Antiretroviral therapy: research, rollout and resistance
Angie N Pinto A and David A Cooper AThe Kirby Institute
Wallace Wurth Building
UNSW Australia
Sydney, NSW 2052, Australia
Email: apinto@kirby.unsw.edu.au
dcooper@kirby.unsw.edu.au
Microbiology Australia 35(2) 79-82 https://doi.org/10.1071/MA14024
Published: 7 May 2014
Antiretroviral therapy has revolutionised the management of human immunodeficiency virus (HIV). Advances in research leading to the development of combination antiretroviral therapies (ARTs) have led to significant decreases in AIDS related morbidity and increases in life expectancy for individuals with access to treatment. The goal of ‘getting to zero : zero AIDs related deaths’ now is within reach. Globally nearly 10 million people have access to ART; however, further rollout efforts are required to reach the 34 million people living with HIV sustainably over the long term. Changing paradigms see a broader scope for ART with a push towards earlier initiation, and even pre-exposure prophylaxis, with public health goals of preventing new infections. In Australia, collaborative research efforts, bipartisan political will and subsidised medication costs have allowed around 13 000 people to be maintained on antiretroviral therapy. Despite this, the challenges of continuous lifelong suppressive therapy remain, as currently there is no cure. Poor adherence can lead to disease progression and drug resistance, limiting future treatment options. Antiretroviral resistance in Australia appears to have been stable, but changing epidemiology and evolving viral subtypes may impact these rates. This article will reflect on the advances in antiretroviral research, rollout and resistance in our region.
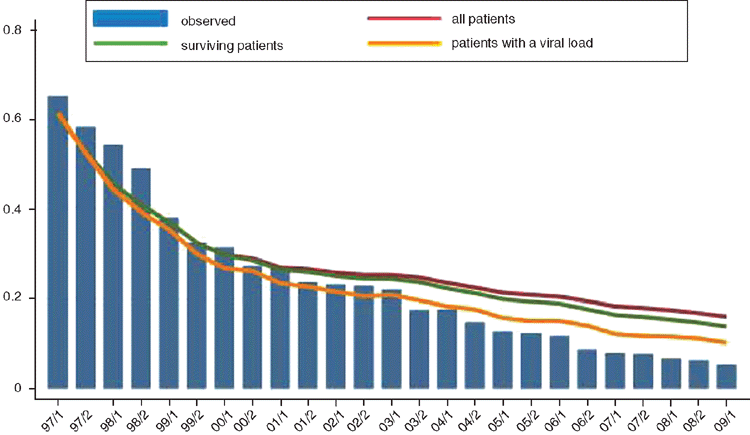
Australia has benefitted from early access and rollout of ART, and now has one of the highest treatment rates in the world, with around 50–70% of those diagnosed receiving treatment, and 85–95% of these with a suppressed viral load (see Figure 1)1. Progression to AIDS has been halved, deaths have fallen by 80%, and life expectancies approaching the general population can now be expected2–4.
