Epstein–Barr virus-associated malignancies: pathobiology and emerging therapeutic options
Corey Smith and Rajiv KhannaCentre for Immunotherapy and Vaccine Development
Queensland Institute of Medical Research
Tumour Immunology Laboratory
Department of Immunology
300 Herston Road, Brisbane
Qld 4006, Australia
Tel: +61 7 3362 0385
Fax: +61 7 3845 3510
Email: rajiv.khanna@qimr.edu.au
Microbiology Australia 34(3) 120-124 https://doi.org/10.1071/MA13041
Published: 4 September 2013
Epstein–Barr virus (EBV) was first identified in malignant Burkitt lymphoma cells in 1964. Since then, EBV has been associated with a number of other malignancies of either lymphocytic origin, including both B cell and NK/T cell cancers, or epithelial origin, predominantly nasopharyngeal and gastric cancers. While a complete understanding of the relationship between EBV-mediated cellular transformation and the oncogenic events that lead to uncontrolled malignant cell growth remains to be determined for a number of these cancers, it is clear in all of these settings that a breakdown in the immune surveillance of virally infected cells contributes to the survival of EBV-bearing malignant cells.
The lifecycle of EBV infection
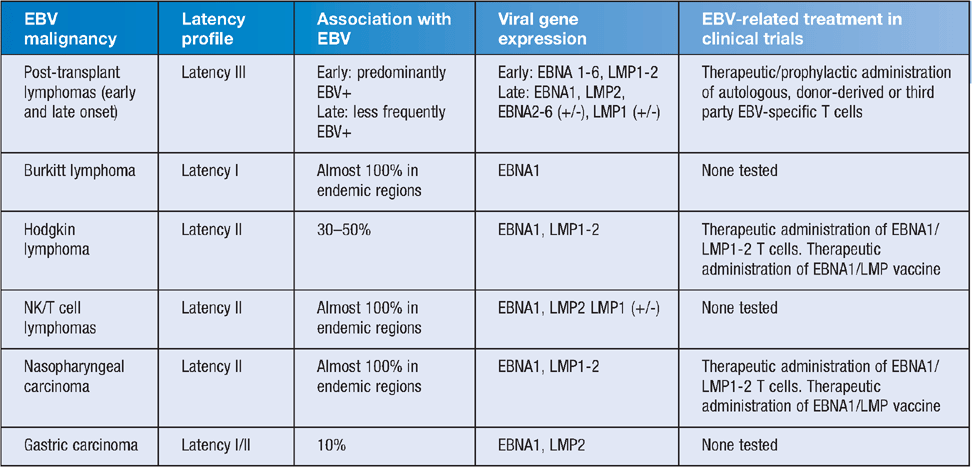
As with most members of the Herpesvirus family, evolutionary adaptation over millions of years has allowed EBV to establish a unique biological niche in humans that allows it to maintain persistent infection for life with typically limited complications for the host and an efficient capacity to infect a new host. As a consequence of this efficient lifecycle, EBV is ubiquitous in the community, infecting 90–95% of the world population1. EBV infection typically occurs via the mucosal surfaces of the oropharynx. Primary lytic infection is followed by the establishment of latent infection of B lymphocytes2,3. Following transition of the linear EBV genome to the nucleus of the B cell, the EBV chromosome is circularised. Latent gene expression is then initiated from the W promoter, inducing the expression of the EBV nuclear antigens (EBNA) 2 and 5, which then induce the expression of other EBNA proteins, including EBNA1, 3, 4 and 6, and the latent membrane proteins (LMP) 1, 2a and 2b. While all of the latent cycle genes play important roles in latent transformation, EBNA1 is indispensible for viral latency and functions by binding to the origin of replication, oriP, on the viral episome. EBNA1 tethers the viral episome to the host chromosome, promoting maintenance of the latent EBV episome in daughter cells. Intermittent lytic replication in the upper respiratory tract promotes viral shedding and spread to a new host. This unique aspect of EBV biology, and that of the related gamma-herpesviruses, distinguishes it from other herpesviruses by promoting B cell proliferation without the need for active viral replication. However, it is also this capacity to efficiently transform B cells and induce lymphoproliferation, which leads to both the malignant and lymphoproliferative disorders, that are associated with EBV-infected cells4–6. A summary of common EBV-associated malignancies and their viral gene expression is shown in Table 1.
