Sneezing leads to wheezing: microorganisms important in asthma
Christiana Willenborg A and Sacha Stelzer-Braid A BA Virology Division
SEALS Microbiology
Prince of Wales Hospital
Randwick, NSW 2031, Australia
Tel: +61 2 9382 9243
Email: Christiana.Willenborg@sesiahs.health.nsw.gov.au
B Virology Division
SEALS Microbiology
Prince of Wales Hospital
Randwick, NSW 2031, Australia
School of Medical Sciences
The University of New South Wales
Kensington, NSW 2033, Australia
Tel: +61 2 9382 9096
Fax: +61 2 9382 8533
Email: Sacha.Stelzer-Braid@sesiahs.health.nsw.gov.au
Microbiology Australia 34(3) 125-129 https://doi.org/10.1071/MA13042
Published: 4 September 2013
Asthma is a common, chronic disease of the airways. Asthmatics can suffer exacerbations (worsening symptoms) due to a range of environmental and occupational factors. At least half of all asthma exacerbations are caused by respiratory viruses. In this article we examine some of the microbiological causes of asthma development and exacerbations.
What is asthma?
Asthma is a chronic inflammatory condition of the airways. It is a heterogeneous disease with several clinical phenotypes described as mild, moderate or severe, although definition can be difficult1. Asthma is often difficult to diagnose as the symptoms (wheeze, difficulty breathing) are common to other illnesses such as respiratory tract infections and obesity2. ‘Viral-induced wheeze’ and cough are common in young children with respiratory infections, but are not necessarily caused by asthma. The disease is characterised by episodes of wheezing, breathlessness and chest tightness due to widespread narrowing of the airways in the lungs, known as exacerbations. Exacerbations can vary from mild to severe and result in periods of incapacity, emergency hospital admissions and, rarely, death.
Exacerbations can be triggered by a number of environmental and occupational allergens3 including:
-
viral infections (which cause the majority of exacerbations in children and adults4)
-
exercise
-
cold weather
-
exposure to specific allergens such as:
-
-
house dust mites
-
pollens
-
mould spores
-
animal dander
-
-
irritants such as:
-
-
tobacco smoke
-
pollution (such as nitrogen dioxide (NO2) from the combustion of natural gas and motor fuel)
-
some food additives
-
-
occupational exposure to:
-
-
specific allergens
-
irritants including dust and fumes.
-
One in 10 Australians suffer from asthma5. The prevalence of asthma in Australian children increased between 1982 and 19922, but has now declined in children since 2001 and is stabilised in adults5,6. Asthma is a significant health problem in Australia, and in 2006–2007 the number of Australians hospitalised due to this disease reached well over 36,000 people. It has been predicted that over the next two decades, asthma will continue to rank as one of the major causes of disease burden in Australia7. With this there is also great cost. The most recent data show that from 2004–2005 $606 million was spent on asthma (1.2% of all health expenditure in that year)5.
While there is currently no cure for asthma, inhaled corticosteroids and other medications are available to control the disease and prevent exacerbations8. The underlying causes of asthma are not yet well understood, but it appears that a combination of pre-disposing genetic factors and certain environmental factors cause an individual to develop chronic asthma9,10.