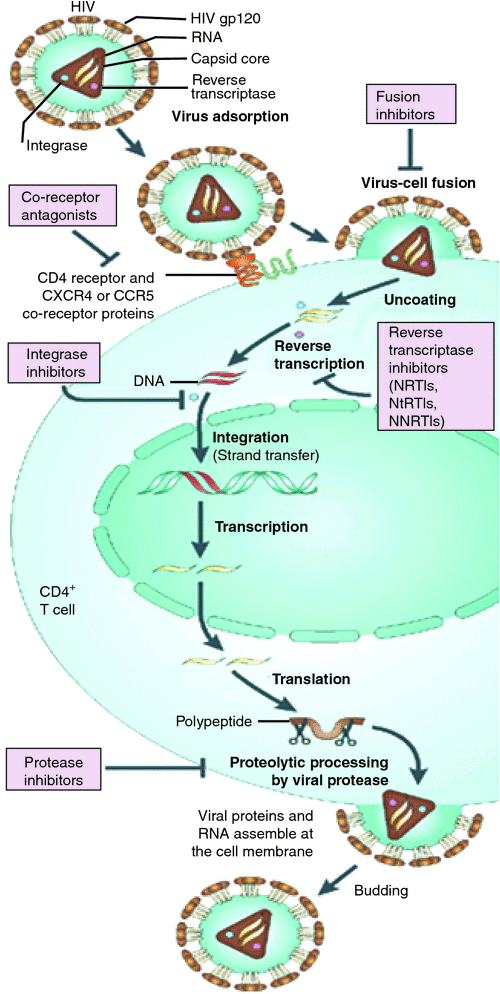
Currently available ART targets five key pathways of the HIV life cycle (see Figure 2). Reverse transcriptase inhibitors prevent transcription of viral RNA to DNA, preventing viral replication. In 1987, treatment was limited to monotherapy with a nucleoside reverse transcriptase inhibitor (NRTI) until the availability of dual NRTI therapy in 1992. The release of protease inhibitors (PIs) in 1995 allowed dual class highly active antiretroviral therapy (HAART) that has transformed the management of HIV. They remain a component of one of the preferred initial treatment regimens. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are also an important part of the antiretroviral landscape, and are currently also recommended as part of the preferred first line regimens for naïve patients5.
|
The longevity of earlier regimens was limited by significant toxicities, leading to poor adherence and ultimately treatment failures. Newer combination therapies are utilising novel classes with unique mechanisms of action and favourable side effect profiles. The fusion inhibitors were FDA approved in 2003 and offered hope for those with drug resistant virus, but their widespread use was restricted by the need for subcutaneous injection. They act by inhibiting the viral cell membrane from fusing with host cell, preventing entry into the host CD4+ T cell (Figure 2). Development of the co-receptor antagonists, approved in 2007, was a step towards personalised medicine, with targeted therapy available to patients whose virus preferentially used the CCR5 co-receptor for host cell entry. The rollout of this class was hampered by the need for a highly specialised test (Trofile® co-receptor tropism assay), initially only available in the United States with a lengthy turnaround time. The newest class available, the integrase inhibitors, have impressive effects on viral kinetics, appear to be well tolerated, and are now included in the Australian guidelines as a preferred regimen for treatment of naïve patients. They act by competing with viral DNA for binding with integrase, preventing integration of virus into host cell DNA. This five class armamentarium of ART, available in high-income countries, including fixed dose combinations (FDCs) has allowed HIV to be managed as a chronic disease with a single daily pill.
The sound evidence base for the development and rollout of ART has been a result of international collaboration, pharmaceutical engagement and participation in clinical trials. Australian clinical and laboratory researchers have been involved in development of almost every antiretroviral available on the market. Much has been learnt from cohort studies, including descriptions of the natural history of HIV, however long-term randomised controlled trials (RCTs) with clinical endpoints were instrumental in providing definitive evidence for ART. As newer drugs became rapidly available, RCTs needed to yield results in a timely manner, rather than waiting for clinical endpoints. This meant that the laboratory marker of suppressed viral load was used as a surrogate endpoint allowing shorter follow up periods of up to 48 weeks. As a result, diagnostic laboratories play an important role in providing HIV viral load monitoring, now recommended as the preferred method to diagnose and confirm treatment failures6. In low-income countries, with access to only three or four classes of ARVs, access to viral load monitoring is not routinely available, hence clinical and CD4 count monitoring may be used to diagnose treatment failure. In this setting, virological failure is more difficult to detect and assess, resulting in heterogeneous failure rates of up to 20%, contributing to the risk of emerging resistance on a global scale7.
Subsidised treatment is available for Medicare eligible Australian citizens and permanent residents via the section 100 scheme of the highly specialised drugs program of the National Health Act 1953, however access is not universal. For approximately 450 Medicare ineligible temporary residents in care, the options include purchasing full cost ART (around $12 000/year), enrolment in clinical trials or sourcing generic ART from overseas. This is often prohibitive, and as a result less than 50% of these patients have an undetectable viral load, increasing transmission risk. Programs such as the Australian HIV observational database Temporary Residents Access Study (ATRAS) that provide compassionate access ART have increased the proportion with viral load suppression, which minimises the risk of transmission8. Despite access to ART (for those Medicare eligible), there are ongoing challenges in maintaining lifelong suppressive combination therapy, including managing chronic toxicity and ensuring adherence.
