Serine proteases and ovine footrot
Xiaoyan Han , Ruth M Kennan and Julian I RoodAustralian Research Council Centre of Excellence in Structural and Functional Microbial Genomics, Department of Microbiology, Monash University, Clayton, Vic. 3800, Australia
Email: julian.rood@monash.edu
Microbiology Australia 34(1) 37-40 https://doi.org/10.1071/MA13012
Published: 20 March 2013
Footrot is a disease that is of importance to the wool and sheep meat industries. The principle causative agent of ovine footrot is the anaerobic bacterium, Dichelobacter nodosus, virulent isolates of which secrete three closely related subtilisin-like proteases, AprV2, AprV5 and BprV1. By constructing isogenic mutants and carrying out virulence tests in sheep it was shown that AprV2 is a major virulence factor of D. nodosus2. Structural analysis of AprV2 has revealed that it contains several novel loops, one of which appears to act as an exosite that may modulate substrate accessibility2. Both elastase activity and protease thermostability have been used for the differential diagnosis of D. nodosus isolates. Analysis of the protease mutants has shown that AprV2 is the thermostable protease and also is responsible for the elastase activity of D. nodosus, while AprV5 is the major extracellular protease2. In addition, AprV5 is required for its own maturation and for the optimal cleavage of AprV2 and BprV to their mature active forms3.
The severity of ovine footrot is a continuum that ranges from benign footrot, which presents as an interdigital dermatitis, to virulent footrot, which involves severe underrunning of the horn of the hoof and the separation of the hoof from the underlying tissue (Fig. 1A), leading to lameness (Fig. 1B) and loss of body weight4–6. Consequently the disease results in significant losses to the sheep industry due to a reduction in meat and wool production and the cost of control and treatment programs7,8.

|
Clinical disease is dependent upon the virulence properties of the causative D. nodosus isolate and the presence of warm and wet climatic conditions. Type IV fimbriae and extracellular serine proteases were shown to be major virulence factors following the development of methods for the genetic manipulation of D. nodosus2,9. Type IV fimbriae also are required for optimal serine protease secretion9–11.
Virulent strains of D. nodosus secrete two acidic proteases, AprV2 and AprV5, and the basic protease BprV, which putatively cause tissue damage during a footrot infection2,12. The equivalent proteases in benign strains are known as AprB2, AprB5 and BprB13,14. Phenotypic characterisation of the extracellular proteases, including analysis of their elastase activity and protease thermostability, has been traditionally used for the differentiation of benign and virulent strains of D. nodosus15,16, with virulent isolates often producing elastase positive, thermostable proteases and benign strains generally having an elastase negative, more thermolabile protease phenotype. Note that these phenotypes do not absolutely correlate with virulence.
These proteases are synthesised as precursors with an N-terminal pre-pro-region, a serine protease domain and a C-terminal extension. The amino acid sequences of the catalytic protease domains are highly conserved (~65% identity)17–19, but the sequences of the C-terminal extensions are less conserved, showing only approximately 35% similarity. The active proteases are produced by cleavage of the pre-pro region and the C-terminal extension17,18,20. Sequence analysis of the C-terminal extensions revealed that they contain a P-domain, which is typically associated with eukaryotic pro-protein convertases that belong to the subtilisin-like protease superfamily6,21.
To determine the role of each of the proteases in virulence, mutants of each of the three protease genes were constructed by allelic exchange in the virulent strain VCS1703A2. Quantitative protease assays of culture supernatants, using azocasein as the substrate, revealed that AprV5 made the major contribution to overall protease activity; followed by AprV2, with BprV only making a minor contribution2. Double mutants had very little protease activity, suggesting that the proteases may act synergistically, or that one or more may be involved in the activation of the other proteases2. The mechanism of processing of the extracellular proteases was further examined by zymogram analysis and Western blotting using AprV5-, AprV2- and BprV-specific antisera3. The results indicated that AprV5 is responsible for its own maturation and for the optimal processing of both AprV2 and BprV. By constructing a series of C-terminal truncated aprV5 mutants in D. nodosus, it also was shown that the C-terminal extension of AprV5 is required for efficient processing of all three enzymes, presumably because it is required for the optimal self-processing of AprV5. In the absence of this domain, protease processing is delayed3. Moreover, it was shown that cleavage of the pro-domain and the C-terminal extensions of the AprV2 and BprV precursors occurs after secretion.
