Measles and SSPE: occurrence and pathogenesis
Jude JayamahaDepartment of Virology
Medical Research Institute
PO Box 527
Colombo 008, Sri Lanka
Email: jayamahacar@gmail.com
Microbiology Australia 34(3) 132-134 https://doi.org/10.1071/MA13044
Published: 4 September 2013
Measles is an acute febrile exanthematous condition that is usually a self-limiting disease, but it can be associated with several complications, one of which is subacute sclerosing panencephalitis (SSPE). It is a rare delayed complication of measles due to persistence of the virus in the central nervous system. All of the genetic analyses of viral material derived from brain tissue of SSPE patients have revealed sequences of wild-type measles virus (MV). There is no evidence that measles vaccine can cause SSPE. Several mutations have been described in genes coding for proteins in SSPE strains of MV. Several host cell modifications, mechanisms of virus reactivation and immunopathology in pathogenesis of SSPE have been explained recently, broadening the understanding of this fatal disease.
Measles is a highly contagious disease caused by the measles virus and is one of the most devastating infectious diseases in humans. Usually it is a self-limiting acute febrile exanthematous condition, but up to 40% of patients can have complications. Common complications mainly occur in the respiratory tract, with pneumonia, laryngotracheobronchitis (croup) and otitis media1. Rare but serious complications of measles usually involve the central nervous system (CNS). Encephalomyelitis occurs within 2 weeks of the onset of rash. Other CNS complications that occur months to years after acute infection are measles inclusion body encephalitis and SSPE, both of which are caused by persistent measles virus infection2.
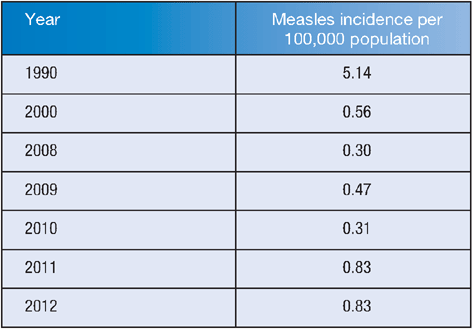
From 1990 to 2010, there has been a decrease in measles incidence in Australia, but the incidence increased in 2011 and 2012, with several imported and local clusters of measles in several territories of Australia (Table 1)3.
|
Subacute sclerosing panencephalitis
The incidence of SSPE varies greatly from approximately 0.2 to 40 cases per million population per year, depending on the country and the time at which the data were collected. Data analyses in the UK and, more recently, the USA have shown the true incidence of SSPE to be approximately 4–11 cases of SSPE per 100,000 cases of measles4. In the nations of India and Eastern Europe the incidence of SSPE remains high5. A study suggests an incidence of 0.02/100,000 per annum on the basis of four cases in Australian children for the period 1995–19986. Measles vaccination programmes have led to a dramatic reduction in the incidence of SSPE7.
Initial symptoms of SSPE typically occur some years after natural measles infection and are usually subtle, with intellectual decline and behavioural changes. Most patients proceed over months or years to generalised convulsions, dementia, coma and death. Death usually occurs within 1–3 years. There is also a higher incidence among males than females, with a ratio of three to one. SSPE is confirmed when there is a recognised clinical course accompanied by one or more of the following: measles antibody detected in the cerebrospinal fluid; a characteristic pattern on electroencephalography; typical histological findings in brain biopsy material or tissue obtained by post-mortem examination4. There is currently no effective treatment for SSPE, although many therapies have been tried. Two case reports have suggested slight improvement of clinical condition with intravenous administration of high-dose ribavirin combined with intraventricular administration of IFN-α. Management largely depends on supportive care8.