|
Post-transplant lymphoproliferative disorders
Post-transplant lymphoproliferative disorders (PTLD) arise in immunocompromised patients who have undergone either solid organ (SOT) or haematopoietic stem cell transplantation (HSCT) and are almost universally associated with EBV infection7. Under normal immunological conditions EBV-infected B cells are controlled by EBV-specific cytotoxic T lymphocytes, which efficiently clear these cells predominantly via the recognition of peptide epitopes encoded by EBNA3-68. However, the immunosuppressive environment associated with SOT and HSCT disrupts this immunological balance and can lead to uncontrolled proliferation of these EBV-infected cells that can typically be characterised with a latency III profile and the expression of the full array of latent genes9 (Table 1). PTLD in SOT patients is usually of recipient origin and is most prevalent in seronegative transplant recipients as a consequence of primary infection following transplant of organs containing EBV-infected cells. However, PTLD can occur in seropositive recipients as a consequence of the immunosuppressive regime used to prevent organ rejection. In Australia, PTLD is typically diagnosed in 1–10% of SOT recipients and is more highly prevalent in children due to an increased likelihood of seronegative status prior to transplant10. PTLD in HSCT patients is usually of donor origin and is most prevalent in seropositive recipients who receive a transplant from a seronegative donor. This is typically facilitated by the reactivation of EBV of recipient origin and subsequent infection of B cells of donor origin and the concomitant immunosuppression of the induction of an EBV-specific cellular immune response. However, PTLD does occur following transplant from seropositive donors and increased risks are also associated with T cell depleted HSCT, commonly used to reduce the risk of graft-versus-host disease and due to HLA mismatch between donor and recipient7.
A reduction in the immunosuppressive regime is usually the first option for treatment of PTLD in both SOT and HSCT patients in order to restore/promote EBV-specific cellular immunity. Rituximab, an antibody that recognises CD20 on the surface of most B cells, is also used to treat PTLD in both SOT and HSCT patients, often in combination with standard chemotherapy used to treat other B cell lymphomas11. In addition, adoptive cellular therapy is emerging as a powerful tool for the treatment of EBV-associated PTLD, particularly in the context of HSCT. Pioneered by Rooney and colleagues at the Baylor College of Medicine in Texas, the transfer of EBV-specific T cells, which classically involves the use of in vitro-generated EBV-infected lymphoblastoid cell lines (LCL) to stimulate donor memory T cells, have been used effectively both therapeutically and prophylactically to treat or prevent PTLD in HSCT patients12–14. However, in the context of PTLD in SOT patients or following a seronegative HSCT transplant, the success of EBV-specific cellular therapy is dependent on the generation of recipient-derived T cells15 (Table 1). While this has been used successfully to resolve PTLD in SOT patients, an emerging approach for the treatment of PTLD in these instances is the use of a bank of third-party T cells16–19. These T cells are generated from another EBV-seropositive healthy donor who shares HLA alleles with the patient. Initial reservations surrounding the potential risk of inducing graft-versus-host disease or organ rejection have proven to be unfounded following the administration of third-party T cells and they have proven effective in the treatment of PTLD in both SOT and HSCT patients7,20.
EBV-associated Hodgkin and non-Hodgkin lymphomas
In Australia, Europe and North America, 30–50% of Hodgkin lymphoma (HL) cases are associated with EBV. In contrast, EBV positivity in HL cases reaches as high as 100% in certain regions of Asia, Africa and Latin America21. A unique characteristic of HL is the large inflammatory infiltrate that is associated with lymphoid organs in HL patients. The malignant Reed–Sternberg cells in HL comprise less than 1% of the cellular mass in inflamed lymphoid organs and the nature of the infiltrate is used histologically to characterise the HL subtype21. While associated with all HL subtypes, EBV infection is most prevalent in classical HL. EBV-infected Reed–Sternberg cells express a latency II gene expression pattern, which is restricted to EBNA1 and LMP1&2 (Table 1), both of which play important roles in the transformation of infected B cells22,23. In addition to their important role in the maintenance and transformation of EBV-infected B cells, EBNA1 and LMP1&2 are poorly immunogenic, relative to the other EBV-latent molecules. T cells specific for EBNA1&LMP1 display a loss of function during acute HL and an increased susceptibility to immunomodulatory molecules generated by HL cells, such as galectin-124–26. These immune evasion strategies are likely important for the maintenance of EBV-infected B cells in immunocompetent hosts, but also provide mechanisms for immune evasion by malignant Reed–Sternberg cells27.
