New Asian pseudoscorpions improve the phylogenetic resolution of Garypinoidea (Pseudoscorpiones)
Zhizhong Gao A B , Feng Zhang B * and Mark S. Harvey C D *
C D *
A
B
C
D
Handling Editor: Gonzalo Giribet
Abstract
The recent subdivision of the pseudoscorpion family Garypinidae into three subfamilies, Garypininae, Amblyolpiinae and Protogarypininae, used a combination of molecular and morphological criteria. Newly obtained sequence data from several new garypinoid pseudoscorpions has helped clarify the relationships between various clades. Most importantly, we were able to include the type species of the family, Garypinus dimidiatus (L. Koch, 1873), and two additional species of Amblyolpium Simon, 1898, including A. shenzhou sp. nov. from southern China, which provided a better resolved phylogeny with Amblyolpium as sister to all other Garypinoidea. We raise the subfamily Amblyolpiinae to full family level, Amblyolpiidae stat. nov. In addition, we describe a new genus and species from the Himalayan Plateau, Absensus zhangi sp. nov., which has a morphological feature that allows placement in Amblyolpiidae.
ZooBank: urn:lsid:zoobank.org:pub:4BDE596B-6EFE-4AB8-932E-1D4379849677
Keywords: classification, identification key, molecular phylogeny, morphology, new genus, new species, new status, taxonomy.
Introduction
A recent molecular phylogeny of the pseudoscorpion superfamily Garypinoidea using two nuclear and one mitochondrial gene found evidence for some remarkable divergences yielding surprising results (Harvey 2023). Most notably, the family Garypinidae was not found to be monophyletic as it included the family Larcidae, a result that was also reported by Benavides et al. (2019) albeit with a much smaller sample size but greater gene sampling. Harvey (2023) proposed a novel classification for Garypinidae by recognising three subfamilies that were also supported by morphological data: Amblyolpiinae for Amblyolpium Simon, 1898, Neoamblyolpium Hoff, 1956 and the Eocene Baltamblyolpium Stanczak, Harvey, Harms, Hammel, Kotthoff & Loria, 2023; Protogarypininae for Neominniza Beier, 1930, Oreolpium Benedict & Malcolm 1978, Protogarypinus Beier, 1954, Teratolpium Beier, 1959 and Thaumatolpium Beier, 1931; and Garypininae for the remaining genera, Aldabrinus Chamberlin, 1930, Caecogarypinus Dashdamirov, 2007, Galapagodinus Beier, 1978, Garypinidius Beier, 1955, Garypinus Daday, 1888, Haplogarypinus Beier, 1959, Hemisolinus Beier, 1977, Nelsoninus Beier, 1967, Nobilipinus Harvey, 2023, Pseudogarypinus Beier, 1931, Serianus Chamberlin, 1930, Solinellus Muchmore, 1979, Solinus Chamberlin, 1930, and the Cretaceous Ajkagarypinus Novák, Harvey, Márton, Hammel, Harms, Kotthoff, Hörweg, Brazidec & Ősi, 2023.
We have studied specimens of a garypinid pseudoscorpion from the eastern Himalayan region that, at first glance, resembled a relatively standard garypinid with deeply divided arolia, divided tergites and sternites, and the sub-basal position of the trichobothria of the prolateral series. However, the presence of long venom ducts and the absence of sternal glandular setae posed a problem in finding a satisfactory subfamily placement, as long venom ducts are characteristic of Amblyolpiinae, but not of Garypininae or Protogarypininae, and the glandular setae have been reported for all garypinid taxa except the garypinine Pseudogarypinus (Harvey 2023). Although sequence data are lacking for the sequenced representative of this genus (P. cooperi Muchmore), we added newly obtained sequence data for several important garypinid taxa to the molecular dataset provided by Harvey (2023), including additional species of Amblyolpium from Australia and China, and Garypinus dimidiatus (L. Koch, 1873) from Greece. The inclusion of these taxa has vastly improved the resolution of the phylogenetic inferences that can be made of Garypinoidea. Most importantly, Amblyolpiinae was recovered as sister to the other garypinoids, confirming the importance of the venom duct length in the systematics of garypinoid pseudoscorpions, which allows us to reexamine the rank of this enigmatic clade, and to describe a new genus from China.
Materials and methods
The specimens are preserved in 75% ethanol and deposited in the Museum of Hebei University, Baoding City, China (MHBU). The methods used for the study follow Gao and Zhang (2019).
Terminology and mensuration mostly follow Chamberlin (1931), with the exception of the nomenclature of the pedipalps, legs and with some minor modifications to the terminology of the trichobothria (Harvey 1992), chelicera (Judson 2007) and faces of the appendages (Harvey et al. 2012). The following abbreviations are used: chelal trichobothria: fixed finger, eb, externo-basal trichobothrium; esb, externo-sub-basal trichobothrium; est, externo-subterminal trichobothrium; et, externo-terminal trichobothrium; ib, interno-basal trichobothrium; isb, interno-sub-basal trichobothrium; ist, interno-subterminal trichobothrium; it, interno-terminal trichobothrium; movable finger, b, basal trichobothrium; sb, sub-basal trichobothrium; st, subterminal trichobothrium; t, terminal trichobothrium; cheliceral setae: ebs, externobasal seta; es, external seta; gs, galeal seta; is, interior seta; ls, laminal seta; sbs, sub-basal seta.
Molecular methods
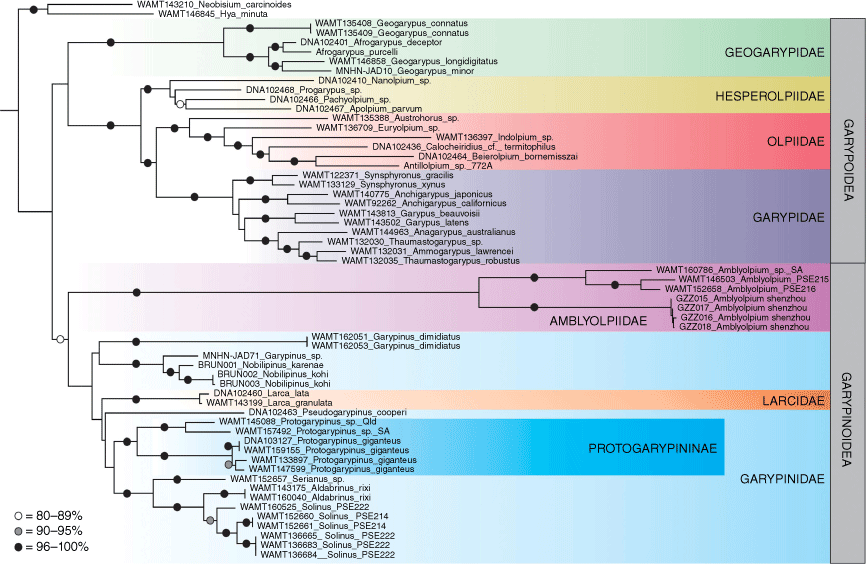
Taxon selection included all of the specimens studied by Harvey (2023) with the addition of two species of Amblyolpium, A. shenzhou sp. nov. from southern China, an undescribed species from South Australia, and Garypinus dimidiatus from Greece (Table 1). The analysis was rooted on Austrochthonius sp. of the superfamily Chthonioidea, and other outgroups included Neopseudogarypus scutellatus Morris, 1948 (Feaelloidea), Hya minuta Tullgren, 1907 and Neobisium carcinoides (Hermann, 1803) (Neobisioidea). For clarity, the phylogram (Fig. 1) omits the chthonioid and feaelloid outgroups.