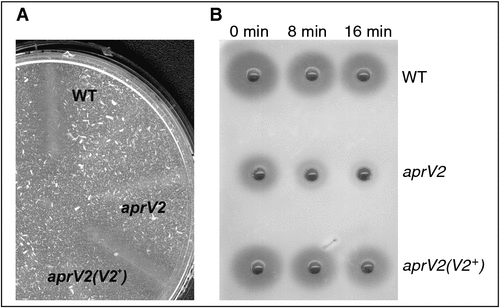
Elastase assays revealed that AprV2 is responsible for the elastase activity found in most virulent isolates of D. nodosus (Fig. 2A)2. AprV2 is also the major thermostable protease (Fig. 2B). Complementation of the aprV2 mutant with the benign protease gene aprB2 restored the overall protease activity to that of the wild type, but not the elastase activity or thermostability; therefore this strain had the phenotype of a benign strain, despite possessing two functional virulent protease genes, aprV5 and bprV.
|
The virulent wild-type strain, each of the protease mutants, and their corresponding complemented derivatives, were examined in a sheep virulence trial to determine the role of the proteases in disease2. The aprV2 mutant was avirulent and complementation of the mutant with the wild-type aprV2 gene restored full virulence. Surprisingly, complementation with aprB2 (the equivalent protease from a wild-type benign strain) also restored full virulence, which indicates that elastase activity is not required for virulence. The aprV5 and the bprV mutants were also avirulent, but unexpectedly their complemented strains were not virulent, possibly due to unstable protease expression in the complemented strains. Therefore, although we cannot conclude that AprV5 and BprV are essential for virulence, it is likely that they do play a role in the disease process.
It has been known for some time that the mature AprV2 and AprB2 proteases differ by a single amino acid, with Tyr92 of AprV2 substituted by an Arg residue in AprB218. To better understand how this single amino acid change could contribute to the substrate specificity of these proteases the crystal structures of both proteases were determined2. The structures were closely related and were similar to other subtilisin-like proteases, although they each had several major insertions (I1 – I4) in the loops surrounding the active site cleft. The largest of these insertions (I2) is tethered by a disulphide bond, and the single amino acid change (Y92R) between AprV2 and AprB2 is located at the end of this loop. Site-directed mutagenesis revealed that Tyr92 does not contribute to catalysis at the active site, but that the I2 loop acts as an exosite and mediates the formation of a stable enzyme-substrate interaction. The tethering of the loop by the disulphide bond appears to be important for this function2. Finally, analysis of the crystal structures of BprV and BprB reveals that differences in the substrate specificity of these enzymes reflects amino acid changes in the S1 pocket of these enzymes and that subtle changes in the D. nodosus proteases may significantly influence tissue destruction22.
Conclusions
For many years extracellular serine proteases had been considered as putative virulence factors of D. nodosus; however, their importance in the pathogenesis of disease has only recently been established. There is now clear evidence that AprV2 is the major thermostable protease and elastase in virulent strains of D. nodosus and is essential for virulence. In addition, it has been shown that AprV5 is required for its self-maturation and that it facilitates the activation of AprV2 and BprV, a process that requires the C-terminal extension of AprV5. The fact that a strain in which an aprV2 mutation was complemented with the aprB2 gene was benign by standard laboratory diagnostic tests, but caused virulent disease in sheep, indicates that elastase activity and thermostability are not direct indicators of the virulence of a strain. This finding is in keeping with the difficult task of precisely defining a virulent isolate of D. nodosus, an issue that has been noted for many years in this field. The presence of other virulence factors, such as the other virulent proteases and type IV fimbriae-mediated twitching motility, also contributes to virulence. Virulence in D. nodosus clearly is multifactorial and we suspect that the pathogenesis of disease is much more complex than currently envisaged.
Acknowledgements
We acknowledge the financial support of the Australian Research Council through the ARC Centre of Excellence in Structural and Functional Microbial Genomics.
References
[1] Kennan, R.M. et al. (2011) The pathogenesis of ovine footrot. Vet. Microbiol. 153, 59–66.| The pathogenesis of ovine footrot.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFKiurzE&md5=cc43f7d331a42d337618e745b78ab8beCAS |
[2] Kennan, R.M. et al. (2010) The subtilisin-like protease AprV2 is required for virulence and uses a novel disulphide-tethered exosite to bind substrates. PLoS Pathog. 6, e1001210.
| The subtilisin-like protease AprV2 is required for virulence and uses a novel disulphide-tethered exosite to bind substrates.Crossref | GoogleScholarGoogle Scholar |
[3] Han, X. et al. (2012) The AprV5 subtilase is required for the optimal processing of all three extracellular serine proteases from Dichelobacter nodosus. PLoS ONE 7, e47932.