Resistance can develop to every class of antiretrovirals, ultimately leading to treatment failure, cross resistance and reduced effectiveness of first line drugs. Combination triple therapy aims to increase the threshold to resistance by including a regimen of at least two classes, usually comprising a ‘backbone’ of reverse transcriptase inhibitors paired with another agent, however this dogma may be challenged in the future9,10.
The introduction of HAART in 1996 was accompanied with a precipitous decline in the rate of RT mutations, with no increases in PI resistance11. In Victoria and NSW, rates of transmitted drug resistance have since remained stable at 10–16% when last analysed11–13. Since then, there appear to be some shifts in the epidemiology of HIV in South Australia, which comprises 4% of the national HIV burden, with an increased incidence of non-B subtypes now accounting for almost half of new diagnoses14. They tend to occur in females born overseas, acquired overseas through heterosexual transmission, and reflect increasing migration from high prevalence countries15,16. Non-B subtypes may have different rates of disease progression, and treatment responses, as transmitted subtype specific polymorphisms can lead to different patterns of secondary resistance. However, the locally predominant subtype B mainly occurs in men who have sex with men and still comprises the majority of new infections in Australia overall. Ongoing surveillance of changing subtypes and patterns of resistance, particularly in higher burden regions of Australia is imperative.
In other regions, such as Asia and Africa, the availability of resistance testing is more limited and virological failures rates due to resistance are much more varied7. In the setting of failure of a first line regimen in resource limited countries, a recent Australian led study has demonstrated that a novel combination of a boosted PI and integrase inhibitor is non-inferior to the WHO recommended nucleotide and PI based regimen10. While providing much needed clinical trial data for the one million people in low to middle income countries requiring second line treatment, this novel regimen currently is not cost effective in some of these countries where ART is provided through donor or publicly funded programmes.
The scope for ART is broadening to settings beyond treatment of the individual living with HIV. ART for post exposure prophylaxis, while not TGA licensed for this indication, has been available in Australia for high risk occupational and non-occupational exposures. Additionally, non-prescribed informal use for recreational or pre exposure prophylaxis has been reported in HIV negative people, sometimes during high risk behaviour17,18.
Treatment paradigms are shifting from being solely focused on the outcomes of the individual to a broader public health approach, with a goal of ‘getting to zero: zero new HIV infections’. The Treatment as Prevention (TasP) trial and the HIV Prevention Trials Network (HPTN052) have clearly demonstrated the role of ART in the prevention of HIV. In regions such as Southern Africa where ART programmes have been rolled out, there has been decreases in the incidence of HIV19. Similarly, regions of Canada where there has been greater ART coverage have seen corresponding declines in rates of new infections20. This approach has led to the concept of ‘community viral load’ where aggregated measures from a community can be used to monitor ART uptake and risks of transmission21. If the ongoing rollout of ART continues towards a ‘test and treat’ and ‘treatment as prevention’ approach, then one of the challenges of increased global demand and costs in the face of reduced donor funding must be addressed.
One consideration is revising pre-existing dose guidelines, in the form of dose optimisation studies that re-evaluate the lowest effective dose, which on a global scale can result in significant cost savings. The ENCORE1 48 week analysis demonstrated non-inferiority of 400mg dose of the NNRTI efavirenz compared to the standard 600mg dose, with significantly less associated toxicity22. Studies such as these breathe extended life into currently available ARVs.
This broader public health approach to treatment must be balanced with the benefits of treatment for the individual patient. Despite all the advances in antiretroviral treatment over the years, one of the most fundamental questions remains unanswered: when to start treatment? The 2013 WHO guidelines have been edging towards earlier treatment, and now recommend ART initiation at CD4 count >350 and ≤ 500 cells/mm3 regardless of clinical stage. When faced with an individual patient, the decision to start earlier must be balanced against a commitment to taking a lifelong medication. The Strategic Timing of AntiRetrovirals Trial (START) due for completion in December 2015 aims to answer this question (NCT00867048).
