation, and changing recreational practices likely involved13.
Balmuthia mandrillaris is most commonly found in soil, though it may also be recovered from water samples4. Infection tends to occur in immunocompetent individuals, most commonly children, probably through inhalation or nasal or cutaneous inoculation. Transmission via organ transplantation has also been described19. Six human cases have been described in Australia20–24 (Western Australia, Tasmania, Victoria and Queensland), with another being described in a Victorian dog25.
Clinical manifestations
Acanthamoeba sp.
The predominant clinical manifestations of Acanthamoeba infection are disseminated disease (e.g. cutaneous, nasopharyngeal or pulmonary infection), GAE and keratitis. With the exception of rare case reports, GAE and disseminated disease occur in the immunocompromised or debilitated. The incubation period is unknown, but thought to be weeks to months in duration. Cutaneous infection usually begins as fibronodular lesions, which progress to non-healing ulcerated lesions over time10. While GAE may be fatal within days of symptom onset, it generally assumes a more chronic course, with slow progression over weeks to months. Clinical features of GAE are myriad and include fever, symptoms of meningism, personality and mental status change and, later, focal neurological deficits, coma and death. Imaging of the brain may show single or multiple space occupying lesions which can be ring enhancing.
Acanthamoeba keratitis is usually a disease of immunocompetent patients, with the strongest risk factor being contact lens use and poor lens hygiene. The disease is usually unilateral, with symptoms including lacrimation, pain, photophobia and foreign body sensation. Signs include a typical corneal ring infiltrate, stromal infiltrates, epitheliopathy and hypopyon26. In the absence of effective therapy, it may progress to corneal perforation and loss of vision. The clinical diagnosis of AK can be difficult, as lesions may resemble bacterial or fungal disease, or the dendritic ulcer of HSV infection26. Furthermore the clinical course may be characterised by periods of temporary remission, leading to false impressions of response to antibacterial or viral agents2.
Naegleria fowleri
Naegleria fowleri causes primary amoebic meningoencephalitis. Symptoms generally occur 2–5 days after exposure and may begin with changes in taste or smell, followed by fever, nausea, vomiting, photophobia and headache. The disease is fulminant, with rapid progression to coma and death.
Balamuthia mandrillaris
Balamuthia mandrillaris causes GAE in immunocompromised and immunocompetent individuals. The onset of meningoencephalitis is often subacute or chronic, with symptoms developing over a period of 2 weeks to 2 years27. It also has the propensity to cause cutaneous lesions that may precede CNS involvement and are similar in appearance to those of Acanthamoeba sp. These lesions appear as poorly defined plaques and may be single or with bordering satellite lesions4. They often involve the central face and appear to be more common in South America4. Cutaneous disease generally progresses to CNS involvement; however, it may resolve with therapy4.
Laboratory diagnosis
Diagnosis of FLA infection, particularly systemic disease, is challenging: it may masquerade as bacterial or viral infection, exposure events may not be apparent and specialised diagnostic testing availability is limited. Unfortunately as a result, many CNS infections are diagnosed post-mortem. Successful early diagnosis depends on appropriate clinical suspicion and collection of suitable diagnostic material, usually tissue or CSF.
Microscopy
CSF samples in cases of PAM appear purulent, with no bacteria evident on gram stain, a polymorphonuclear pleocytosis, elevated protein and decreased glucose. Wet preparations may show motile Naegleria fowleri trophozoites (Figure 1a), as there are usually large numbers of organisms in the CSF. These findings contrast with those of GAE caused by Acanthamoeba or Balamuthia. While CSF from cases of GAE also demonstrates elevated protein and lowered glucose, these changes are more modest, and a mononuclear, rather than polymorphonuclear inflammatory response is seen. Furthermore, Acanthamoeba or Balamuthia trophozoites are not typically seen in CSF preparations.