| Family | Species | Locality | Repository and number | COI | 18S rRNA | 28S rRNA | |
|---|---|---|---|---|---|---|---|
| Superfamily Chthonioidea | |||||||
| Chthoniidae | Austrochthonius sp. | AUSTRALIA: Western Australia: Millstream-Chichester National Park, George River, 2.2 km SE of Mt Montagu | WAM T135835 | OR067318 | OR039602 | OR059142 | |
| Superfamily Feaelloidea | |||||||
| Pseudogarypidae | Neopseudogarypus scutellatus Morris, 1948 | AUSTRALIA: Tasmania: Launceston, Cataract Gorge | WAM T104213 | OR067276 | OR039591 | OR059173 | |
| Superfamily Neobisioidea | |||||||
| Hyidae | Hya minuta (Tullgren, 1905) | BRUNEI DARUSSALAM: Mengkubau, off Jalan Penghubang mentiri | WAM T146845 | OR067360 | OR039622 | OR059105 | |
| Neobisiidae | Neobisium carcinoides (Hermann, 1804) | DENMARK: Stampeskov at Raadvad | WAM T143210 | OR067357 | OR039620 | OR059108 | |
| Superfamily Garypinoidea | |||||||
| Amblyolpiidae | Amblyolpium sp. ‘PSE215’ | AUSTRALIA: Western Australia: Paraburdoo Range, ~12.0 km SW of Paraburdoo | WAM T146503 | OR359473 | OR359932 | OR372787 | |
| Amblyolpium sp. ‘PSE216’ | AUSTRALIA: Western Australia: ~44.4 km SSE of Menzies | WAM T152658 | OR359474 | OR359933 | – | ||
| Amblyolpium sp. ‘SA’ | AUSTRALIA: South Australia: Brookfield Conservation Park | WAM T160786 | PQ834287* | PQ834286* | PQ834287* | ||
| Amblyolpium shenzhou sp. nov. | CHINA: Guizhou Province, Tongren City, Jiangkou County | GZ015 | PQ814591* | PQ810648* | PQ810644* | ||
| CHINA: Guizhou Province, Tongren City, Jiangkou County | GZ016 | PQ814592* | PQ810649* | PQ810645* | |||
| CHINA: Guizhou Province, Tongren City, Jiangkou County | GZ017 | PQ814593* | PQ810650* | PQ810646* | |||
| CHINA: Guizhou Province, Tongren City, Jiangkou County | GZ018 | PQ814594* | PQ810651* | PQ810647* | |||
| Garypinidae | Aldabrinus rixi Harvey, 2023 | AUSTRALIA: Western Australia: Torndirrup National Park, Sharp Point at end of Eclipse Island Road | WAM T143175 | OR359471 | OR359931 | OR372788 | |
| AUSTRALIA: Western Australia: Denmark | WAM T160040 | OR359472 | – | – | |||
| Garypinus dimidiatus (L. Koch, 1873) | GREECE: Naxos, 1.7 km ENE of Kastraki Beach | WAM T162051 | PQ834815 | PQ834284 | PQ834288 | ||
| GREECE: Naxos, 1.7 km ENE of Kastraki Beach | WAM T162053 | PQ834816* | PQ834285* | PQ834289* | |||
| Garypinus sp. JA-2011 | [Unknown] | MNHN-JAD71 | JN018179 | JN018296 | JN018393 | ||
| Nobilipinus karenae Harvey, 2023 | BRUNEI DARUSSALAM: Ulu Temberong National Park, near Kuala Belalong Field Studies Centre | BRUN001 | OR359476 | OR359936 | OR372784 | ||
| Nobilipinus kohi Harvey, 2023 | BRUNEI DARUSSALAM: Mengkubau, off Jalan Penghubang mentiri | BRUN002 | OR359477 | OR359934 | – | ||
| BRUNEI DARUSSALAM: Mengkubau, off Jalan Penghubang mentiri | BRUN003 | OR359478 | OR359935 | – | |||
| Protogarypinus giganteus Beier, 1954 | AUSTRALIA: Western Australia: Walpole-Nornalup National Park, The Tingle Tree | DNA103127 | EU559565 | EU559377 | EU559484 | ||
| AUSTRALIA: Western Australia: Fitzgerald River National Park, summit of West Mt Barren | WAM T133897 | OR359479 | OR359937 | – | |||
| AUSTRALIA: Western Australia: Kuch Road | WAM T147599 | OR359480 | OR359938 | OR372783 | |||
| AUSTRALIA: Western Australia: Walpole-Nornalup National Park, Cemetery Road | WAM T159155 | OR359481 | OR359939 | OR372782 | |||
| Protogarypinus sp. ‘QLD’ | AUSTRALIA: Queensland: O’Reilly’s, Wishing Tree Track | WAM T145088 | OR359482 | OR359940 | OR372781 | ||
| Protogarypinus sp. ‘SA’ | AUSTRALIA: South Australia: Burnside Quarry Track | WAMT157492 | OR359483 | OR359941 | OR372780 | ||
| Pseudogarypinus cooperi Muchmore, 1980 | USA: California: Riverside County: James Reserve, Lake Fulor | DNA102463 | EU559566 | EU559423 | EU559485 | ||
| Serianus sp. | CHILE: La Araucania: Comunidad Indigena Quinquén | WAM T152657 | OR359484 | OR359942 | OR372779 | ||
| Solinus pingrup Harvey, 2023 | AUSTRALIA: Western Australia: Allanson Primary School | WAM T160525 | OR359490 | – | – | ||
| Solinus sp. ‘PSE214’ | AUSTRALIA: Western Australia: 22 km E of Mundrabilla Roadhouse, Eyre Highway | WAM T152660 | OR359485 | OR359943 | OR372777 | ||
| AUSTRALIA: Western Australia: 24 km SSW of Eucla, Eyre Highway | WAM T152661 | OR359486 | OR359944 | OR372776 | |||
| Solinus sp. ‘PSE222’ | AUSTRALIA: Western Australia: Strelley River | WAM T136665 | OR359487 | OR359945 | OR372778 | ||
| AUSTRALIA: Western Australia: Strelley River | WAM T136683 | OR359488 | OR359946 | – | |||
| AUSTRALIA: Western Australia: Strelley River | WAM T136684 | OR359489 | OR359947 | – | |||
| Larcidae | Larca granulata (Banks, 1891) | USA: New York: E.N. Huyck Preserve, near Research Center | WAM T143199 | – | OR359930 | OR372789 | |
| Larca lata (Hansen, 1885) | CZECHIA: Jihomoravský kraj: Lanzhot, Chanov Reserve | DNA102460 | EU559563 | EU559425 | – | ||
| Superfamily Garypoidea | |||||||
| Garypidae | Anchigarypus californicus (Banks, 1909) | USA: California: Marin County: Bolinas Point | WAM T92262 | MN058668 | MN065584 | MN065610 | |
| Anchigarypus japonicus (Beier, 1952) | JAPAN: Ehime Prefecture: O-shima Island, 3 km E of Myakubo | WAM T140775 | MN058687 | MN065603 | MN065628 | ||
| Ammogarypus lawrencei Beier, 1962 | NAMIBIA: Erongo: Gobabeb | WAM T132031 | MN058676 | MN065589 | MN065614 | ||
| Anagarypus australianus Muchmore, 1982 | AUSTRALIA: Northern Territory: Bumtja Beach, Garrthalala | WAM T144963 | MN058692 | MN065607 | MN065632 | ||
| Garypus beauvoisii (Audouin, 1826) | CYPRUS: Akanas | WAM T143813 | MN058691 | MN065606 | MN065631 | ||