The development of ART has led to significant improvements for individuals living with HIV, and could lead to an AIDS free generation. On a community level, the rollout of ART has had significant impacts on transmission and rates of new infections. The current goal of ‘getting to zero’ requires further ART rollout efforts and healthcare support spending from a shrinking funding pool. Renewed political will, financial commitment and innovative solutions are needed to bridge this gap.
References
[1] Australian Government Standing Council on Health Report on Progress on the Australian Response to HIV and AIDS. pp. 20.[2] Correll, P.K. et al. (1998) HIV disease progression in Australia in the time of combination antiretroviral therapies. Med. J. Aust. 169, 469–472.
| 1:STN:280:DyaK1M%2FmsVeltg%3D%3D&md5=353951feec702d23344fcafb6e52d7e3CAS | 9847898PubMed |
[3] van Sighem, A.I. et al. (2010) Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 24, 1527–1535.
| Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals.Crossref | GoogleScholarGoogle Scholar | 20467289PubMed |
[4] McManus, H. et al. (2012) Long-term survival in HIV positive patients with up to 15 years of antiretroviral therapy. PLoS ONE 7, e48839.
| Long-term survival in HIV positive patients with up to 15 years of antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhslehs7zJ&md5=2bd3cf95af62c83e767edefa1b7a8b49CAS | 23144991PubMed |
[5] ASHM (2013) What to start: initial combination regimens for the antiretroviral-naïve patient. ASHM http://arv.ashm.org.au/arv-guidelines/what-to-start
[6] WHO (2013) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. WHO Press, World Health Organization. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/
[7] Aghokeng, A.F. et al. (2014) Extraordinary heterogeneity of virological outcomes in patients receiving highly antiretroviral therapy and monitored with the World Health Organization public health approach in sub-saharan Africa and southeast Asia. Clin. Infect. Dis. 58, 99–109.
| Extraordinary heterogeneity of virological outcomes in patients receiving highly antiretroviral therapy and monitored with the World Health Organization public health approach in sub-saharan Africa and southeast Asia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvFeqt77O&md5=98d6061ac3ee53d062dc2954bab265d5CAS | 24076968PubMed |
[8] Petoumenos, K. (2013) The Australian HIV Observational Database Temporary Residents Access Study (ATRAS) In One year follow-up, The Kirby Institute University of New South Wales
[9] Boyd, M.A. and Cooper, D.A. (2013) SPRING-2 the future of antiretroviral therapy. Lancet Infect. Dis. 13, 908–909.
| SPRING-2 the future of antiretroviral therapy.Crossref | GoogleScholarGoogle Scholar | 24074643PubMed |
[10] Boyd, M.A. et al. (2013) Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet 381, 2091–2099.
| Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXpsV2mt78%3D&md5=3fc6d7cc5358c9dfbb542314b7e18a6fCAS | 23769235PubMed |
[11] Ammaranond, P. et al. (2003) No increase in protease resistance and a decrease in reverse transcriptase resistance mutations in primary HIV-1 infection: 1992–2001. AIDS 17, 264–267.
| No increase in protease resistance and a decrease in reverse transcriptase resistance mutations in primary HIV-1 infection: 1992–2001.Crossref | GoogleScholarGoogle Scholar | 12545090PubMed |
[12] Russell, J.S. et al. (2009) Prevalence of transmitted HIV drug resistance since the availability of highly active antiretroviral therapy. Commun. Dis. Intell. Q. Rep. 33, 216–220.
| 19877541PubMed |
[13] Ammaranond, P. et al. (2003) Rates of transmission of antiretroviral drug resistant strains of HIV-1. J. Clin. Virol. 26, 153–161.