| The AprV5 subtilase is required for the optimal processing of all three extracellular serine proteases from Dichelobacter nodosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1KisLrE&md5=2455f525aeb6d140a31273814358ab63CAS |
[4] Egerton, J.R. et al. (1969) The aetiology and pathogenesis of ovine footrot. I.A histological study of the bacterial invasion. J. Comp. Pathol. 79, 207–216.
| The aetiology and pathogenesis of ovine footrot. I.A histological study of the bacterial invasion.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF1M7mtF2kug%3D%3D&md5=3ffcbfdf72ed5c39f550f33faebce145CAS |
[5] Stewart, D.J. et al. (1984) Differences between strains of Bacteroides nodosus in their effects on the severity of footrot, bodyweight and wool growth on Merino sheep. Aust. Vet. J. 61, 348–352.
| Differences between strains of Bacteroides nodosus in their effects on the severity of footrot, bodyweight and wool growth on Merino sheep.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2M7kvFSgtg%3D%3D&md5=0aae009db086336de864a7beecd8411cCAS |
[6] Siezen, R.J. and Leunissen, J.A. (1997) Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 6, 501–523.
| Subtilases: the superfamily of subtilisin-like serine proteases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhvVOlsrY%3D&md5=49f8a5cf7b34bf7f4599a9bcc40f4ad3CAS |
[7] Egerton, J.R. (1986) Control and eradication of ovine footrot. In Footrot in Ruminants. Proceedings of a Workshop, Melbourne 1985 (Stewart, D.J. et al., eds), pp. 35–42, CSIRO Division of Animal Health and Australian Wool Corporation.
[8] Green, L.E. and George, T.R. (2008) Assessment of current knowledge of footrot in sheep with particular reference to Dichelobacter nodosus and implications for elimination or control strategies for sheep in Great Britain. Vet. J. 175, 173–180.
| Assessment of current knowledge of footrot in sheep with particular reference to Dichelobacter nodosus and implications for elimination or control strategies for sheep in Great Britain.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c7jtlersg%3D%3D&md5=1005a27013f5ede4f78a62b843b29f1aCAS |
[9] Kennan, R.M. et al. (2001) The type IV fimbrial subunit gene (fimA) of Dichelobacter nodosus is essential for virulence, protease secretion, and natural competence. J. Bacteriol. 183, 4451–4458.
| The type IV fimbrial subunit gene (fimA) of Dichelobacter nodosus is essential for virulence, protease secretion, and natural competence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXlsVensrw%3D&md5=f36d3b3677639f3cedb0446294f5de2cCAS |
[10] Han, X. et al. (2008) Twitching motility is essential for virulence in Dichelobacter nodosus. J. Bacteriol. 190, 3323–3335.
| Twitching motility is essential for virulence in Dichelobacter nodosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlt1Kqu7k%3D&md5=6089142890fd1ff4fdb87362380e7ff2CAS |
[11] Han, X. et al. (2007) Type IV fimbrial biogenesis is required for protease secretion and natural transformation in Dichelobacter nodosus. J. Bacteriol. 189, 5022–5033.
| Type IV fimbrial biogenesis is required for protease secretion and natural transformation in Dichelobacter nodosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXotFaksbY%3D&md5=7741937e70b741ba397e4cb5fa1564e3CAS |
[12] Thomas, J.H. (1964) The pathogenesis of footrot in sheep with reference to proteases of Fusiformis nodosus. Aust. J. Agric. Res. 15, 1001–1016.
| The pathogenesis of footrot in sheep with reference to proteases of Fusiformis nodosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2MXosFWksw%3D%3D&md5=c8be4ef868474f578be33414b60d23c6CAS |
[13] Kortt, A.A. et al. (1993) Amino acid sequence of extracellular acidic protease V5 of Dichelobacter nodosus, the causative organism of ovine footrot. Biochem. Mol. Biol. Int. 29, 989–998.
| 1:CAS:528:DyaK3sXkvFSjsrs%3D&md5=6689f78b40e0d0d5c2d11b05f037ef59CAS |
[14] Kortt, A.A. et al. (1994) Characterization of a basic serine protease (pI ~ 9.5) secreted by virulent strains of Dichelobacter nodosus and identification of a distinct, but closely related, proteinase secreted by benign strains. Biochem. J. 299, 521–525.