| Garypus latens Harvey, 2020 | AUSTRALIA: Western Australia: Barrow Island | WAM T143502 | MN058690 | MN065605 | MN065630 | ||
| Synsphyronus gracilis Harvey, 1987 | AUSTRALIA: Western Australia: Mudlark, 92.3 km NW of Newman | WAM T122371 | MN058669 | MN065585 | MN065611 | ||
| Synsphyronus xynus Cullen & Harvey | AUSTRALIA: Western Australia: near Sulfur Springs | WAM T133129 | MN058679 | MN065595 | MN065620 | ||
| Thaumastogarypus robustus Beier, 1947 | NAMIBIA: Hardap: Hardap Dam near Marieantal | WAM T132035 | MN058678 | MN065592 | MN065617 | ||
| Thaumastogarypus sp. | NAMIBIA: Erongo: Uis (on the road C35) | WAM T132030 | MN058675 | MN065588 | MN065613 | ||
| Geogarypidae | Afrogarypus deceptor Neethling & Haddad, 2016 | SOUTH AFRICA: KwaZuluNatal: Ndumo Game Reserve | DNA102401 | EU559560 | EU559385 | EU559436 | |
| Afrogarypus purcelli (Ellingsen, 1912) | SOUTH AFRICA: Western Cape Province: De Hoop Nature Reserve | Afro_purcelli | KP331817 | – | KP297850 | ||
| Geogarypus connatus Harvey, 1986 | AUSTRALIA: South Australia: 14 km WNW of Yalata | WAM T135409 | – | OR359929 | OR372785 | ||
| Geogarypus connatus Harvey, 1986 | AUSTRALIA: South Australia: 14 km WNW of Yalata | WAM T135408 | OR359475 | – | OR372786 | ||
| Geogarypus longidigitatus (Rainbow, 1897) | SINGAPORE: Bukit Timah Nature Reserve, South view path | WAM T146858 | MN058693 | MN065608 | – | ||
| Geogarypus minor (L. Koch, 1873) | [Unknown] | MNHN-JAD10 | JN018180 | JN018297 | JN018394 | ||
| Hesperolpiidae | Apolpium parvum Hoff, 1945 | TRINIDAD & TOBAGO: Mt Saint Benedict | DNA102467 | EU559541 | EU559380 | EU559489 | |
| Nanolpium sp. | ZAMBIA: Kafue National Park, Chibila Camp | DNA102410 | EU559543 | EU559390 | EU559445 | ||
| Pachyolpium sp. | TRINIDAD & TOBAGO: Mt Saint Benedict | DNA102466 | EU559542 | EU559421 | EU559488 | ||
| Progarypus sp. | CHILE: Concepción: Cerro Caracol | DNA102468 | EU559538 | EU559420 | EU559490 | ||
| Olpiidae | Antillolpium sp. 772A | CUBA: [no further data] | sp. 772A | KX263395 | – | KX263356 | |
| Austrohorus sp. | AUSTRALIA: Western Australia: Madura Pass, 800 m NE of Madura | WAM T135388 | MN058681 | MN065597 | MN065622 | ||
| Beierolpium bornemisszai (Beier, 1966) | AUSTRALIA: Western Australia: Gleneagle State Forest | DNA102464 | EU559545 | EU559378 | EU559486 | ||
| Calocheiridius cf. termitophilus Beier, 1964 | EQUATORIAL GUINEA: Bata District: near Rio Utonde, Bata | MCZIZ-130511 | EU559544 | EU559359 | EU559460 | ||
| Euryolpium sp. | AUSTRALIA: Western Australia: Steep Head Island | DNA102465 | MN058685 | MN065601 | MN065626 | ||
| Indolpium sp. | AUSTRALIA: Western Australia: 79.1 km SW of Tom Price | WAM T136397 | MN058683 | MN065599 | MN065624 | ||
The majority of the data is derived from Harvey (2023), with the sequence data that is newly added for this project marked with an asterisk.
Maximum likelihood phylogeny of Garypoidea and Garypinoidea, based on alignment of concatenated COI, 18S and 28S. Bootstrap values are presented for nodes greater than 79%.
We included fragments of the cytochrome c oxidase subunit I (COI), 18S ribosomal RNA (18S) and 28S ribosomal RNA (28S), which were sequenced using standard Sanger methodology.
In China, the DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany) was used for total genomic DNA extraction. The sequences of primers and polymerase chain reaction (PCR) conditions for three gene regions followed (Folmer et al. 1994; Giribet et al. 1996; Whiting et al. 1997; Murienne et al. 2008). The 25-μL PCR reactions included 12.5 μL of 2×Taq MasterMix (KangWei Biotech, Beijing, China), 0.8 μL of each forward and reverse 10 μM of primer, 4 μL of genomic DNA, and 6.9 μL of double-distilled H2O. The PCR products were visualised by agarose gel electrophoresis (1% agarose). All PCR products were purified and sequenced at Sangon Biotech (Shanghai, China) Co., Ltd. Sequences of the fragment were obtained by using the Chromaseq package in Mesquite (ver. 3.81, W. P. Maddison and D. R. Maddison, see https://www.mesquiteproject.org/) from chromatograms by Phred (ver. 0.020425c, P. Green and B. Ewing, see https://www.phrap.com) and Phrap (ver. 1.090518, P. Green, see https://www.phrap.com). Sequence alignments were carried out using MAFFT (ver. 7.313, see https://mafft.cbrc.jp/alignment/software/; Katoh and Standley 2013) with the L-INS-I strategy, and checked for the presence of stop codons of COI by translating them into amino acid sequence using Geneious Prime (ver. 2024.0.7, Biomatters Ltd, see https://www.geneious.com/, accessed June 2021; Kearse et al. 2012). Ambiguously aligned positions were culled using trimAl (ver. 1.2, see http://trimal.cgenomics.org/; Capella-Gutiérrez et al. 2009) with default parameters.
In Australia, the molecular methods used for the polymerase chain reaction (PCR) amplification of each of these genes followed Harvey et al. (2015, 2016, 2020), Harvey (2023) with PCR purification and Sanger bidirectional sequencing conducted by the Australian Genome Research Facility (AGRF; Perth). Chromatograms were edited using the Geneious software package, and resulting nucleotide sequences for all taxa are deposited in GenBank (Table 1). The sequences were aligned using the MAFFT (ver. 7.490; Katoh et al. 2002; Katoh and Standley 2013) plug-in within Geneious with the default settings. The concatenated alignments were analysed using maximum likelihood (ML) methodology in the web version of IQ-TREE (ver. 2.2.0, see http://www.iqtree.org/; Trifinopoulos et al. 2016; Minh et al. 2020). The substitution model option was set at Auto, and the branch support analysis was performed with 5000 bootstrap alignments.