|
Trophozoites from all pathogenic FLA species can be difficult to differentiate from host inflammatory cells, especially in stained tissue sections. The nuclear characteristics of amoeba can be helpful in differentiating these parasites from host cells, with Naegleria fowleri possessing a nucleus with a large, round, central nucleolus and Acanthamoeba and Balamuthia (Figure 1b) a rounded nucleus with large, central nucleolus forming a halo. Polyclonal and monoclonal antibodies, with a secondary detecting fluorescent anti-IgG antibody (such as FITC), may be used to identify and differentiate each of these amoebae in tissue specimens28 (as can molecular methods), though availability is limited to the CDC, Atlanta, USA.
In Acanthamoeba keratitis, a diagnosis may be made by demonstrating trophozoites and/or cysts in corneal samples. It is possible to directly identify Acanthamoeba trophozoites within the cornea using confocal microscopy and in experienced hands this technique is sensitive and specific29. Trophozoites and cysts may be revealed by staining the smear with H&E or Giemsa, while cysts are also readily identified using PAS and fluorescent stains, such as calcofluor white and acridine orange30. On occasion, non-specific fluorescence or binding to fungi in mixed infections can lead to diagnostic errors31, especially when used by inexperienced microscopists.
Culture
Samples intended for amoebic culture should be kept at room temperature and processed as quickly as possible. Freezing should be avoided, particularly for samples where Naegleria is suspected (the cyst stage is more fragile), as this compromises organism viability. Naegleria fowleri and Acanthamoeba sp. (Figure 1c) can be readily cultured using non-nutrient media containing live or killed non-mucoid bacteria (usually E. coli or Enterobacter sp) as a food source32. Acanthamoeba and Naegleria will cover the agar surface in 1–2 days when incubated at 37°C and their presence can be confirmed by examination of the plate with a plate microscope, or by performing microscopy of a wet mount from the agar plate. Balamuthia do not appear to use bacteria as a food source and therefore cannot be cultivated in the same fashion32. They may be successfully cultured using axenic and tissue culture methods32, however with generation times of around 25 h, culture is a lengthy process and not part of routine diagnostic testing.
Nucleic acid testing
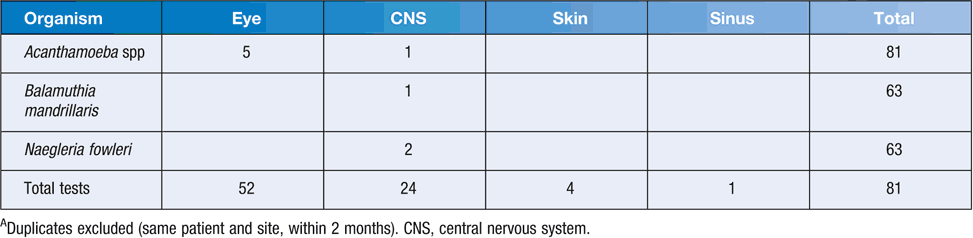
The use of molecular testing to diagnose and confirm infections with FLA has transformed diagnostics in this area. It allows more rapid diagnosis, with greater sensitivity than other methods and reduces the requirement for specialist, experienced staff to discern subtle microscopic features. Most molecular assays use ribosomal genes, such as the 18S rRNA or ITS repeat regions, as targets for PCR. However, as requests are infrequent, these tests are generally only offered by reference or research laboratories. Of particular note is a multiplex PCR, described by Qvarnstrom et al.33. This assay uses 18S rRNA primer/probe sets to accurately identify Acanthamoeba to the genus level and Naegleria fowleri and Balamuthia mandrillaris species. The sensitivity is reported at one amoeba per sample. Sullivan Nicolaides Pathology instituted this test in 2010 and our experience is summarised in Table 1. As a significant number of ocular specimens have been tested against all three targets, a large number of negatives for Naegleria fowleri and Balamuthia mandrillaris are expected. It is also notable that we have recently reported a probable false negative Acanthamoeba result. The referred CSF specimen tested negative at our laboratory, but positive at the CDC (using the same multiplex PCR). It is possible the small volume of CSF received at our laboratory contributed to this result (only 100 µL was received, where 200 µL is the standard volume for extraction).
|
Treatment and prognosis
Primary amoebic meningitis
There have been few survivors of primary amoebic meningitis and the factors that have resulted in successful therapy are poorly defined, though early diagnosis and institution of therapy appears critical. The treatment of choice is the antifungal amphotericin B, which is often used both intravenously and intrathecally. Other agents used in survivors include miltefosine, sulfisoxazole, fluconazole, miconazole and rifampicin.