The incidence of HL is bimodal in nature, with a peak in incidence in children under 10 years and in adults over 50 years. An increased risk of developing EBV-associated HL has also been linked to infectious mononucleosis during primary EBV infection28. HL is highly amenable to current chemotherapeutic regimes, with 5-year survival rates approaching 90%. However, EBV infection is associated with more rapid progression in HL patients and reduced overall survival29. The current chemotherapeutic regimes used to treat HL are also associated with an increased risk of the development of secondary cancers and strategies to reduce the dependence of the current level of chemotherapy are under development, particularly for children30. EBV-associated HL, often when refractory to chemotherapy, has also been successfully treated with adoptive cellular therapy29,31–36. While initial studies focussed on the LCL-based approach used to treat PTLD, more recent approaches have specifically targeted the induction of T cells specific for EBNA1 and LMP1&237. These approaches have proved to be successful in phase 1 clinical studies and are currently being evaluated in phase II studies31.
EBV infection is also associated with a number of non-Hodgkin’s lymphomas (NHL), including Burkitt lymphoma (BL) and diffuse large B cell lymphoma (DLBCL)38. Although rare in Australia, EBV-associated BL is endemic in regions of Africa and Papua New Guinea22. It arises primarily in young children and its incidence is closely associated with malaria endemic regions, which has been attributed to a loss of functionality in EBV-specific T cells in children with co-exposure to malaria39,40. Burkitt lymphoma cells typically display an EBV latency I profile, characterised by the expression of only EBNA1 (Table 1). In association with reduced expression of surface HLA molecules, this renders BL cells highly immunoevasive41–43. Burkitt lymphoma is also characterised by the translocation of the gene encoding the oncogenic c-MYC protein44.
Nasopharyngeal carcinoma
EBV infection is associated with the majority of undifferentiated nasopharyngeal carcinoma (NPC)45–47. While rare in the Australian population, with an incidence of less than 1 in 100,000, EBV-associated NPC is endemic in regions of south-east Asia and North Africa. It reaches its highest prevalence in southern China with incidence rates of 15–50 cases per 100,000 within some communities48. An increased incidence of NPC is also evident in Australia in immigrant populations from these regions; however, this incidence is reduced in first generation Australians, indicative of the role both genetics and environment play in the development of NPC.
Similar to HL, EBV gene expression in malignant NPC cells is restricted to EBNA1 and LMP1&2. Current treatment options for NPC typically involve surgical intervention, radiotherapy and/or chemotherapy49. Standard treatment options have seen response rates in primary disease improve to greater than 90%. Despite this, a significant proportion of patients, particularly those who initially present with late stage disease, will relapse, which often leads to distance metastases that are refractory to conventional therapy49. EBV-targeted treatment options are therefore in development as adjunct approaches to current radio/chemotherapeutic regimes. While some strategies focus on the induction of lytic viral reactivation as a potential mechanism of enhancing cellular immunity and inducing lysis of malignant cells, the majority of these approaches focus on using EBV-specific immunotherapy to treat NPC33 (Table 1). Augmentation of cellular immunity to EBNA1 and the LMP antigens have emerged as potential adjunct or alternative approach to chemo/radiotherapy. A number of phase I clinical studies treating NPC with a range of immunotherapeutic strategies have provided evidence that these approaches are safe and might have some clinical benefit, particularly in patients with locoregional disease33,50. Current efforts are being placed on validating these observations in larger cohorts of patients.
Other EBV-associated malignancies
EBV-infection has also been associated with a number of other malignancies of both lymphocytic and epithelial cell origin, including a range of NK/T cell lymphomas, lymphoepithelial-like carcinomas and gastric carcinomas. While not considered a normal target for EBV infection, EBV is associated with a subset of lymphomas of NK and T cell origin51. The most prevalent of these lymphomas are the nasal NK/T lymphomas that are rare in Australia, but more common in parts of south-east Asia. EBV-associated NK/T cell lymphomas have a very poor prognosis, with current 5-year survival rates of less than 50%51. EBV infection in NK/T cell lymphomas is characterised with a latency I/II gene expression profile, characterised predominantly by the expression of EBNA1 and LMP252 (Table 1). Despite this, due to the lack of an in vitro model, the role EBV and these latent antigens play in the tumorigenesis of NK/T cell lymphomas is not clear.
The EBV-associated lymphoepithelial-like carcinomas share features with NPC but are found in a range of other anatomical sites, including the stomach53,54. These cancers also show a distinct geographical distribution and are endemic in regions of Asia and indigenous communities in America, further evidence for the influence of environment and genetics on the incidence of EBV-associated carcinoma. EBV-infection is also associated with a small percentage of non-lymphoepithelial-like gastric carcinoma cases55. While the incidence of gastric carcinoma has reduced significantly in most developed countries, the incidence remains high in developing countries and in some developed countries, including Japan and Korea56. EBV infection in gastric carcinoma cells is associated with a latency I/II gene expression profile, characterised by the expression of EBNA1 and in some instances LMP2A57,58. Although no aetiological relationship has been established between EBV infection and the transformation of gastric carcinoma cells, it will be important to explore therapeutic options that specifically target EBV to improve clinical outcome59.