Results
The phylogeny (Fig. 1) resulting from the molecular analysis recovered a monophyletic Garypinoidea with moderate Bootstrap support (80%), and a monophyletic Garypoidea with poor support (48%). Whereas no differences in the Garypoidea topology were found in comparison to the phylogeny provided by Harvey (2023), there were significant differences in the topology of the garypinoid taxa. Garypinus dimidiatus was found to be the sister group to Garypinus sp. + Nobilipinus karenae + N. kohi (76%). The provenance of the specimen identified as Garypinus sp. was not stated by Arabi et al. (2012) but it seems likely to represent a species of Nobilipinus, a genus that was only recently recognised (Harvey 2023). This clade was sister to Larcidae + Pseudogarypinus + Protogarypinus + Serianus + Aldabrinus + Solinus. The low support value of 44% diminishes the confidence that can be placed on the monophyly of this clade, but internally there is strong support for Aldabrinus + Serianus + Solinus (100%) and a monophyletic Protogarypinus (98%). The four species of Amblyolpium included in the analysis formed a well-supported monophyletic clade (100%), and this clade was in turn sister to the remaining garypinoids but with low support (63%). In the earlier analysis, Amblyolpium and Larca formed a poorly supported clade that was sister to Nobilipinus + Garypinus sp., which was suggested to be due to long-branch attraction (Harvey 2023). The new analysis provides a better resolved phylogenetic scenario, at least in relation to the position of Amblyolpium. Indeed, the significant morphological differences shared by Amblyolpium, Neo-amblyolpium, Absensus and Baltamblyolpium when compared with the remaining garypinids allows us to elevate the subfamily Amblyolpiinae to full family level. Although this partly resolves the conundrum of the non-monophyly of Garypinoidea faced by Harvey (2023), the position of Larca, the sole genus of Larcidae (Harvey and Wynne 2014), still poses significant problems with the family level classification as currently perceived. Likewise, the subfamily Garypininae was not recovered as monophyletic as it also included Larcidae and the garypinid subfamily Protogarypininae (Fig. 1). The low support values suggest that further taxon sampling will provide better resolution to this part of the phylogeny, although it does not fully contradict the phylogeny recovered by Benavides et al. (2019) who used a 210-gene dataset and found Larcidae as sister to Pseudogarypinus, which in turn was sister to Protogarypinus.
Biogeography
The presence of a phylogenetically significant garypinoid pseudoscorpion highlights the importance of the Himalayan Plateau and southern China as a cradle for relictual taxa. The specimens of Amblyolpium shenzhou were collected from three provinces (Guangdong, Hunan and Guizhou) in southern China, and it may have a wide distribution in the Oriental Realm. Absensus zhangi was only found in the Xizang Autonomous region on the Himalayan Plateau, a region that also includes species of genus Centrochthonius Beier, 1931 (family Pseudotyrannochthoniidae), which is known from high-altitude regions of Asia (Harvey and Harms 2022; Kolesnikov et al. 2023), as well as from Eocene Bitterfeld Amber in northern Europe (Schwarze et al. 2022).
Taxonomy
Family AMBLYOLPIIDAE Harvey, 2023, stat. nov.
Amblyolpiinae Harvey, 2023, p. 631.
The family Amblyolpiidae differs from Garypinidae by the long venom ducts, which are short in Garypinidae, and from Larcidae by the rectangular carapace, which is sub-triangular in Larcidae.
As discussed above, the new molecular analysis (Fig. 1) recovered the species of Amblyolpium as sister to the remaining Garypinoidea. Owing to the presence of short femorae on legs I and II, and the positions of the trichobothria, these features must be excluded as diagnostic for Amblyolpiidae.
The inclusion of the Eocene genus Baltamblyolpium in Amblyolpiidae can be justified by the distal position of trichobothrium it that in Amblyolpium and Neoamblyolpium is situated medially (Stanczak et al. 2023). In all other garypinoids, including the Upper Cretaceous genus Ajkagarypinus (Novák et al. 2023), trichobothrium it is situated in a basal position alongside ib, isb and ist.
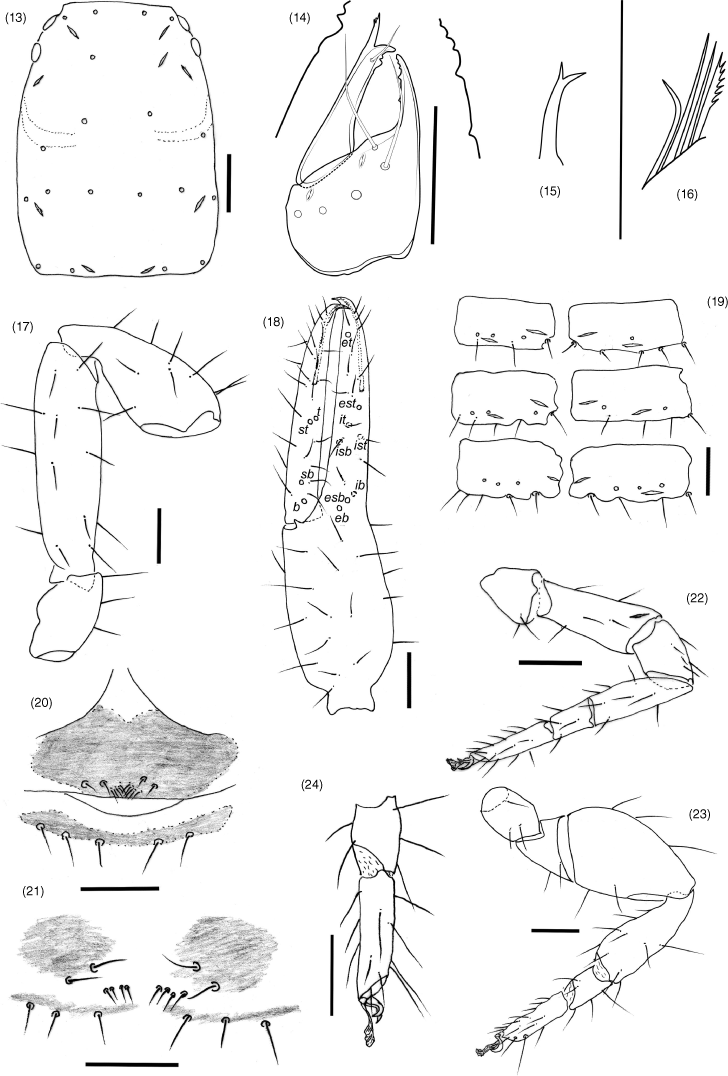
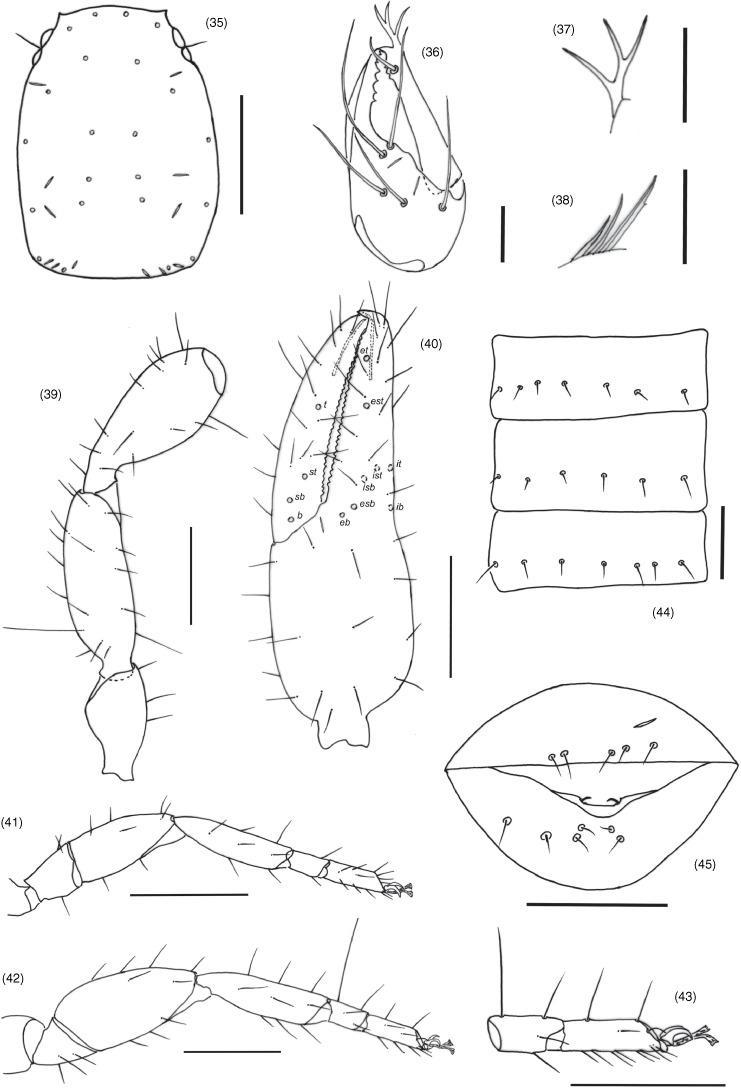
| 1. | Trichobothrium it situated in basal half of finger (Fig. 40); trichobothrium est situated much closer to et than esb (Fig. 40); sternites without glandular setae (Fig. 44)...Absensus gen. nov. (China) Trichobothrium it situated in medial or distal half of finger (Fig. 18); trichobothrium est situated either much closer to esb than et, or midway between esb and et (Fig. 18); sternites VI, VII and sometimes VIII with glandular setae (Fig. 19)...2 |
| 2. | Trichobothrium st closer to sb than t; trichobothrium it situated subdistally; legs I and II with femur approximately same length as patella; sternites VI–VIII with glandular setae...Baltamblyolpium Stanczak, Harvey, Harms, Hammel, Kotthoff & Loria (Eocene) Trichobothrium st closer to t than sb (Fig. 18); trichobothrium it situated sub-medially (Fig. 18); legs I and II with femur longer than patella (Fig. 22); sternites VI and VII, but not VIII, with glandular setae (Fig. 19)...3 |