Granulomatous amoebic encephalitis
Acanthamoeba
Suboptimal efficacy of antimicrobial agents, the high morbidity of patients affected and tendency to late diagnosis contribute to a poor prognosis in Acanthamoeba GAE, with a mortality rate of >90%34. In the few reported survivors of GAE or cutaneous infection, most have received combination therapy. The agents used have included trimethoprim-sulfamethoxazole, flucytosine, sulfadiazine, penicillin G, chloramphenicol, pentamidine, fluconazole and itraconazole. More recently, it appears that the inclusion of miltefosine in combination regimens may result in improved survival35.
Balamuthia
Early recognition of cutaneous disease is critical to allow early therapy and prevent progression to CNS infection. Unfortunately, the overall prognosis in Balamuthia CNS infection is extremely poor. However, there are several case reports of survival in the literature36, including an Australian case24. These cases have received varied combination therapy regimens, with agents including pentamidine, flucytosine, fluconazole, macrolides, sulfadiazine, miltefosine, thioridazine, amphotericin B, albendazole and trimethoprim-sulfamethoxazole. Miltefosine, in particular, has demonstrated amoebicidal activity in vitro37 and its inclusion in combination regimens may offer a survival advantage35.
Acanthamoeba keratitis
Like disseminated disease, success in treatment is dependent on early diagnosis and institution of therapy. Fortunately, success rates in treating Acanthamoebic keratitis are more promising, with cure rates in the literature generally greater than 75–85%38,39. Topical chlorhexidine and polyhexamethylenebiguanide (PHMB) are effective against trophozoites and cysts and form the mainstay of therapy. These are usually used in combination with diamidine, propamidine or hexamidine, though other agents including ketoconazole, itraconazole, voriconazole and topical imidazoles have been used. Surgical intervention, including enucleation, is sometimes required in severe cases40.
References
[1] Qvarnstrom, Y. et al. (2009) Molecular confirmation of Sappinia pedata as a causative agent of amoebic encephalitis. J. Infect. Dis. 199, 1139–1142.| Molecular confirmation of Sappinia pedata as a causative agent of amoebic encephalitis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXltVOms7w%3D&md5=3bd48f55663dd45d9624cca171020b39CAS | 19302010PubMed |
[2] Mandell, G.L. et al. (2015) Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Philadelphia, PA: Churchill Livingstone/Elsevier.
[3] Chappell, C.L. et al. (2001) Standardized method of measuring Acanthamoeba antibodies in sera from healthy human subjects. Clin. Diagn. Lab. Immunol. 8, 724–730.
| 1:CAS:528:DC%2BD3MXltlehsrk%3D&md5=46196aaa3eded85bf9175bd4ca3cd576CAS | 11427418PubMed |
[4] Bravo, F.G. and Seas, C. (2012) Balamuthia mandrillaris amoebic encephalitis: an emerging parasitic infection. Curr. Infect. Dis. Rep. 14, 391–396.
| Balamuthia mandrillaris amoebic encephalitis: an emerging parasitic infection.Crossref | GoogleScholarGoogle Scholar | 22729402PubMed |
[5] Marciano-Cabral, F. et al. (1987) Specificity of antibodies from human sera for Naegleria species. J. Clin. Microbiol. 25, 692–697.
| 1:CAS:528:DyaL2sXhslCgt70%3D&md5=37de77e5c5428a767a1a153311881ddcCAS | 2437151PubMed |
[6] Carter, R.F. et al. (1981) A fatal case of meningoencephalitis due to a free-living amoeba of uncertain identity–probably Acanthamoeba Sp. Pathology 13, 51–68.