Asthma and respiratory viruses
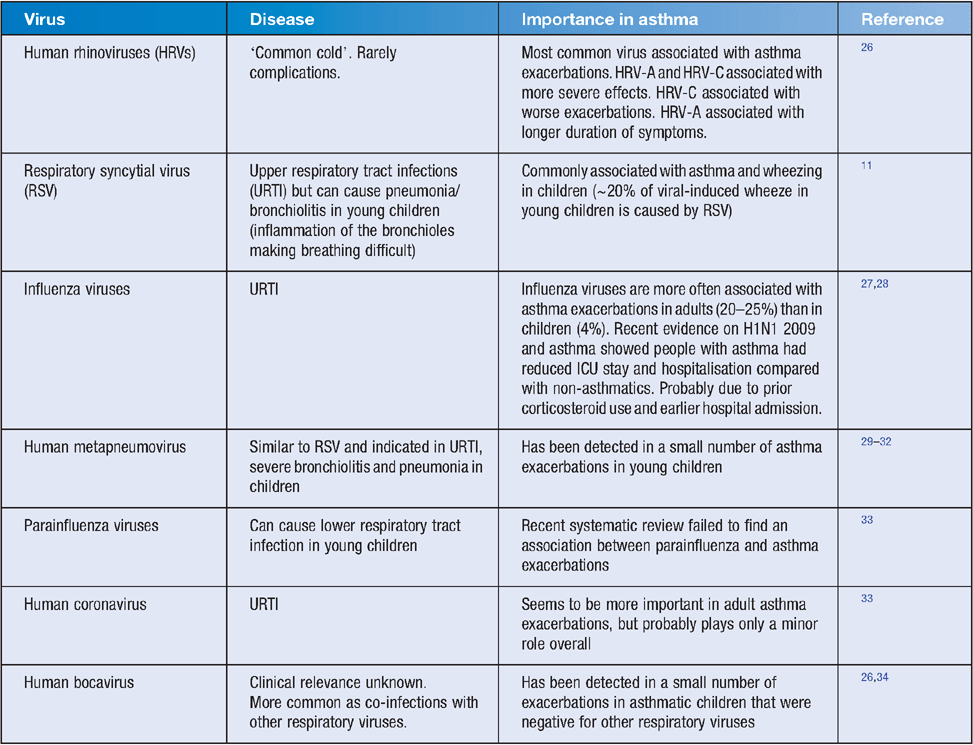
Respiratory viruses cause up to 90% of asthma exacerbations in children11. The most common viruses responsible are human rhinoviruses (HRVs), respiratory syncytial virus (RSV), human metapneumovirus (hMPV) and influenza viruses (Table 1).
|
HRVs are the most frequently detected respiratory pathogen, and typically cause the common cold12,13. Annually, HRVs infect billions of people worldwide14 and cost billions in healthcare dollars15. HRVs were discovered as the common cold pathogen over 50 years ago, with the first classical strain discovered in 195616,17. There are approximately 160 types and together they cause a wide range of clinical outcomes infecting both upper and lower respiratory tracts13–15. In addition to the common cold, infections with HRV can be asymptomatic but can also cause severe lower respiratory illnesses such as exacerbations of asthma and even pneumonia14. HRVs are a large group of genetically diverse RNA viruses and are classified into three different species (A, B and C). The classical serotypes are found within species A and B with another 50 additional strains recently identified as being part of species C13,14. They are part of the Picornaviridae family, have a 7200-nucleotide mRNA positive sense genome and are classified due to their sequence variations with HRV-C showing substantial sequence divergence from the other classified species13,18,19. The development of more sensitive molecular techniques has enabled scientists to detect more HRVs and other respiratory virus infections20 and gain a greater appreciation of the broad range of clinical illnesses caused by HRVs13,14. By using these techniques, viral respiratory infections have been detected in up to 85% of asthma exacerbations in children and approximately 50% in adults13. Of these infections, approximately two-thirds are caused by HRVs. Of the three species, HRV-A and HRV-C are more common in infections and exacerbations. Miller et al. showed that almost half of all hospitalisations due to HRV infections were associated with HRV-C suggesting that this group causes a substantial burden of paediatric disease21.
Multiple strains of HRV circulate at any one time during a season13,22, with children having several HRV infections per year23. HRVs are present year-round with all three species (HRV-A, HRV-B and HRV-C) being represented24,25. In temperate climates there are peaks of HRV infections in autumn and spring, coinciding with return to school after holidays. This is known as ‘the back-to-school effect’ and it is seen globally23. Exacerbations of asthma and hospital admissions for asthma also show distinct peaks in autumn and spring, suggesting that viral infections could be major contributors to seasonal asthma morbidity13. The seasonal prevalence of different HRV subtypes has also been examined with the proportion of respiratory infections in which HRV-B and HRV-C were detected being the lowest in summer, and more common in autumn21,25. It also appears that with this seasonal variation, HRV-C seems to exchange its dominance with HRV-A.
Evidence for early life viral infections and development of asthma
Approximately one-third of infants who have an acute viral wheezing illness will go on to develop more common wheezing events13; however, most wheezing illnesses in infancy will resolve with no long-term effects. A number of birth cohort studies have shown that viral respiratory illnesses early on in life might promote asthma in some children13.