Concluding remarks
Over the past two decades considerable knowledge has been accumulated on EBV pathogenesis in different malignancies. This knowledge has recently been successfully exploited to develop new diagnostic and therapeutic tools that have significantly impacted on the clinical management of patients with EBV-associated diseases.
References
[1] Rickinson, A.B. et al. (1996) Epstein–Barr virus. In Fields Virology (Vol. 3), pp. 2397–2446, Philadelphia, Lippincott–Raven Publishers.[2] Rickinson, A.B. and Moss, D.J. (1997) Human cytotoxic T lymphocyte responses to Epstein–Barr virus infection. Annu. Rev. Immunol. 15, 405–431.
| Human cytotoxic T lymphocyte responses to Epstein–Barr virus infection.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXisVCmtrc%3D&md5=89a536220f6497d4fe5f6a407a96a2b1CAS | 9143694PubMed |
[3] Thorley-Lawson, D.A. (2001) Epstein–Barr virus: exploiting the immune system. Nat. Rev. Immunol. 1, 75–82.
| Epstein–Barr virus: exploiting the immune system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlvF2qtb8%3D&md5=0ee4637828a32562ad99bf4b7d42dc87CAS | 11905817PubMed |
[4] Moss, P. and Rickinson, A. (2005) Cellular immunotherapy for viral infection after HSC transplantation. Nat. Rev. Immunol. 5, 9–20.
| Cellular immunotherapy for viral infection after HSC transplantation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXlvVOg&md5=876ed1005ac42986a8c584dbb7d44ecbCAS | 15630425PubMed |
[5] Rickinson, A.B. (1992) Introduction: viruses and human cancer. Semin. Cancer Biol. 3, 249–251.
| 1:STN:280:DyaK3s7itVGisA%3D%3D&md5=57a2c3fe2512ba63106bfaa41fb186daCAS | 1477330PubMed |
[6] Kieff, E. et al. (1996) Epstein–Barr virus and its replication. In Virology (Vol. 3), pp. 2343–2396, Philadelphia, Raven Press.
[7] Bollard, C.M. et al. (2012) T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat. Rev. Clin. Oncol. 9, 510–519.
| T-cell therapy in the treatment of post-transplant lymphoproliferative disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xht1Kjs7zP&md5=f53de565415f6789d7d746d20cc8b07cCAS | 22801669PubMed |
[8] Young, L. et al. (1989) Expression of Epstein–Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N. Engl. J. Med. 321, 1080–1085.
| Expression of Epstein–Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3c%2FhsV2rug%3D%3D&md5=6886e917c7ee95b6b1f1d786cb421e72CAS | 2552313PubMed |
[9] Razonable, R.R. and Paya, C.V. (2003) Herpesvirus infections in transplant recipients: current challenges in the clinical management of cytomegalovirus and Epstein–Barr virus infections. Herpes 10, 60–65.
| 14759337PubMed |
[10] Khanna, R. et al. (2001) Immunotherapeutic strategies for EBV-associated malignancies. Trends. Mol. Med. 7, 270–276.
| Immunotherapeutic strategies for EBV-associated malignancies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXltVahu7c%3D&md5=f14d2c857968899cd7db7eb3f2ff29b8CAS | 11378517PubMed |
[11] Kuehnle, I. et al. (2000) CD20 monoclonal antibody (rituximab) for therapy of Epstein–Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood 95, 1502–1505.
| 1:CAS:528:DC%2BD3cXhtFKitbc%3D&md5=b0b2d3a507c04f5420541aa70f9fbeb2CAS | 10666232PubMed |
[12] Rooney, C.M. et al. (1995) Use of gene-modified virus-specific T lymphocytes to control Epstein–Barr-virus-related lymphoproliferation. Lancet 345, 9–13.
| Use of gene-modified virus-specific T lymphocytes to control Epstein–Barr-virus-related lymphoproliferation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2M%2FpslOgsA%3D%3D&md5=700cbb8a0a6995f8e3357607a82377a4CAS | 7799740PubMed |
[13] Rooney, C.M. et al. (1998) Infusion of cytotoxic T cells for the prevention and treatment of Epstein–Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 92, 1549–1555.
| 1:CAS:528:DyaK1cXls1Siu7w%3D&md5=ad77222e0572e741b38f7bfd403fa636CAS | 9716582PubMed |
[14] Heslop, H.E. et al . (2010) Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 115, 925–935.
| Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhvFOitr0%3D&md5=700eca907b91dd49d9c55e4348049daaCAS | 19880495PubMed |
[15] Khanna, R. et al. (1999) Activation and adoptive transfer of Epstein–Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc. Natl Acad. Sci. USA 96, 10391–10396.
| Activation and adoptive transfer of Epstein–Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlvFentL8%3D&md5=eb708ad3ef95545a0225e0f72b5aa5cfCAS | 10468618PubMed |
[16] Haque, T. et al. (2001) Complete regression of posttransplant lymphoproliferative disease using partially HLA-matched Epstein Barr virus-specific cytotoxic T cells. Transplantation 72, 1399–1402.
| Complete regression of posttransplant lymphoproliferative disease using partially HLA-matched Epstein Barr virus-specific cytotoxic T cells.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MnhtV2ntw%3D%3D&md5=efaac24c150f4820f1d4b1938d5009a0CAS | 11685111PubMed |
[17] Haque, T. et al. (2007) Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 110, 1123–1131.
| Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXptFKmu78%3D&md5=ad1949940f8b84cea2ffa9d818b57679CAS | 17468341PubMed |
[18] Haque, T. et al. (2002) Treatment of Epstein–Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet 360, 436–442.
| Treatment of Epstein–Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells.Crossref | GoogleScholarGoogle Scholar | 12241714PubMed |
[19] Leen, A.M. et al. (2013) Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121, 5113–5123.
| Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFSnt7fM&md5=d22b9634b7f385bfb8b45bc3f4429739CAS | 23610374PubMed |
[20] Bollard, C.M. et al. (2008) Immunotherapy targeting EBV-expressing lymphoproliferative diseases. Best Pract. Res. Clin. Haematol. 21, 405–420.
| Immunotherapy targeting EBV-expressing lymphoproliferative diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFanurzP&md5=77633262283fa820cd8fb9ef5906ebbdCAS | 18790446PubMed |
[21] Gandhi, M.K. et al. (2004) Epstein–Barr virus‐associated Hodgkin’s lymphoma. Br. J. Haematol. 125, 267–281.
| Epstein–Barr virus‐associated Hodgkin’s lymphoma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXkvFChsLo%3D&md5=8d53383a68d5b7f57427e69cd4d473e4CAS | 15086409PubMed |
[22] Ahmed, N. and Heslop, H.E. (2006) Viral lymphomagenesis. Curr. Opin. Hematol. 13, 254–259.
| Viral lymphomagenesis.Crossref | GoogleScholarGoogle Scholar | 16755222PubMed |
[23] Koduru, P.R.K. et al. (1993) Phenotypic and genotypic characterization of Hodgkins disease. Am. J. Hematol. 44, 117–124.
| Phenotypic and genotypic characterization of Hodgkins disease.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2c%2FptFarsg%3D%3D&md5=445d45a11e3cf733edc0742df51db5b7CAS |
[24] Gandhi, M.K. et al. (2007) Galectin-1 mediated suppression of Epstein–Barr virus-specific T-cell immunity in classic Hodgkin lymphoma. Blood 110, 1326–1329.
| Galectin-1 mediated suppression of Epstein–Barr virus-specific T-cell immunity in classic Hodgkin lymphoma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXptFKls7g%3D&md5=ee887f366367d7857e7bb8ec5018c769CAS | 17438085PubMed |
[25] Gandhi, M.K. et al. (2006) Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood 108, 2280–2289.
| Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVCgur7F&md5=dac7354c5a937ac4dc0efaf41dba2f4fCAS | 16757686PubMed |
[26] Smith, C. et al. (2009) Acquisition of polyfunctionality by Epstein–Barr virus-specific CD8+ T cells correlates with increased resistance to galectin-1-mediated suppression. J. Virol. 83, 6192–6198.
| Acquisition of polyfunctionality by Epstein–Barr virus-specific CD8+ T cells correlates with increased resistance to galectin-1-mediated suppression.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntFWqur4%3D&md5=bdb9a6c1bdc0d3ea4db679a6736d2e96CAS | 19357166PubMed |
[27] Poppema, S. and van den Berg, A. (2000) Interaction between host T cells and Reed–Sternberg cells in Hodgkin lymphomas. Semin. Cancer Biol. 10, 345–350.
| Interaction between host T cells and Reed–Sternberg cells in Hodgkin lymphomas.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M7js1ejtQ%3D%3D&md5=a9e653e338cd698271075a8a49ae29b0CAS | 11100882PubMed |
[28] Rosdahl, N. et al. (1974) Hodgkin’s disease in patients with previous infectious mononucleosis: 30 years’ experience. BMJ 2, 253–256.