| A fatal case of meningoencephalitis due to a free-living amoeba of uncertain identity–probably Acanthamoeba Sp.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M7nsVentw%3D%3D&md5=6fd0bf3794356b8bcb3228a30b405943CAS | 6261208PubMed |
[7] Harwood, C.R. et al. (1988) Isolation of Acanthamoeba from a cerebral abscess. Med. J. Aust. 148, 47.
| 1:STN:280:DyaL1c%2Fptl2lsg%3D%3D&md5=5cc379f9e14b83569f2c664848b92a32CAS | 3336301PubMed |
[8] Azzam, R. et al. (2015) Acanthamoeba encephalitis: isolation of genotype T1 in mycobacterial liquid culture medium. J. Clin. Microbiol. 53, 735–739.
| Acanthamoeba encephalitis: isolation of genotype T1 in mycobacterial liquid culture medium.Crossref | GoogleScholarGoogle Scholar | 25502534PubMed |
[9] Trabelsi, H. et al. (2012) Pathogenic free-living amoebae: epidemiology and clinical review. Pathol. Biol. (Paris) 60, 399–405.
| Pathogenic free-living amoebae: epidemiology and clinical review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38rmslCnsg%3D%3D&md5=9cb335aec83229377047961df064ff71CAS | 22520593PubMed |
[10] Marciano-Cabral, F. and Cabral, G. (2003) Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16, 273–307.
| Acanthamoeba spp. as agents of disease in humans.Crossref | GoogleScholarGoogle Scholar | 12692099PubMed |
[11] Siddiqui, R. and Khan, N.A. (2012) Biology and pathogenesis of Acanthamoeba. Parasit. Vectors 5, 6.
| Biology and pathogenesis of Acanthamoeba.Crossref | GoogleScholarGoogle Scholar | 22229971PubMed |
[12] Kemble, S.K. et al. (2012) Fatal Naegleria fowleri infection acquired in Minnesota: possible expanded range of a deadly thermophilic organism. Clin. Infect. Dis. 54, 805–809.
| Fatal Naegleria fowleri infection acquired in Minnesota: possible expanded range of a deadly thermophilic organism.Crossref | GoogleScholarGoogle Scholar | 22238170PubMed |
[13] Siddiqui, R. and Khan, N.A. (2014) Primary amoebic meningoencephalitis caused by Naegleria fowleri: an old enemy presenting new challenges. PLoS Negl. Trop. Dis. 8, e3017.
| Primary amoebic meningoencephalitis caused by Naegleria fowleri: an old enemy presenting new challenges.Crossref | GoogleScholarGoogle Scholar | 25121759PubMed |
[14] Cooter, R. (2002) The history of the discovery of primary amoebic meningoencephalitis. Aust. Fam. Physician 31, 399–400.
| 12043140PubMed |
[15] Fowler, M. and Carter, R.F. (1965) Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report. BMJ 2, 734–742.
| Acute pyogenic meningitis probably due to Acanthamoeba sp.: a preliminary report.Crossref | GoogleScholarGoogle Scholar |
[16] Carter, R.F. (1968) Primary amoebic meningo-encephalitis: clinical, pathological and epidemiological features of six fatal cases. J. Pathol. Bacteriol. 96, 1–25.
| Primary amoebic meningo-encephalitis: clinical, pathological and epidemiological features of six fatal cases.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF1czmtlOjtQ%3D%3D&md5=8f42c04805a3dfa8268c002b3960f8ebCAS | 5667848PubMed |
[17] Dorsch, M.M. et al. (1983) The epidemiology and control of primary amoebic meningoencephalitis with particular reference to South Australia. Trans. R. Soc. Trop. Med. Hyg. 77, 372–377.
| The epidemiology and control of primary amoebic meningoencephalitis with particular reference to South Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c%2FhvVKrsQ%3D%3D&md5=bdaa299998f7cf0162b58b88ee0754dbCAS | 6623596PubMed |
[18] National Health and Medical Research Council Australia (2011) Natural Resource Management Ministerial C. Australian drinking water guidelines 2011: national water quality management strategy. Canberra: National Health and Medical Research Council.