Measles virus proteins and SSPE virus strains
Measles virus is composed of six structural proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin (H) and large protein (L). The N, P and L proteins are essential for viral replication and transcription. Sequences of viral genomes of SSPE cases are typically not related to circulating wild-type viruses when patients developed SSPE, but instead to those in circulation when patients developed an acute MV infection some years back. This is consistent with other evidence that SSPE is caused by persistent MV infection and that this is partly dependent on the infecting strain9. Genetic analyses have also revealed that persistent MV derived from SSPE cases (SSPE virus strains, SSPEV) contain numerous mutations. The M gene of SSPEV appears to be particularly vulnerable to mutation and its expression is restricted. Other changes in SSPEV structural proteins have been found in the F and H proteins. The base pairs difference in N, P, M, F and H genes of the SSPE strains from standard Edmonston measles strain are 2.3, 3.3, 2.1, 3.3 and 2.5% respectively10.
There is no evidence that measles vaccine can cause SSPE. Sequence analyses of 57 SSPE viral strains derived from brain tissue of SSPE patients from 1955–1998 have revealed sequences of wild-type measles virus (genotype C1, C2, D1, D3, D5, E and F) never vaccine virus (genotype A)4,11.
Pathogenesis
Several host cell modifications, mechanisms of virus reactivation and immunopathology in pathogenesis of SSPE have been explained recently, broadening the understanding of the disease.
Host cell modifications in MV persistence
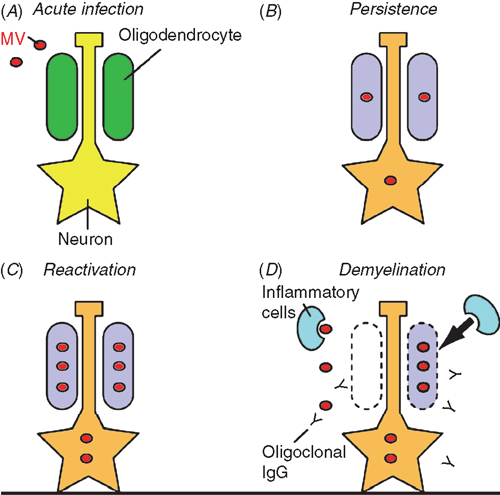
Modulation of gene expression patterns in MV-infected dendritic and other CNS cells that upregulate certain cytokines (e.g. interferon α) have been reported12. Alterations in molecules (e.g. NF-κB transcription factors) in post-transcription of MV-infected cells might be involved in SSPE pathogenesis. NF-κB is also implicated in susceptibility to multiple sclerosis. Glial cells appear to be vulnerable to endoplasmic reticulum (ER) stress, altered expression of the above molecules involved in ER stress can perturb myelination13. Myelination is a complex process that requires a precise stoichiometry for gene dosage, along with protein and lipid synthesis. Alterations in lipid metabolism, such as decreased cholesterol synthesis and impaired β-oxidation are associated with MV persistence14. An alteration in lipid metabolism during persistent MV infection would affect the maintenance of myelin in the CNS (Figure 1B).
|
Reactivation mechanisms of persistent MV
It is known that persistent MV infection is asymptomatic, but can eventually result in SSPE. The latent MV should be reactivated at the onset of disease, resulting in clinical signs of SSPE (Figure 1C). Several molecules and cellular mechanisms have been implicated on reactivation recently. Potential molecules involved in MV reactivation in SSPE are heat shock protein 72 and peroxiredoxin 1. Age-related modifications such as hyperoxidisation might explain why it takes several years after an acute MV infection for the first symptoms of SSPE to appear15.
Pathogenesis of persistent MV infection
The immune system appears to be involved in SSPE pathogenesis (Figure 1D). Three mechanisms have been explained in immunopathology of SSPE: direct cytopathic effects, autoantigen and superantigen.
Direct cytopathic effects: Persistent MV infection might destroy infected cells, including oligodendrocytes, and damage inflammatory responses, thereby resulting in demyelination. Consistent with this idea, there is a strong correlation among the extent of viral fusion activity, cytopathic effects of MV and severity of neurovirulence in a hamster model16.