| 3. | Trichobothrium ib situated on same level as, or slightly anterior to level of, eb and esb (Fig. 19)...Amblyolpium Simon (cosmopolitan) Trichobothrium ib situated posterior to level of eb and esb...Neoamblyolpium Hoff (western USA) |
Genus Amblyolpium Simon, 1898
Type species: Amblyolpium dollfusi Simon, 1898, by monotypy.
The genus Amblyolpium is widely distributed around the world, with 16 named species known from Asia, southern Europe, northern Africa and South America (World Pseudoscorpiones Catalog 2024), and several unnamed species from Australia (Harvey 2023).
Amblyolpium shenzhou sp. nov.
ZooBank: urn:lsid:zoobank.org:act:818D25B7-D2A6-410F-9C6A-365E37AEE02F
Holotype. CHINA: Guangdong Province: ♂, Meizhou City, Mt Qingliangshan: [24°14′28.46″N, 116°08′22.61″E], elev. 232 m, 10 April 2018, under bark, Xiangbo Guo (Ps.–MHBU–GD18041001).
Paratypes. CHINA: Guangdong Province: 1 ♂, 1 ♀, same data as holotype (Ps.–MHBU–GD18041002 and GD18041003). Hunan Province: 1 ♂, Huaihua City, Yuanling County [28°34′52.25″N, 110°27′28.04″E], elev. 139 m, 9 July 2022, under bark, Yanmeng Hou, Nana Zhan and Jianzhou Sun (Ps.–MHBU–HN22070901). Guizhou Province: 3 ♂, 1 ♀, Tongren City, Jiangkou County [27°49′35.79″N, 108°51′42.32″E], elev. 403 m, 4 July 2022, under bark, Yanmeng Hou, Nana Zhan and Jianzhou Sun (Ps.–MHBU–GZ22070401–03 and GZ22070404).
The specific name, shenzhou, is derived from the Chinese mandarin phrase ‘Shénzhōu’ (神舟), referring to the Shenzhou Crewed Spacecraft, a Chinese spacecraft developed for the nation’s crewed space program. Shenzhou-19 manned spacecraft was successfully launched in 2024. The name is a noun in apposition.
Amblyolpium shenzhou closely resembles A. japonicum, but differs by having stouter pedipalps (patella 2.5× in A. japonicum), and by the different carapaceal chaetotaxy (4 in each row, with a total of 28 setae in A. japonicum) (Morikawa 1960); it differs from A. biaroliatum (Tömösváry, 1884) and A. birmanicum (With, 1906) by the stout pedipalps (femur 4.3, patella 2.9× in A. biaroliatum and A. birmanicum) (Beier 1932); it differs from A. bellum Chamberlin, 1930 in the shape of the marginal teeth on the fixed chelal finger (retrorse pointed teeth in A. shenzhou, normal teeth in A. bellum) and the chaetotaxy of tergites I–III (4: 4: 4–6 v. 4–6–6), also by the slender pedipalps (femur 3.1× in A. bellum) (Chamberlin 1930a, 1930b; Harvey 1988); A. shenzhou shares a similar trichobothrial pattern with A. atropatesi Nassirkhani & Doustaresharaf, 2019 but can be distinguished by the different carapaceal chaetotaxy (4: 6: 4: 4–6: 4 (22–24) in the latter) and the stout chelal patella (2.07–2.14 v. 2.47–2.67×) (Nassirkhani and Doustaresharaf 2019); A. shenzhou resembles A. goldastehae Nassirkhani, Shoushtari & Abadi, 2016, but differs by the different trichobothrial pattern (est at the same level as t in A. goldastehae, distal to t in A. shenzhou) and the stouter pedipalpal patella (2.07–2.14 v. 2.69×) (Nassirkhani et al. 2016).
Body moderately flattened. Colour with sclerotised portions generally yellowish-brown, pedipalps, carapace and tergites darker.
With 5 setae on hand (Fig. 14), all setae acuminate, ls and is situated adjacent to each other; movable finger with one subdistal seta; subterminal tooth of movable finger not bifurcate and not enlarged; with two lyrifissures on dorsal face; galea with bifurcate tip (Fig. 15); rallum composed of 4 blades, distal blade with serrations on anterior margin, basal blade 1–3 smooth (Fig. 16); serrula exterior with 10–13 blades; lamina exterior present, moderately broad.
All segments completely smooth; setae very long and acicular; trochanter elongate, without small and round tubercles; trochanter 1.73, femur 3.73–3.91, patella 2.07–2.14, chela (with pedicel) 3.57–3.79, chela (without pedicel) 3.39–3.58, hand (with pedicel) 1.70–1.74, hand (without pedicel) 1.48–1.53× longer than broad, movable finger 1.12–1.15× longer than hand with pedicel. Femur without tactile seta on dorso-basal face. Fixed chelal finger with eight trichobothria, movable chelal finger with four trichobothria (Fig. 18): eb, esb and ib situated basally; esb in the midway of eb and isb; isb, ist and it grouped sub-medially; trichobothrium est in the midway of fixed chelal finger, and situated closer to et than to esb; ist slightly distal to isb; et situated near sub-distal end of finger; sb situated closer to b than to st; t situated closer to st, both in the midway of movable chelal finger, away from the tip of movable finger; no microsetae present on either finger. Venom apparatus present in both chelal fingers, venom ducts long and slender, terminating in nodus ramosus near one-third of the way from the tip of the finger. Fixed finger with 30–34 retrorse pointed teeth; movable finger with 21–24 teeth, distal five pointed and straight, the remainder retrorse and obtuse; without accessory teeth.