[19] Schlessinger, S. et al. (2010) Balamuthia mandrillaris transmitted through organ transplantation – Mississippi, 2009. Morbidity and Mortality Weekly Report 59, 1165–1170.
[20] Carter, R.F. et al. (1981) A fatal case of meningoencephalitis due to a free-living amoeba of uncertain identity–probably Acanthamoeba sp. Pathology 13, 51–68.
| A fatal case of meningoencephalitis due to a free-living amoeba of uncertain identity–probably Acanthamoeba sp.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3M7nsVentw%3D%3D&md5=6fd0bf3794356b8bcb3228a30b405943CAS | 6261208PubMed |
[21] Reed, R.P. et al. (1997) Fatal granulomatous amoebic encephalitis caused by Balamuthia mandrillaris. Med. J. Aust. 167, 82–84.
| 1:STN:280:DyaK2szpsFGmtw%3D%3D&md5=c04c4179ececb1c906fd9a9b1d827bf7CAS | 9251693PubMed |
[22] Hill, C.P. et al. (2011) Balamuthia amebic meningoencephalitis and mycotic aneurysms in an infant. Pediatr. Neurol. 45, 45–48.
| Balamuthia amebic meningoencephalitis and mycotic aneurysms in an infant.Crossref | GoogleScholarGoogle Scholar | 21723460PubMed |
[23] Doyle, J.S. et al. (2011) Balamuthia mandrillaris brain abscess successfully treated with complete surgical excision and prolonged combination antimicrobial therapy. J. Neurosurg. 114, 458–462.
| Balamuthia mandrillaris brain abscess successfully treated with complete surgical excision and prolonged combination antimicrobial therapy.Crossref | GoogleScholarGoogle Scholar | 21073255PubMed |
[24] Moriarty, P. et al. (2014) Balamuthia mandrillaris encephalitis: survival of a child with severe meningoencephalitis and review of the literature. J. Pediatric Infect. Dis. Soc. 3, e4–e9.
| Balamuthia mandrillaris encephalitis: survival of a child with severe meningoencephalitis and review of the literature.Crossref | GoogleScholarGoogle Scholar | 26624913PubMed |
[25] Finnin, P.J. et al. (2007) Multifocal Balamuthia mandrillaris infection in a dog in Australia. Parasitol. Res. 100, 423–426.
| Multifocal Balamuthia mandrillaris infection in a dog in Australia.Crossref | GoogleScholarGoogle Scholar | 17033842PubMed |
[26] Patel, D.V. and McGhee, C.N. (2009) Acanthamoeba keratitis: a comprehensive photographic reference of common and uncommon signs. Clin. Experiment. Ophthalmol. 37, 232–238.
| Acanthamoeba keratitis: a comprehensive photographic reference of common and uncommon signs.Crossref | GoogleScholarGoogle Scholar | 19723132PubMed |
[27] Visvesvara, G.S. et al. (2007) Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 50, 1–26.
| Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXmtVOhsb8%3D&md5=244349c111afc5e8f2750c7014c89954CAS | 17428307PubMed |
[28] da Rocha-Azevedo, B, Tanowitz, HB and Marciano-Cabral, F (2009) Diagnosis of infections caused by pathogenic free-living amoebae. Interdisciplinary perspectives on infectious diseases 2009, 251406.
| Diagnosis of infections caused by pathogenic free-living amoebae.Crossref | GoogleScholarGoogle Scholar | 19657454PubMed |
[29] Tu, E.Y. et al. (2008) The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of Acanthamoeba keratitis. Cornea 27, 764–772.
| The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of Acanthamoeba keratitis.Crossref | GoogleScholarGoogle Scholar | 18650660PubMed |
[30] Hahn, T.-W. et al. (1998) Acridine orange staining for rapid diagnosis of Acanthamoeba keratitis. Jpn. J. Ophthalmol. 42, 108–114.