Sigurs et al.35 studied 47 children hospitalised due to RSV infection in their first year of life, and 93 age- and gender-matched controls, prospectively for 18 years. The cohort infected with RSV had high prevalence of early onset allergy-associated wheeze, increased airway hyper-responsiveness (AHR) and reduced airway function at 18 years of age35. The RSV group had a higher incidence of parental asthma/atopy compared with the control group (indicating the children were at ‘high-risk’ of developing asthma), but the difference was not significant.
Jackson et al.11 enrolled 289 genetically ‘high-risk’ children at birth into the NIH-funded Childhood Origins of ASThma study (COAST) based on one or both of their parents having asthma/allergies. Children who had a wheezing respiratory illness caused by HRV in the first 3 years of life had a 10-fold increase in asthma risk at age 6 years. HRV infection without wheeze was not associated with an increased asthma diagnosis at age 6 years. RSV wheezing illness was associated with a smaller increase in asthma risk at 6 years. An Australian birth cohort study has also found that more wheezing illness in infants is caused by HRV than RSV36.
But do early life infections with HRV or RSV necessarily cause asthma? It is the age-old question: Which came first, the chicken or the egg? Whether it is causal or genetic factors linking HRV and RSV to asthma later in life is still not known and much debated. Kuehni and colleagues37 argued that existing evidence shows it is more likely that a genetic predisposition for asthma renders an individual more susceptible to worse illness caused by RSV in early life, rather than RSV being the cause of asthma development. It might be that, as hypothesised by Jackson et al.11, in genetically high-risk individuals, HRV and RSV infections cause pathological changes in the lungs that have lasting effects and lead to asthma. However, not all infants who wheeze with a viral infection will go on to develop asthma. Further studies are needed to resolve this question.
Asthma and other microorganisms
Respiratory viruses are important in children with asthma, but bacteria and fungi can also cause exacerbations of asthma and are more important in older children and adults1.
Recent studies of Mycoplasma pneumoniae, Chlamydia pneumoniae and Legionella pneumophila have looked at the associations of these atypical bacteria with both acute exacerbations and chronic cases of asthma38. Evidence from human studies have linked both M. pneumoniae and C. pneumoniae to cases of prevalent asthma and even new cases of wheezing indicating they could play possible roles in promoting airway inflammation38. Gil et al. found that M. pneumoniae colonised at a higher rate in patients with asthma (24.7%) than those without (5.7%), possibly inducing the wheezing events39. Qasem and colleagues, in a study of both asthmatic and non-asthmatic patients, found that M. pneumoniae was more common in asthmatic patients and was also related to the exacerbation of asthma symptoms (patients with suspected viral infections were excluded from this study)38. In a study conducted in France of children with known asthma and children with a new diagnosis of asthma, Biscardi et al. found that M. pneumoniae and C. pneumoniae were found in both groups of children, but at higher infection rates in those children with newly diagnosed asthma (50% of newly diagnosed patients had M. pneumoniae infection compared with 5.2% of stable asthma patients). Along with the different bacteria, respiratory viruses were tested for within the participants; however, major comparisons were not made and coinfections were not looked at40. Further to this, of those children infected with both bacteria and who were experiencing their first attack, 62% had asthma recurrences compared with only 27% who were not infected40.
Some individuals with asthma (approximately 2.5%41) can become chronically infected with the fungus Aspergillus fumigatus, causing the diseases allergic bronchopulmonary aspergillosis (ABPA). ABPA can cause bronchiecstasis (chronic inflammation of the airways, decreased mucus clearance, leading to chronic lung infection) and sometimes death. A. fumigatus acts as an allergen and pathogen and also sensitises the sufferer to several other fungal pathogens.
Conclusions
Clinical data now indicate microorganisms are the main cause of asthma exacerbations, with many cases of wheeze in children <1 year linked to asthma later in life. However, many aspects of the interaction between asthma and microorganisms are still not well understood. Why do some children with wheezing HRV/RSV infections go on to develop asthma and some do not? Why do some children with viral respiratory infections have more severe exacerbations of their asthma (leading to hospitalisations) and some do not? Further studies are needed on the severity of these infections and resulting impact on asthma symptoms. The likely benefits of such studies are enormous in terms of reducing the clinical impact of asthma.