Autoantigen: Autoimmune responses to myelin proteins are considered to be possible causes of some demyelinating diseases including SSPE. It has also been suggested that autoimmunity could arise as a result of cross-reactivity between viral and myelin antigens17. Myelin basic protein (MBP)-homologous sequences in the N and C proteins in measles might account not only for encephalomyelitis in humans, but also for cross-reactions as detected by delayed skin tests with MBP in measles-sensitised guinea pigs18 (Figure 1D).
Superantigen: A whole class of T lymphocyte cells can activate by superantigens (which might produce certain bacteria, mycoplasma or viruses) in a distinctive mode irrespective of antigen specificity. Activated T lymphocyte cells can cross the blood–brain barrier, enter the brain parenchyma and initiate inflammatory lesions. The permeability of the blood–brain barrier increases, leading to an influx of soluble factors, such as tumor necrosis factor, into the CNS, which will result in extensive CNS lesions19.
Conclusions
Several host cell modifications, mechanisms of virus reactivation and immunopathology in pathogenesis of SSPE have been explained recently broadening our understanding of the disease. However, there could be unidentified mechanisms involved in disease progression during measles virus persistence and pathogenicity. Future research should focus on these aspects and address on early markers of disease, possible novel therapeutic agents in prevention and treating this fatal condition.
References
[1] Duke, T. and Mgone, C.S. (2003) Measles: not just another viral exanthem. Lancet 361, 763–773.| Measles: not just another viral exanthem.Crossref | GoogleScholarGoogle Scholar | 12620751PubMed |
[2] Johnson, R.T. et al. (1984) Measles encephalomyelitis—clinical and immunologic studies. N. Engl. J. Med. 310, 137–141.
| Measles encephalomyelitis—clinical and immunologic studies.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2c%2FptlCnsg%3D%3D&md5=ee62c2f3327f30457072c4913ede306dCAS | 6197651PubMed |
[3] World Health Organization (2013) WHO vaccine-preventable diseases: monitoring system. 2013 global summary. http://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria[country]=AUS (accessed 14 July 2013).
[4] Campbell, H. et al. (2007) Review of the effect of measles vaccination on the epidemiology of SSPE. Int. J. Epidemiol. 36, 1334–1348.
| Review of the effect of measles vaccination on the epidemiology of SSPE.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2sjitFyiuw%3D%3D&md5=d06d5036dfdd18ff47b7fa282dad3356CAS | 18037676PubMed |
[5] http://www.disabled-world.com/disability/types/sspe.php (accessed 24 May 2013).
[6] Hanna, J. and Messer, R. Subacute sclerosing panencephalitis. Australian Paediatric Surveillance Unit. http://www.apsu.org.au/assets/past-studies/sspe.pdf (accessed 30 July 2013).
[7] Moss, W.J. and Griffin, D.E. (2012) Measles. Lancet 379, 153–164.
| Measles.Crossref | GoogleScholarGoogle Scholar | 21855993PubMed |
[8] Hosoya, M. and Shigeta, S. (2001) High-dose intravenous ribavirin therapy for subacute sclerosing panencephalitis. Antimicrob. Agents Chemother. 45, 943–945.
| High-dose intravenous ribavirin therapy for subacute sclerosing panencephalitis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhsFWgu7c%3D&md5=1337c41cf48a88f6ad3d10852ea8180aCAS | 11181386PubMed |
[9] Patterson, J.B. et al. (2001) Evidence that the hypermutated M protein of a subacute sclerosing panencephalitis measles virus actively contributes to the chronic progressive CNS disease. Virology 291, 215–225.
| Evidence that the hypermutated M protein of a subacute sclerosing panencephalitis measles virus actively contributes to the chronic progressive CNS disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xit1ylug%3D%3D&md5=aca2eb5a3a1ead6ed67545390e4ccd23CAS | 11878891PubMed |
[10] Cattaneo, R. and Rose, J.K. (1993) Cell fusion by the envelope glycoproteins of persistent measles viruses which caused lethal human brain disease. J. Virol. 67, 1493–1502.