| Hodgkin’s disease in patients with previous infectious mononucleosis: 30 years’ experience.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaE2c7mtVWktQ%3D%3D&md5=284a31f4e351216f071d9f6235c59ec6CAS | 4406463PubMed |
[29] Kanakry, J.A. and Ambinder, R.F. (2013) EBV-related lymphomas: new approaches to treatment. Curr. Treat. Options Oncol. 14, 224–236.
| EBV-related lymphomas: new approaches to treatment.Crossref | GoogleScholarGoogle Scholar | 23549980PubMed |
[30] Kelly, K.M. et al. (2011) BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: a report from the Children’s Oncology Group. Blood 117, 2596–2603.
| BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: a report from the Children’s Oncology Group.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsVWguro%3D&md5=8fc06af55ed51dce9bdc88323206d781CAS | 21079154PubMed |
[31] Gottschalk, S. et al. (2006) T cell therapies. Ernst Schering Found Symp Proc. , 69–82.
| 1:STN:280:DC%2BD2srislKktA%3D%3D&md5=a3cfb0c04bfc5f1100e55aeb7a8fbe67CAS | 17824182PubMed |
[32] Bollard, C.M. et al. (2006) Administration of latent membrane protein 2-specific cytotoxic T lymphocytes to patients with relapsed Epstein–Barr virus-positive lymphoma. Clin. Lymphoma Myeloma 6, 342–347.
| Administration of latent membrane protein 2-specific cytotoxic T lymphocytes to patients with relapsed Epstein–Barr virus-positive lymphoma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XisFWktbc%3D&md5=5a27089829a7b3bc9ebc62869cc1efeeCAS | 16507214PubMed |
[33] Gottschalk, S. et al. (2005) Adoptive immunotherapy for EBV-associated malignancies. Leuk. Lymphoma 46, 1–10.
| Adoptive immunotherapy for EBV-associated malignancies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFSntr3J&md5=b224fd899268216268dde48a5947e402CAS | 15621775PubMed |
[34] Bollard, C.M. et al. (2004) The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J. Immunother. 27, 317–327.
| The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease.Crossref | GoogleScholarGoogle Scholar | 15235393PubMed |
[35] Bollard, C.M. et al. (2004) Cytotoxic T lymphocyte therapy for Epstein–Barr virus+ Hodgkin’s disease. J. Exp. Med. 200, 1623–1633.
| Cytotoxic T lymphocyte therapy for Epstein–Barr virus+ Hodgkin’s disease.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtFGmurbI&md5=32f7f7903ad5424c42a641481533a6e6CAS | 15611290PubMed |
[36] Straathof, K.C. et al. (2003) Immunotherapy for Epstein–Barr virus-associated cancers in children. Oncologist 8, 83–98.
| Immunotherapy for Epstein–Barr virus-associated cancers in children.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhvVyqtr0%3D&md5=1e00b7370485ffb839c5df675c1a8e36CAS | 12604735PubMed |
[37] Smith, C. et al. (2006) Functional reversion of antigen-specific CD8+ T cells from patients with Hodgkin lymphoma following in vitro stimulation with recombinant polyepitope. J. Immunol. 177, 4897–4906.
| 1:CAS:528:DC%2BD28Xps1GnsLk%3D&md5=f8c327b8ded39d6142eaf06f93ae61e8CAS | 16982932PubMed |
[38] Heslop, H.E. (2005) Biology and treatment of Epstein–Barr virus-associated non-Hodgkin lymphomas. Hematology (Am Soc Hematol Educ Program) , 260–266.
| Biology and treatment of Epstein–Barr virus-associated non-Hodgkin lymphomas.Crossref | GoogleScholarGoogle Scholar | 16304390PubMed |
[39] Njie, R. et al. (2009) The effects of acute malaria on Epstein–Barr virus (EBV) load and EBV-specific T cell immunity in Gambian children. J. Infect. Dis. 199, 31–38.
| The effects of acute malaria on Epstein–Barr virus (EBV) load and EBV-specific T cell immunity in Gambian children.Crossref | GoogleScholarGoogle Scholar | 19032105PubMed |
[40] Chattopadhyay, P.K. et al. (2013) Holoendemic malaria exposure is associated with altered Epstein–Barr virus-specific CD8(+) T-cell differentiation. J. Virol. 87, 1779–1788.