References
[1] Edwards, M.R. et al. (2012) The microbiology of asthma. Nat. Rev. Microbiol. 10, 459–471.| 1:CAS:528:DC%2BC38XotVOrtbo%3D&md5=a166e8a884f870ae3be8175754f84299CAS | 22669219PubMed |
[2] Poulos, L.M. et al. (2005) The burden of asthma in children: an Australian perspective. Paediatr. Respir. Rev. 6, 20–27.
| The burden of asthma in children: an Australian perspective.Crossref | GoogleScholarGoogle Scholar | 15698810PubMed |
[3] Australian Institute of Health and Welfare 2013 http://www.aihw.gov.au/what-is-asthma/.
[4] Jackson, D.J. et al. (2011) Asthma exacerbations: origin, effect, and prevention. J. Allergy Clin. Immunol. 128, 1165–1174.
| Asthma exacerbations: origin, effect, and prevention.Crossref | GoogleScholarGoogle Scholar | 22133317PubMed |
[5] Australian Centre for Asthma Monitoring (2011) Asthma in Australia 2011. AIHW Asthma Series no. 4. Cat. no. ACM 22, Canberra, AIHW.
[6] Toelle, B.G. et al. (2004) Prevalence of asthma and allergy in schoolchildren in Belmont, Australia: three cross sectional surveys over 20 years. BMJ 328, 386–387.
| Prevalence of asthma and allergy in schoolchildren in Belmont, Australia: three cross sectional surveys over 20 years.Crossref | GoogleScholarGoogle Scholar | 14962876PubMed |
[7] Australian Institute of Health and Welfare: Australian Centre for Asthma Monitoring (2009) Burden of disease due to asthma in Australia 2003. Cat. no. ACM 16, Canberra, AIHW.
[8] Powell, H. and Gibson, P.G. (2003) Inhaled corticosteroid doses in asthma: an evidence-based approach. Med. J. Aust. 178, 223–225.
| 12603186PubMed |
[9] Daley, D. et al. (2012) Associations and interactions of genetic polymorphisms in innate immunity genes with early viral infections and susceptibility to asthma and asthma-related phenotypes. J. Allergy Clin. Immunol. 130, 1284–1293.
| Associations and interactions of genetic polymorphisms in innate immunity genes with early viral infections and susceptibility to asthma and asthma-related phenotypes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVyht7fO&md5=a4d0ab1bc5c2a8efb264ed41f2269fa6CAS | 23063165PubMed |
[10] Çalışkan, M. et al. (2013) Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N. Engl. J. Med. 368, 1398–1407.
| Rhinovirus wheezing illness and genetic risk of childhood-onset asthma.Crossref | GoogleScholarGoogle Scholar | 23534543PubMed |
[11] Jackson, D.J. et al. (2008) Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 178, 667–672.
| Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children.Crossref | GoogleScholarGoogle Scholar | 18565953PubMed |
[12] McErlean, P. et al. (2008) Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C). PLoS ONE 3, e1847.
| Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C).Crossref | GoogleScholarGoogle Scholar | 18382652PubMed |
[13] Gern, J.E. (2010) The ABCs of rhinoviruses, wheezing, and asthma. J. Virol. 84, 7418–7426.
| The ABCs of rhinoviruses, wheezing, and asthma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVejtrbF&md5=232301f50a81689e74a3545722dd6308CAS | 20375160PubMed |
[14] Lee, W.M. et al. (2012) Human rhinovirus species and season of infection determine illness severity. Am. J. Respir. Crit. Care Med. 186, 886–891.
| Human rhinovirus species and season of infection determine illness severity.Crossref | GoogleScholarGoogle Scholar | 22923659PubMed |
[15] Arden, K.E. and Mackay, I.M. (2009) Human rhinoviruses: coming in from the cold. Genome Medicine 1, 44.
| Human rhinoviruses: coming in from the cold.Crossref | GoogleScholarGoogle Scholar | 19439028PubMed |
[16] Price, W.H. (1956) The isolation of a new virus associated with respiratory clinical disease in humans. Proc. Natl. Acad. Sci. USA 42, 892–896.