| 1:CAS:528:DyaK3sXitFSnurY%3D&md5=b908f1e60a897e906aa0c09185289b7cCAS | 8437226PubMed |
[11] Duclos, P. and Ward, B.J. (1998) Measles vaccines: a review of adverse events. Drug Saf. 19, 435–454.
| Measles vaccines: a review of adverse events.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M%2FpsVKqtQ%3D%3D&md5=ac1058d344b7b10108ec773b4b143abcCAS | 9880088PubMed |
[12] Bolt, G. et al. (2002) Measles virus-induced modulation of host cell gene expression. J. Gen. Virol. 83, 1157–1165.
| 1:CAS:528:DC%2BD38XjsFWmsb8%3D&md5=415f614d92ee34c7b211acb1bad55a63CAS | 11961271PubMed |
[13] Honda, T. et al. (2013) Pathogenesis of encephalitis caused by persistent measles virus infection. In Encephalitis (Tkachev, S., ed.), pp. 251–262, InTech. http://www.intechopen.com/books/encephalitis/pathogenesis-of-encephalitis-caused-by-persistent-measles-virus-infection (accessed 12 August 2013).
[14] Robinzon, S. et al. (2009) Impaired cholesterol biosynthesis in a neuronal cell line persistently infected with measles virus. J. Virol. 83, 5495–5504.
| Impaired cholesterol biosynthesis in a neuronal cell line persistently infected with measles virus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmtlGksr4%3D&md5=6e595532a40ead7e2d41a024d4116214CAS | 19297498PubMed |
[15] Watanabe, A. et al. (2011) Peroxiredoxin 1 is required for efficient transcription and replication of measles virus. J. Virol. 85, 2247–2253.
| Peroxiredoxin 1 is required for efficient transcription and replication of measles virus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVWlt7fE&md5=4cce00bf563b12a8ac66647df3b17820CAS | 21159870PubMed |
[16] Ayata, M. et al. (2010) The F gene of the Osaka-2 strain of measles virus derived from a case of subacute sclerosing panencephalitis is a major determinant of neurovirulence. J. Virol. 84, 11189–11199.
| The F gene of the Osaka-2 strain of measles virus derived from a case of subacute sclerosing panencephalitis is a major determinant of neurovirulence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVeisb7P&md5=ba9a3c4ea6f465a510f911f5b5a59c43CAS | 20719945PubMed |
[17] Wucherpfennig, K.W. and Strominger, J.L. (1995) Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80, 695–705.
| Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXksVahs74%3D&md5=0c78c1607d1b0caade525608bc1180a6CAS | 7534214PubMed |
[18] Jahnke, U. et al. (1985) Sequence homology between certain viral proteins and proteins related to encephalomyelitis and neuritis. Science 229, 282–284.
| Sequence homology between certain viral proteins and proteins related to encephalomyelitis and neuritis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2MXkvVCktb4%3D&md5=e8cd97324e9bc10c33dcdf7e34cc3a18CAS | 2409602PubMed |
[19] Brocke, S. et al. (1994) Infection and multiple sclerosis: a possible role for superantigens? Trends Microbiol. 2, 250–254.
| Infection and multiple sclerosis: a possible role for superantigens?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2czmtlWhsA%3D%3D&md5=4659ba805fed98fa4d1797c2dd02e31cCAS | 8081652PubMed |
Biography
Jude Jayamaha is Head of the National Influenza Centre, Sri Lanka, Consultant Medical Virologist at Department of Virology and was the Acting Virologist, National Measles and Rubella Reference Laboratory, Sri Lanka. His post-MD training was at the Prince of Wales Hospital, Sydney and the WHO Collaborating Centre for Reference and Research on Influenza, Melbourne. His research interests include respiratory viruses’ morbidity in children, novel diagnostic methods of measles infection and CMV diseases in immunocompromised individuals.