| 1:CAS:528:DyaK2cXktVeluro%3D&md5=f065d8f405fd83969f0dc1812bbaa1aeCAS |
[15] Stewart, D.J. (1979) The role of elastase in the differentiation of Bacteroides nodosus infections in sheep and cattle. Res. Vet. Sci. 27, 99–105.
| 1:CAS:528:DyaL3cXitFejtbk%3D&md5=67f705ffcbc1bc86e4f865b20f8bf825CAS |
[16] Palmer, M.A. (1993) A gelatin test to detect activity and stability of proteases produced by Dichelobacter nodosus. Vet. Microbiol. 36, 113–122.
| A gelatin test to detect activity and stability of proteases produced by Dichelobacter nodosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXivFamt7o%3D&md5=928cd38c94288b014469d89e2b4d8caeCAS |
[17] Riffkin, M.C. et al. (1993) Cloning, sequence and expression of the gene (aprV5) encoding extracellular serine acidic protease V5 from Dichelobacter nodosus. Gene 137, 259–264.
| Cloning, sequence and expression of the gene (aprV5) encoding extracellular serine acidic protease V5 from Dichelobacter nodosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXhvVCgu78%3D&md5=76bd81aa8113ead1768d9acdb03bec6fCAS |
[18] Riffkin, M.C. et al. (1995) A single amino-acid change between the antigenically different extracellular serine proteases V2 and B2 from Dichelobacter nodosus. Gene 167, 279–283.
| A single amino-acid change between the antigenically different extracellular serine proteases V2 and B2 from Dichelobacter nodosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlvVOgtw%3D%3D&md5=2b28ac11f16bf810391f47f62f41ab51CAS |
[19] Lilley, G.G. et al. (1995) Nucleotide and deduced protein sequence of the extracellular, serine basic protease gene bprB from Dichelobacter nodosus strain 305: comparison with the basic protease gene bprV from virulent strain 198. Biochem. Mol. Biol. Int. 36, 101–111.
| 1:CAS:528:DyaK2MXpt1Ciu7s%3D&md5=16870b29dde5ef44544c1b9471e8c8e7CAS |
[20] Lilley, G.G. et al. (1992) Amino acid and DNA sequences of an extracellular basic protease of Dichelobacter nodosus show that it is a member of the subtilisin family of proteases. Eur. J. Biochem. 210, 13–21.
| Amino acid and DNA sequences of an extracellular basic protease of Dichelobacter nodosus show that it is a member of the subtilisin family of proteases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXltlChtrg%3D&md5=b5d724976db772d312aed75fa4d085a4CAS |
[21] Shinde, U. and Thomas, G. (2011) Insights from bacterial subtilases into the mechanisms of intramolecular chaperone-mediated activation of furin. Methods Mol. Biol. 768, 59–106.
| Insights from bacterial subtilases into the mechanisms of intramolecular chaperone-mediated activation of furin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XkvV2gt78%3D&md5=031975c480da932994cc82fb8243b8feCAS |
[22] Wong, W. et al. (2011) S1 pocket of a bacterially derived subtilisin-like protease underpins effective tissue destruction. J. Biol. Chem. 286, 42 180–42 187.
| S1 pocket of a bacterially derived subtilisin-like protease underpins effective tissue destruction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFKjsr%2FP&md5=cbd6f7befceac810cf9c1e10cb6b9557CAS |
Biographies
Dr Xiaoyan Han is a Research Fellow within the ARC Centre of Excellence at Monash University. She has studied the pathogenesis of the ovine footrot pathogen Dichelobacter nodosus since 2002. Her studies focused on the identification of genes involved in type IV fimbrial biogenesis and function in D. nodosus, developing an understanding of the molecular mechanisms by which type IV fimbrial biogenesis, natural transformation, and protease secretion are linked, and an investigation of the role of carboxyl-terminal extensions of proteases in secretion and function.
Dr Ruth Kennan is a Research Fellow within the ARC Centre of Excellence in the Department of Microbiology at Monash University. Her research interests are in the pathogenesis and virulence of Dichelobacter nodosus, the causative agent of ovine footrot. She is responsible for the development of methods for the genetic manipulation of D. nodosus. Current work involves looking at regulation of virulence factors and looking for genomic differences between benign and virulent strains.
Prof Julian Rood is a Chief Investigator of the ARC Centre of Excellence and Deputy Head of the Department of Microbiology at Monash University. His research group has worked for many years on the genetics and pathogenesis of anaerobic bacteria, particularly the pathogenic clostridia and Dichelobacter nodosus. He is a Past President and Fellow of the Australian Society for Microbiology and a Fellow of the American Academy of Microbiology.