| Acridine orange staining for rapid diagnosis of Acanthamoeba keratitis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3kvFChsQ%3D%3D&md5=0e8e77b4bf69fbe8c8123a2f205fb4bfCAS | 9587842PubMed |
[31] Grossniklaus, H.E. et al. (2003) Evaluation of hematoxylin and eosin and special stains for the detection of Acanthamoeba keratitis in penetrating keratoplasties. Am. J. Ophthalmol. 136, 520–526.
| Evaluation of hematoxylin and eosin and special stains for the detection of Acanthamoeba keratitis in penetrating keratoplasties.Crossref | GoogleScholarGoogle Scholar | 12967807PubMed |
[32] Schuster, F.L. (2002) Cultivation of pathogenic and opportunistic free-living amebas. Clin. Microbiol. Rev. 15, 342–354.
| Cultivation of pathogenic and opportunistic free-living amebas.Crossref | GoogleScholarGoogle Scholar | 12097243PubMed |
[33] Qvarnstrom, Y. et al. (2006) Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J. Clin. Microbiol. 44, 3589–3595.
| Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFyktLzM&md5=d39ea1cf068516c3087f0cbe4fdf1994CAS | 17021087PubMed |
[34] Barratt, J.L.N. et al. (2010) Importance of nonenteric protozoan infections in immunocompromised people. Clin. Microbiol. Rev. 23, 795–836.
| Importance of nonenteric protozoan infections in immunocompromised people.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3cfnvFOquw%3D%3D&md5=aa2e6831f43a67eb1252f602598e654bCAS |
[35] Cope, J. et al. (2013) Increased patient survival: miltefosine for treatment of free-living ameba infections caused by Acanthamoeba and Balamuthia [Poster]. Council of State and Territorial Epidemiologists’ Annual Conference. Pasadena, California.
[36] Deetz, T.R. et al. (2003) Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin. Infect. Dis. 37, 1304–1312.
| Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases.Crossref | GoogleScholarGoogle Scholar | 14583863PubMed |
[37] Schuster, F.L. et al. (2006) In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J. Eukaryot. Microbiol. 53, 121–126.
| In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjsVGgsbk%3D&md5=f41ad3752c4cf40fb568c7d09d7773f8CAS | 16579814PubMed |
[38] Dart, J. K. et al. (2009) Acanthamoeba keratitis: diagnosis and treatment update 2009. Am. J. Ophthalmol. 148, 487–99e2.
| Acanthamoeba keratitis: diagnosis and treatment update 2009.Crossref | GoogleScholarGoogle Scholar | 19660733PubMed |
[39] Butler, T.K.H. et al. (2005) Six‐year review of Acanthamoeba keratitis in New South Wales, Australia: 1997–2002. Clin. Experiment. Ophthalmol. 33, 41–46.
| Six‐year review of Acanthamoeba keratitis in New South Wales, Australia: 1997–2002.Crossref | GoogleScholarGoogle Scholar |
[40] Vemuganti, G.K. et al. (2005) Granulomatous inflammation in Acanthamoeba keratitis: an immunohistochemical study of five cases and review of literature. Indian J. Med. Microbiol. 23, 231–238.
| 1:STN:280:DC%2BD2MnksV2quw%3D%3D&md5=44458773b9231d1ac1a4f77e75fe7fa5CAS | 16327118PubMed |
Biographies
Dr Evan Bursle, BSc, MBBS, is a microbiology and infectious diseases registrar based at Sullivan Nicolaides Pathology. Although his microbiological tastes are broad and still being refined, he has a keen interest in parasitology.
Dr Jenny Robson, MBBS (Hons I); FRACP, FRCPA, FACTM, is an infectious disease physician and microbiologist who has worked for the past 26 years at Sullivan Nicolaides Pathology. She has a broad range of interests, which includes travel and tropical medicine.