| The isolation of a new virus associated with respiratory clinical disease in humans.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28zitFajsA%3D%3D&md5=5b822b97877d971c2c3ccf8bb9d5a16cCAS | 16589969PubMed |
[17] Pelon, W. et al. (1957) A cytopathogenic agent isolated from naval recruits with mild respiratory illnesses. Exp. Biol. Med. 94, 262.
| A cytopathogenic agent isolated from naval recruits with mild respiratory illnesses.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaG2s%2FlsVGisA%3D%3D&md5=162dab32256c7339cf0618972a43ae7cCAS |
[18] Lee, W.M. et al. (2007) A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE 2, e966.
| A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants.Crossref | GoogleScholarGoogle Scholar | 17912345PubMed |
[19] Simmonds, P. et al. (2010) Proposals for the classification of human rhinovirus species C into genotypically assigned types. J. Gen. Virol. 91, 2409–2419.
| Proposals for the classification of human rhinovirus species C into genotypically assigned types.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht12qs73K&md5=d8c3da10df976f9af582e8c543feb03bCAS | 20610666PubMed |
[20] Bizzintino, J. et al. (2011) Association between human rhinovirus C and severity of acute asthma in children. Eur. Respir. J. 37, 1037–1042.
| Association between human rhinovirus C and severity of acute asthma in children.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mvmtlaitw%3D%3D&md5=b07b3e12072829c32a3b79bc36ece9e5CAS | 20693244PubMed |
[21] Miller, E.K. et al. (2009) A novel group of rhinoviruses is associated with asthma hospitalizations. J. Allergy Clin. Immunol. 123, 98–104.e101.
| A novel group of rhinoviruses is associated with asthma hospitalizations.Crossref | GoogleScholarGoogle Scholar |
[22] Linder, J.E. et al. (2013) Human rhinovirus C: age, season, and lower respiratory illness over the past 3 decades. J. Allergy Clin. Immunol. 131, 69–77.e1-6.
| Human rhinovirus C: age, season, and lower respiratory illness over the past 3 decades.Crossref | GoogleScholarGoogle Scholar | 23146382PubMed |
[23] Tovey, E.R. and Rawlinson, W.D. (2011) A modern miasma hypothesis and back-to-school asthma exacerbations. Med. Hypotheses 76, 113–116.
| A modern miasma hypothesis and back-to-school asthma exacerbations.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M%2FltVWltQ%3D%3D&md5=69693c0f2d616f40cc65b76fde29bb1dCAS | 20869177PubMed |
[24] Lambert, S.B. et al. (2007) Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool-aged children using parent-collected specimens. Pediatrics 120, e929–e937.
| Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool-aged children using parent-collected specimens.Crossref | GoogleScholarGoogle Scholar | 17875651PubMed |
[25] Mackay, I.M. et al. (2013) Community-wide, contemporaneous circulation of a broad spectrum of human rhinoviruses in healthy Australian preschool-aged children during a 12-month period. J. Infect. Dis. 207, 1433–1441.
| Community-wide, contemporaneous circulation of a broad spectrum of human rhinoviruses in healthy Australian preschool-aged children during a 12-month period.Crossref | GoogleScholarGoogle Scholar | 22829638PubMed |
[26] Arden, K.E. et al. (2010) Newly identified respiratory viruses in children with asthma exacerbation not requiring admission to hospital. J. Med. Virol. 82, 1458–1461.
| Newly identified respiratory viruses in children with asthma exacerbation not requiring admission to hospital.Crossref | GoogleScholarGoogle Scholar | 20572080PubMed |
[27] Papadopoulos, N.G. et al. (2011) Viruses and bacteria in acute asthma exacerbations--a GA(2) LEN-DARE systematic review. Allergy 66, 458–468.
| Viruses and bacteria in acute asthma exacerbations--a GA(2) LEN-DARE systematic review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mrlt1ahsw%3D%3D&md5=4ee6bd5505f3f5384925d976d1aacc7fCAS | 21087215PubMed |
[28] Myles, P. et al. (2013) Differences between asthmatics and nonasthmatics hospitalised with influenza a infection. Eur. Respir. J. 41, 824–831.