Longer than broad (1.37–1.39); sub-rectangular; with two pairs of eyes, each with developed lenses and situated near anterior margin of carapace; with 20 setae, anterior and posterior margin respectively with four setae; without transverse furrows; with four pairs of lyrifissures. Manducatory process with two simple and acute setae; remainder of maxilla with 7–8 setae. Chaetotaxy of coxae I–IV: 5–6: 6–7: 3–4: 5–6.
Pleural membrane longitudinally striate, without setae on membrane. Tergites (some incompletely divided, see Fig. 2) and sternites with narrow medial suture. Tergal chaetotaxy: 4: 4: 4-6: 6: 6: 6: 6–7: 7: 7: 8–9: 4; uniseriate; all setae acicular. Sternal chaetotaxy (IV–XI): 6–8: 8: 8–9: 10: 9–10: 8–9: 7–8: 4; both males and females without patches of chemosensorial setae in the middle of sternites VI–VIII (Fig. 19); setae uniseriate and acuminate; glandular setae absent; anus not surrounded by sternite XI.
Junction between femora and patellae I and II broad and apparently sub-mobile; patella I distinctly shorter than femur I; femur + patella of leg IV 2.11× longer than broad; metatarsus and tarsus IV see Fig. 12, 24; subterminal tarsal setae arcuate and acute; arolium much longer than claws, distinctly divided into two branches.
Male. Body length 1.90–1.99. Pedipalps: trochanter 0.19/0.11 (1.73), femur 0.41–0.43/0.11 (3.73–3.91), patella 0.29–0.30/0.14 (2.07–2.14), chela (with pedicel) 0.72–0.83/0.19–0.23 (3.57–3.79), chela (without pedicel) 0.68–0.78 (3.39–3.58), hand length (with pedicel) 0.33–0.39 (1.70–1.74), hand length (without pedicel) 0.29–34 (1.48–1.53); movable finger length 0.38–0.39. Carapace 0.46–0.48/0.33–0.35. Leg I trochanter 0.08–0.10/0.08 (1.00–1.25), femur 0.28–0.20/0.07 (2.57–2.86), patella 0.10/0.07 (1.43), tibia 0.17/0.05 (3.40), metatarsus 0.07/0.03 (2.33), tarsus 0.12/0.03 (4.00); leg IV trochanter 0.13–0.14/0.09 (1.44–1.56), femur + patella 0.38/0.18 (2.11), tibia 0.25/0.08 (3.13), metatarsus 0.10/0.02 (2.00), tarsus 0.16/0.04 (4.00).
Mostly same as holotype, Body length 2.06–2.49. Carapace 0.51–0.53/0.37–0.38 (1.38–1.39). Pedipalps: trochanter 0.22–0.24/0.11 (2.00–2.18), femur 0.47–0.48/0.12–0.13 (3.69–3.92), patella 0.34–0.35/0.15 (2.27–2.37), chela (with pedicel) 0.83/0.24 (3.46), chela (without pedicel) 0.78 (3.25), hand length (with pedicel) 0.39–0.40, hand length (without pedicel) 0.34–0.35, movable finger length 0.43–0.44. Leg I femur 0.20–0.21/0.08 (2.50–2.63), patella 0.11/0.08 (1.38), tibia 0.18–0.20/0.05-0.06 (3.33–3.60), metatarsus 0.07–0.08/0.04 (1.75–2.00), tarsus 0.13/0.03 (4.33); leg IV trochanter 0.15/0.10 (1.50), femur + patella 0.42/0.19–0.20 (2.10–2.21), tibia 0.27/0.09–0.10 (2.70–3.00), metatarsus 0.11/0.06 (1.83), tarsus 0.17/0.05 (3.40).
Amblyolpium shenzhou has been collected from three provinces (Guizhou, Hunan and Guangdong) in southern China, including the Yunnan–Guizhou Plateau.
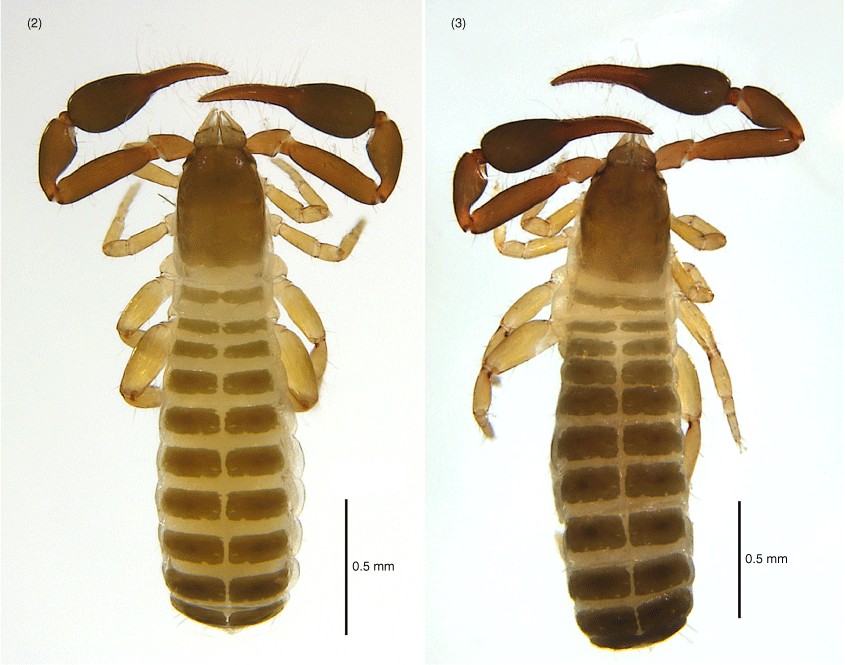
Amblyolpium shenzhou sp. nov.: male holotype except Fig. 9: 4, carapace, dorsal; 5, left pedipalp, dorsal; 6, left chela, retrolateral; 7, left chelicera, dorsal; 8, genital area, ventral; 9, genital area of female paratype, ventral.
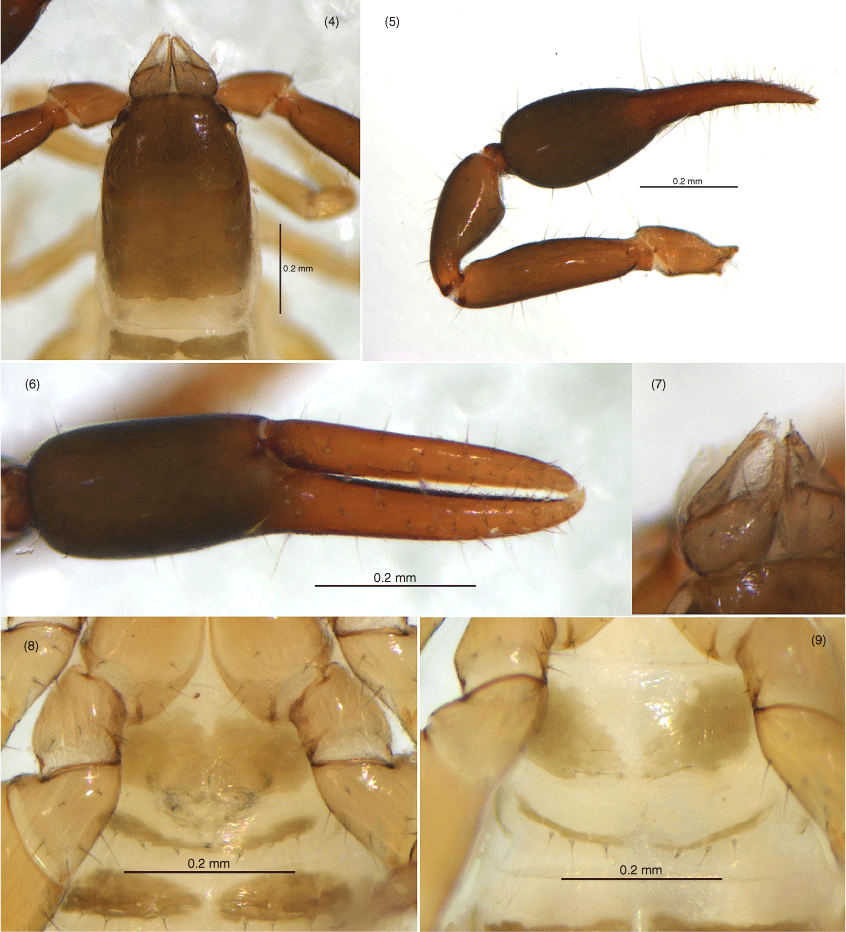
Amblyolpium shenzhou sp. nov.: holotype: 10, left leg I, lateral; 11, left leg IV, lateral; 12, left leg IV, metatarsus and tarsus, lateral.