| Holoendemic malaria exposure is associated with altered Epstein–Barr virus-specific CD8(+) T-cell differentiation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltV2itL0%3D&md5=ad3db2d2841e0bfb60896be1622ac8e1CAS | 23175378PubMed |
[41] Münz, C. et al. (2000) Human CD4(+) T lymphocytes consistently respond to the latent Epstein–Barr virus nuclear antigen EBNA1. J. Exp. Med. 191, 1649–1660.
| Human CD4(+) T lymphocytes consistently respond to the latent Epstein–Barr virus nuclear antigen EBNA1.Crossref | GoogleScholarGoogle Scholar | 10811859PubMed |
[42] Khanna, R. and Burrows, S.R. (2000) Role of cytotoxic T lymphocytes in Epstein–Barr virus-associated diseases. Annu. Rev. Microbiol. 54, 19–48.
| Role of cytotoxic T lymphocytes in Epstein–Barr virus-associated diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXotFWrsrc%3D&md5=6eb6c377f365217cf00e30da6e92b27cCAS | 11018123PubMed |
[43] Moss, D.J. et al. (1999) Developing immunotherapeutic strategies for the control of Epstein–Barr virus-associated malignancies. J. Acquir. Immune. Defic. Syndr. 21, S80–S83.
| 1:CAS:528:DC%2BD3cXjtlKktbk%3D&md5=61a47019a3807bfb3224591ce0484663CAS | 10430223PubMed |
[44] Haluska, F.G. et al. (1986) The t(8; 14) chromosomal translocation occurring in B-cell malignancies results from mistakes in V-D-J joining. Nature 324, 158–161.
| The t(8; 14) chromosomal translocation occurring in B-cell malignancies results from mistakes in V-D-J joining.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXivVCjtg%3D%3D&md5=abf3905aeb5b991479eaefc3b36a6652CAS | 3097550PubMed |
[45] Razak, A.R. et al. (2010) Nasopharyngeal carcinoma: the next challenges. Eur. J. Cancer 46, 1967–1978.
| Nasopharyngeal carcinoma: the next challenges.Crossref | GoogleScholarGoogle Scholar | 20451372PubMed |
[46] Cho, W.C. (2007) Nasopharyngeal carcinoma: molecular biomarker discovery and progress. Mol. Cancer 6, 1.
| Nasopharyngeal carcinoma: molecular biomarker discovery and progress.Crossref | GoogleScholarGoogle Scholar | 17199893PubMed |
[47] Chan, A.T. et al. (2002) Nasopharyngeal carcinoma. Ann. Oncol. 13, 1007–1015.
| Nasopharyngeal carcinoma.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38vitFWgtg%3D%3D&md5=8c528d87fc489cf1c787f1d8786406eaCAS | 12176778PubMed |
[48] Raab-Traub, N. (2002) Epstein–Barr virus in the pathogenesis of NPC. Semin.Cancer Biol. 12, 431–441.
| Epstein–Barr virus in the pathogenesis of NPC.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xosl2gu7o%3D&md5=26786e326927f07b6442c3f76ec3e6aeCAS | 12450729PubMed |
[49] Bensouda, Y. et al. (2011) Treatment for metastatic nasopharyngeal carcinoma. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 128, 79–85.
| Treatment for metastatic nasopharyngeal carcinoma.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mvls1eruw%3D%3D&md5=a68031c3795ee1d6d90e2a0ef02b9291CAS | 21177151PubMed |
[50] Smith, C. et al. (2012) Effective treatment of metastatic forms of Epstein–Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res. 72, 1116–1125.
| Effective treatment of metastatic forms of Epstein–Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjt1Wisrw%3D&md5=026d538092744dbfb38b82bdf333beabCAS | 22282657PubMed |
[51] Tse, E. and Kwong, Y.L. (2013) How I treat NK/T-cell lymphomas. Blood 121, 4997–5005.
| How I treat NK/T-cell lymphomas.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtVartL%2FF&md5=0eeb3c9aee3fb4037aece653b2503aedCAS | 23652805PubMed |
[52] Bugalia, A. et al. (2013) Immunomorphologic profile and Epstein–Barr virus status of a cohort of 35 cases of extranodal natural killer/T-cell lymphoma, nasal type of upper aerodigestive tract from a tertiary care center in South India. Leuk. Lymphoma 54, 1201–1207.
| Immunomorphologic profile and Epstein–Barr virus status of a cohort of 35 cases of extranodal natural killer/T-cell lymphoma, nasal type of upper aerodigestive tract from a tertiary care center in South India.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXns1ahu78%3D&md5=0c9964b85d27f3216a6fe057bb4c711eCAS | 23098105PubMed |
[53] Hildesheim, A. (2013) Invited commentary: Epstein–Barr virus-based screening for the early detection of nasopharyngeal carcinoma: a new frontier. Am. J. Epidemiol. 177, 251–253.