| Differences between asthmatics and nonasthmatics hospitalised with influenza a infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnt12rt78%3D&md5=a16f2fb95b25641195ac77aa6902a136CAS | 22903963PubMed |
[29] Rawlinson, W.D. et al. (2003) Asthma exacerbations in children associated with rhinovirus but not human metapneumovirus infection. J. Infect. Dis. 187, 1314.
| Asthma exacerbations in children associated with rhinovirus but not human metapneumovirus infection.Crossref | GoogleScholarGoogle Scholar | 12696012PubMed |
[30] García-García, M.L. et al. (2007) Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5. Pediatr. Pulmonol. 42, 458–464.
| Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5.Crossref | GoogleScholarGoogle Scholar | 17427899PubMed |
[31] Nissen, M.D. et al. (2002) Evidence of human metapneumovirus in Australian children. Med. J. Aust. 176, 188.
| 11913922PubMed |
[32] van den Hoogen, B.G. et al. (2001) A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7, 719–724.
| A newly discovered human pneumovirus isolated from young children with respiratory tract disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnsVCqtbY%3D&md5=fd17c8867237425132438635d816e5d2CAS | 11385510PubMed |
[33] Papadopoulos, N.G. et al. (2011) Viruses and bacteria in acute asthma exacerbations – a GA2LEN-DARE* systematic review. Allergy 66, 458–468.
| Viruses and bacteria in acute asthma exacerbations – a GA2LEN-DARE* systematic review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mrlt1ahsw%3D%3D&md5=4ee6bd5505f3f5384925d976d1aacc7fCAS | 21087215PubMed |
[34] Allander, T. et al. (2007) Human bocavirus and acute wheezing in children. Clin. Infect. Dis. 44, 904–910.
| Human bocavirus and acute wheezing in children.Crossref | GoogleScholarGoogle Scholar | 17342639PubMed |
[35] Sigurs, N. et al. (2010) Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 65, 1045–1052.
| Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life.Crossref | GoogleScholarGoogle Scholar | 20581410PubMed |
[36] Kusel, M.M.H. et al. (2012) Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur. Respir. J. 39, 876–882.
| Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38zlvVWntw%3D%3D&md5=7e303b8a7b4b4984830ad2881e1fd907CAS |
[37] Kuehni, C.E. et al. (2009) Causal links between RSV infection and asthma: no clear answers to an old question. Am. J. Respir. Crit. Care Med. 179, 1079–1080.
| Causal links between RSV infection and asthma: no clear answers to an old question.Crossref | GoogleScholarGoogle Scholar | 19498062PubMed |
[38] Qasem, J.A. et al. (2013) Application of three uniplex polymerase chain reaction assays for the detection of atypical bacteria in asthmatic patients in Kuwait. J. Infect. Public Health 6, 134–141.
| Application of three uniplex polymerase chain reaction assays for the detection of atypical bacteria in asthmatic patients in Kuwait.Crossref | GoogleScholarGoogle Scholar | 23537827PubMed |
[39] Gil, J.C. et al. (1993) Isolation of mycoplasma pneumoniae from asthmatic patients. Ann. Allergy 70, 23–25.
| 1:STN:280:DyaK3s7ktlWitg%3D%3D&md5=e954b83aa49ef78d06fe77fd6d74da23CAS | 8424592PubMed |
[40] Biscardi, S. et al. (2004) Mycoplasma pneumoniae and asthma in children. Clin. Infect. Dis. 38, 1341–1346.
| Mycoplasma pneumoniae and asthma in children.Crossref | GoogleScholarGoogle Scholar | 15156467PubMed |
[41] Denning, D.W. et al. (2013) Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med. Mycol. 51, 361–370.
| Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults.Crossref | GoogleScholarGoogle Scholar | 23210682PubMed |
Biographies
Christiana Willenborg is a Research Assistant in the Virology Research Laboratory at Prince of Wales Hospital. Her current research involves examining the respiratory viruses that infect children at risk of an asthma exacerbation and how these viruses affect their asthma. The aim is to learn why colds and flu make asthma worse and to use the information to reduce or prevent exacerbations.
Sacha Stelzer-Braid is a Postdoctoral Scientist in the Virology Research laboratory at Prince of Wales Hospital and University of New South Wales. Her research interests include novel sampling and diagnostic methods for respiratory viruses, and examining the role of respiratory viruses in exacerbations of asthma and cystic fibrosis.