| Rates of transmission of antiretroviral drug resistant strains of HIV-1.Crossref | GoogleScholarGoogle Scholar | 12600647PubMed |
[14] Hawke, K.G. et al. (2013) HIV non-B subtype distribution: emerging trends and risk factors for imported and local infections newly diagnosed in South Australia. AIDS Res. Hum. Retroviruses 29, 311–317.
| 23098890PubMed |
[15] Chibo, D. and Birch, C. (2012) Increasing diversity of human immunodeficiency virus type 1 subtypes circulating in Australia. AIDS Res. Hum. Retroviruses 28, 578–583.
| Increasing diversity of human immunodeficiency virus type 1 subtypes circulating in Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XotVOgsL4%3D&md5=838c8d704e34b582fd816fd0e61b2535CAS | 22077905PubMed |
[16] Lemoh, C. et al. (2013) Acquisition of HIV by African-born residents of Victoria, Australia: insights from molecular epidemiology. PLoS ONE 8, e84008.
| Acquisition of HIV by African-born residents of Victoria, Australia: insights from molecular epidemiology.Crossref | GoogleScholarGoogle Scholar | 24391866PubMed |
[17] Grelotti, D.J. et al. (2014) Whoonga: potential recreational use of HIV antiretroviral medication in South Africa. AIDS Behav. 18, 511–518.
| Whoonga: potential recreational use of HIV antiretroviral medication in South Africa.Crossref | GoogleScholarGoogle Scholar | 23955659PubMed |
[18] Zablotska, I.B. et al. (2013) The informal use of antiretrovirals for preexposure prophylaxis of HIV infection among gay men in Australia. J. Acquir. Immune Defic. Syndr. 62, 334–338.
| The informal use of antiretrovirals for preexposure prophylaxis of HIV infection among gay men in Australia.Crossref | GoogleScholarGoogle Scholar | 23187947PubMed |
[19] Delva, W. and Abdool Karim, Q. (2014) The HIV epidemic in Southern Africa – is an AIDS-free generation possible? Curr. HIV/AIDS Rep. , .
| The HIV epidemic in Southern Africa – is an AIDS-free generation possible?Crossref | GoogleScholarGoogle Scholar | 24676559PubMed |
[20] Hogg, R.S. et al. (2013) Rates of new infections in British Columbia continue to decline at a faster rate than in other Canadian regions. HIV Med. 14, 581–582.
| Rates of new infections in British Columbia continue to decline at a faster rate than in other Canadian regions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3sbosVCnuw%3D%3D&md5=4c2167a7b04ed05ba522c3ca7e26f563CAS | 24033869PubMed |
[21] Miller, W.C. et al. (2013) Community viral load as a measure for assessment of HIV treatment as prevention. Lancet Infect. Dis. 13, 459–464.
| Community viral load as a measure for assessment of HIV treatment as prevention.Crossref | GoogleScholarGoogle Scholar | 23537801PubMed |
[22] ENCORE1StudyGroup (2014) Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet 383, 1474–1482.
| Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial.Crossref | GoogleScholarGoogle Scholar | 24522178PubMed |
Biographies
Angie N Pinto BSc MBBS MPHTM FRACP FRCPA is an infectious diseases physician and microbiologist. She is the recipient of an NHMRC postgraduate scholarship investigating the long term effects of primary HIV infection and trends in HIV resistance.
David A Cooper AO, BSc(Med) MBBS(Syd), MD, DSc (UNSW), FRACP, FRCPA, FRCP, FAA Scientia Professor of Medicine at the University of New South Wales and a Fellow of the Australian Academy of Science (FAA), is Director of the Kirby Institute for infection and immunity in society, Sydney, Australia. He is a past President of the International AIDS Society, a member of WHO Strategic Advisory Committee on HIV/AIDS and a Director of HIV-NAT, a clinical research and trials collaboration based at the Thai Red Cross AIDS Research Centre in Bangkok, Thailand. He is actively involved in studies of biomedical prevention strategies for HIV infection in the developing world, including vaccines and chemoprophylaxis and is an author on more than 700 published scientific papers.