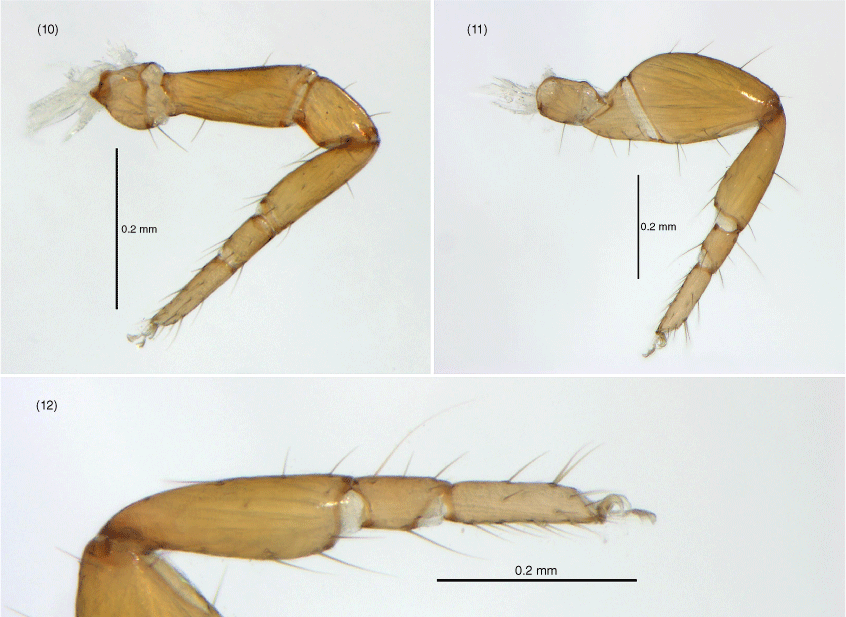
Amblyolpium shenzhou sp. nov.: holotype except Fig. 21: 13, carapace, position of lyrifissures indicated by black bars, dorsal; 14, right chelicerae, dorsal; 15, right galea; 16, right rallum; 17, left pedipalp (minus chela), dorsal; 18, left chela, retrolateral; 19, sternites VI-VIII, ventral; 20, genital area, ventral; 21, genital area of female paratype, ventral; 22, left leg I, lateral; 23, left leg IV, lateral; 24, left leg IV, metatarsus and tarsus. Scale bars: 0.10 mm.
Genus Absensus gen. nov.
ZooBank: urn:lsid:zoobank.org:act:4F082DC6-7B5C-4C74-842C-EBECB04C61C5
Type species: Absensus zhangi sp. nov.
The genus name is derived from the Latin word ‘absens’, means absence, referring to the lack of glandular setae on the abdominal sternites. It is to be treated as masculine.
Absensus can be assigned to Amblyolpiidae due to the presence of long venom ducts (Fig. 40). It differs from the other three genera, Amblyolpium, Neoamblyolpium and Baltamblyolpium, by the lack of glandular setae on the abdominal sternites (Fig. 44), and the basal position of trichobothrium it, which is situated medially in Amblyolpium and Neoamblyolpium and distally in Baltamblyolpium. It further differs from Amblyolpium and Neoamblyolpium by the femorae of the anterior legs being shorter than the patellae (femorae much longer than patellae in Amblyolpium and Neoamblyolpium).
Hand with 5 long, acuminate setae, ls and is situated adjacent to each other; movable finger with 1 long subdistal seta; rallum of 4 blades, distal blade with several spinules on anterior margin, others smooth; with 2 dorsal and 1 ventral lyrifissures; lamina exterior present; serrula interior modified to form velum; galea of ♀ with 2 long distal rami and a long sub-basal ramus, of ♂ with two distal rami.
Femur with a single sub-medial tactile seta; patella without disto-prolateral excavation; chelal hand ovate; pedicel prolaterally medially inserted. Fixed chelal finger with 8 trichobothria, movable chelal finger with 4 trichobothria: eb and esb situated at base of fingers; est situated sub-distally, closer to et than to esb; et situated sub-distally; ib, isb, ist and it situated sub-basally, with ib separated from the others; b, sb and st situated sub-basally, with st situated dorso-distally to sb; t situated sub-medially. Venom apparatus present in both chelal fingers, venom ducts long, terminating in nodus ramosus near tip of fingers. Chelal teeth juxtadentate; accessory teeth absent; chelal hand with retrolateral condyle rounded.
Sub-rectangular; with 4 large eyes situated close to anterior margin of carapace; epistome absent; without furrows.
Manducatory process with 1–2 long distal setae; maxillary shoulder absent; median maxillary lyrifissure present and situated sub-medially; all coxae approximately same width.
Femora I and II shorter than patellae I and II; junction between anterior femora and patellae perpendicular; oblique suture line between femora III and IV and patella III and IV; tarsi slightly longer than metatarsi; metatarsi III and IV with a long basal tactile seta; tibia III and IV with a long medial tactile seta; subterminal tarsal setae acuminate; arolium deeply divided and longer than claws; claws slender and simple.
Most tergites and sternites strongly divided. Pleural membrane longitudinally striate, and lacking setae. Sternites lacking glandular setae. Stigmatic sclerites with a single seta; spiracles with spiracular helix. Setae of anterior genital operculum (sternite II) approximately same size as other sternal setae. Posterior tergites and sternites with tactile setae. Anus (tergite XII and sternite XII) without raised rim; anus situated between tergite XI and sternite XI.
Absensus zhangi sp. nov.
ZooBank: urn:lsid:zoobank.org:act:C1C02372-6719-4812-B67B-A9F9E0D30414
Holotype. CHINA: Xizang Autonomous Region: ♂, Luozha County [28°12.752′N, 91°00.770′E], elev. 3225 m, 7 August 2014, Chao Zhang (Ps.–MHBU–XZ14080701).
Paratypes. CHINA: Xizang Autonomous Region: 1 ♂, 2 ♀, same data as holotype (Ps.–MHBU–XZ14080702–04).
The specific epithet is a patronym in honour of Dr Chao Zhang, who collected the specimens.
Body moderately flattened. Colour with sclerotised portions generally yellowish-brown, pedipalps, carapace and tergites darker.