| Invited commentary: Epstein–Barr virus-based screening for the early detection of nasopharyngeal carcinoma: a new frontier.Crossref | GoogleScholarGoogle Scholar | 23255781PubMed |
[54] Lee, J.H. et al. (2009) Clinicopathological and molecular characteristics of Epstein–Barr virus-associated gastric carcinoma: a meta-analysis. J. Gastroenterol. Hepatol. 24, 354–365.
| Clinicopathological and molecular characteristics of Epstein–Barr virus-associated gastric carcinoma: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 19335785PubMed |
[55] Shah, K.M. and Young, L.S. (2009) Epstein–Barr virus and carcinogenesis: beyond Burkitt’s lymphoma. Clin. Microbiol. Infect. 15, 982–988.
| Epstein–Barr virus and carcinogenesis: beyond Burkitt’s lymphoma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFChtbrM&md5=390a84bf0368e2ccb5837865273a0d28CAS | 19874382PubMed |
[56] Trimeche, M. et al. (2009) Prevalence and characteristics of Epstein–Barr virus-associated gastric carcinomas in Tunisia. Eur. J. Gastroenterol. Hepatol. 21, 1001–1007.
| Prevalence and characteristics of Epstein–Barr virus-associated gastric carcinomas in Tunisia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXps1Cjur4%3D&md5=f050f013863d11672245665a4513c996CAS | 19491698PubMed |
[57] Kim, D.N. et al. (2013) Characterization of naturally Epstein–Barr virus-infected gastric carcinoma cell line YCCEL1. J. Gen. Virol. 94, 497–506.
| Characterization of naturally Epstein–Barr virus-infected gastric carcinoma cell line YCCEL1.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltVyqsrk%3D&md5=1308d13ef04b9ccaeed590ae2047d907CAS |
[58] Wang, Y. et al. (2010) Variations of Epstein–Barr virus nuclear antigen 1 gene in gastric carcinomas and nasopharyngeal carcinomas from Northern China. Virus Res. 147, 258–264.
| Variations of Epstein–Barr virus nuclear antigen 1 gene in gastric carcinomas and nasopharyngeal carcinomas from Northern China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpt1Wi&md5=be23ad8c58894ed3e67b0c923fce4b02CAS | 19941915PubMed |
[59] Saito, M. et al. (2013) Role of DNA methylation in the development of Epstein–Barr virus-associated gastric carcinoma. J. Med. Virol. 85, 121–127.
| Role of DNA methylation in the development of Epstein–Barr virus-associated gastric carcinoma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1Kms73O&md5=3e497020045eacee5301a75fa1e056a5CAS | 23073987PubMed |
Biographies
Dr Corey Smith completed his PhD in 2004 at the University of Melbourne and then took up a research position in the Tumour Immunology Laboratory at the Queensland Institute of Medical Research where his work focuses on the development of immunotherapeutic approaches to treat cancers associated with viral infection. This work led to a phase I clinical study in Hong Kong, using a novel immunotherapeutic approach to treat Epstein–Barr virus associated nasopharyngeal carcinoma. His work has also focused on understanding the mechanisms that influence the efficient induction of T cell responses to persistent human viral infections and the role immune evasion strategies developed by virally associated proteins play in promoting the survival of infected malignant cells.
Professor Rajiv Khanna obtained his doctorate degree from India and undertook his post-doctoral training at the Queensland Institute of Medical Research (QIMR), Brisbane, Australia. He is currently appointed as the Coordinator of the Centre for Immunotherapy and Vaccine Development at QIMR and also holds Senior Principal Research Fellowship from the National Health and Medical Research Council of Australia. The major goals of his research group are to obtain a deeper understanding of the mechanisms by which an immune response to human herpesviruses-associated diseases might be generated, augmented and applied to control these diseases. Over the past few years, Professor Khanna has successfully translated his research towards the development of novel immune-based therapeutic strategies for the treatment of patients with herpesvirus-associated complications and is currently lead investigator on five clinical trials. He has published more than 150 scientific papers in leading journals and holds numerous international patents on EBV and human cytomegalovirus (CMV) and has successfully co-developed a diagnostic kit (QuantiFERON-CMV) in collaboration with Cellestis Ltd (now QIAGEN). QuantiFERON-CMV has been CE marked in Europe for diagnostic application and is expected to be launched in the US in late 2013. Currently, he is also collaborating with Intercell AG (now Valneva SE) to develop a new prophylactic vaccine against CMV.