With 5 setae on hand (Fig. 36), all setae acuminate, ls and is situated adjacent to each other; movable finger with one subdistal seta; subterminal tooth of movable finger not bifurcate and not enlarged; with two lyrifissures on dorsal face; galea with bifurcate tip and one sub-medial ramus (Fig. 37); rallum composed of 3–4 blades, distal blade with 1–2 basal serrations on anterior margin, basal blade smooth (Fig. 38); serrula exterior with 18–19 blades; lamina exterior present, moderately broad.
All segments completely smooth; setae very long and acicular; trochanter elongate, without small and round tubercles; trochanter 1.75, femur 2.71–2.88, patella 2.25–2.35, chela (with pedicel) 3.19–3.27, chela (without pedicel) 2.96–3.00, hand (with pedicel) 1.50–1.52, hand (without pedicel) 1.23–1.30× longer than broad, movable finger 1.12–1.15× longer than hand with pedicel. Femur with one tactile seta on dorso-basal face (Fig. 39). Fixed chelal finger with eight trichobothria, movable chelal finger with four trichobothria (Fig. 40): eb, esb and ib situated basally; esb closer to eb than to isb; isb, ist and it grouped sub-basally; trichobothrium est situated closer to et than to esb; ist distal to isb; et situated near sub-distal end of finger; sb situated closer to b than to st; t situated slightly closer to st than to tip of movable finger; no microsetae present on either finger. Venom apparatus present in both chelal fingers, venom ducts long and slender, terminating in nodus ramosus near one-third of the way from the fingertip. Fixed finger with 26–28 pointed teeth; movable finger with 29–30 obtuse teeth; without accessory teeth.
Longer than broad (1.37–1.54); sub-rectangular; with two pairs of eyes, each with developed lenses and situated near anterior margin of carapace; with 22 setae, anterior and posterior margin respectively with four setae; without transverse furrows; with 6 pairs of lyrifissures. Manducatory process with one long distal, and one long sub-distal setae; remainder of maxilla with eight setae. Chaetotaxy of coxae I–IV: 6: 7: 5: 5.
Pleural membrane longitudinally striate, without setae on membrane. Tergites (some incompletely divided, see Fig. 25) and sternites with narrow medial suture. Tergal chaetotaxy: 3–3: 2–2: 2–2: 2–2: 2–2: 2–2: 2–2: 2–2: 2–2: 5–5: 10; uniseriate; all setae acicular. Sternal chaetotaxy (IV–XI): 1–1: 3–3: 3–3: 3–3: 3–3: 3–3: 4–5: 10; both males and females without patches of chemosensorial setae in the middle of sternites VI–VIII; setae uniseriate and acuminate; glandular setae absent; anus not surrounded by sternite XI.
Junction between femora and patellae I and II broad and apparently sub-mobile; femur I distinctly shorter than patella I; femur + patella of leg IV 2.61–2.67× longer than broad; metatarsus and tarsus IV see Fig. 43; subterminal tarsal setae arcuate and acute; arolium much longer than claws, distinctly divided into two branches.
Measurements (length/breadth or depth in millimetres, ratios in parentheses). Male. Body length 2.61–2.68. Pedipalps: trochanter 0.28/0.16 (1.75), femur 0.46–0.49/0.17 (2.71–2.88), patella 0.45–0.47/0.20 (2.25–2.35), chela (with pedicel) 0.85–0.86/0.26–0.27 (3.19–3.27), chela (without pedicel) 0.78–0.80 (2.96–3.00), hand length (with pedicel) 0.39–0.41 (1.50–1.52), hand length (without pedicel) 0.32–0.35 (1.23–1.30); movable finger length 0.45–0.46. Carapace 0.56-0.60/0.39–0.41. Leg I femur 0.12–0.13/0.10–0.11 (1.18–1.20), patella 0.21–0.22/0.10–0.11 (2.00–2.10), tibia 0.25–0.26/0.06 (4.17–4.33), metatarsus 0.08–0.09/0.04–0.05 (1.80–2.00), tarsus 0.13–0.14/0.04 (3.25–3.50); leg IV femur + patella 0.47–0.48/0.18 (2.61–2.67), tibia 0.34–0.35/0.09–0.10 (3.50–3.78), metatarsus 0.11–0.12/0.06 (3.20–3.40), tarsus 0.16–0.17/0.05 (3.20–3.40).
Mostly same as holotype, Body length 2.94–3.17. Carapace 0.59–0.64/0.43–0.45 (1.37–1.42). Pedipalps: trochanter 0.31/0.16 (1.94), femur 0.48–0.54/0.18–0.19 (2.67–2.84), patella 0.47–0.53/0.21–0.23 (2.24–2.30), chela (with pedicel) 0.87–0.96/0.27–0.32 (3.00–3.22), chela (without pedicel) 0.81–0.90 (2.81–3.00), hand length (with pedicel) 0.43–0.46, hand length (without pedicel) 0.36–0.38, movable finger length 0.45–0.51. Leg I femur 0.13–0.14/0.12 (1.08–1.17), patella 0.22–0.24/0.11–0.12 (2.00), tibia 0.25–0.28/0.07 (3.58–4.00), metatarsus 0.08–0.09/0.05 (1.60–1.80), tarsus 0.12–0.14/0.04 (3.00–3.50); leg IV trochanter 0.17–0.18/0.09–0.11 (1.64–1.89), femur + patella 0.48–0.52/0.17–0.19 (2.74–2.82), tibia 0.36–0.39/0.09–0.11 (3.55–4.00), metatarsus 0.11–0.12/0.06 (1.83–2.00), tarsus 0.13–0.15/0.05 (2.60–3.00).
Specimens of Absensus zhangi have been collected from a single locality in southern China on the Himalayan Plateau.
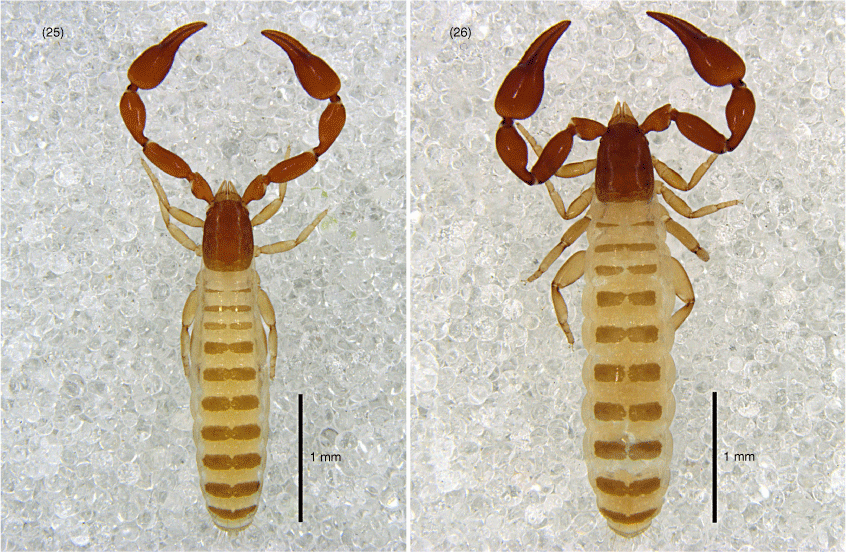
Absensus zhangi sp. nov.: holotype except 31: 27, carapace, dorsal; 28, left pedipalp, dorsal; 29, left chela, retrolateral; 30, genital area, ventral; 31, genital area of female paratype, ventral; 32, left leg I, lateral; 33, left leg IV, lateral; 34, left leg IV, metatarsus and tarsus, lateral.
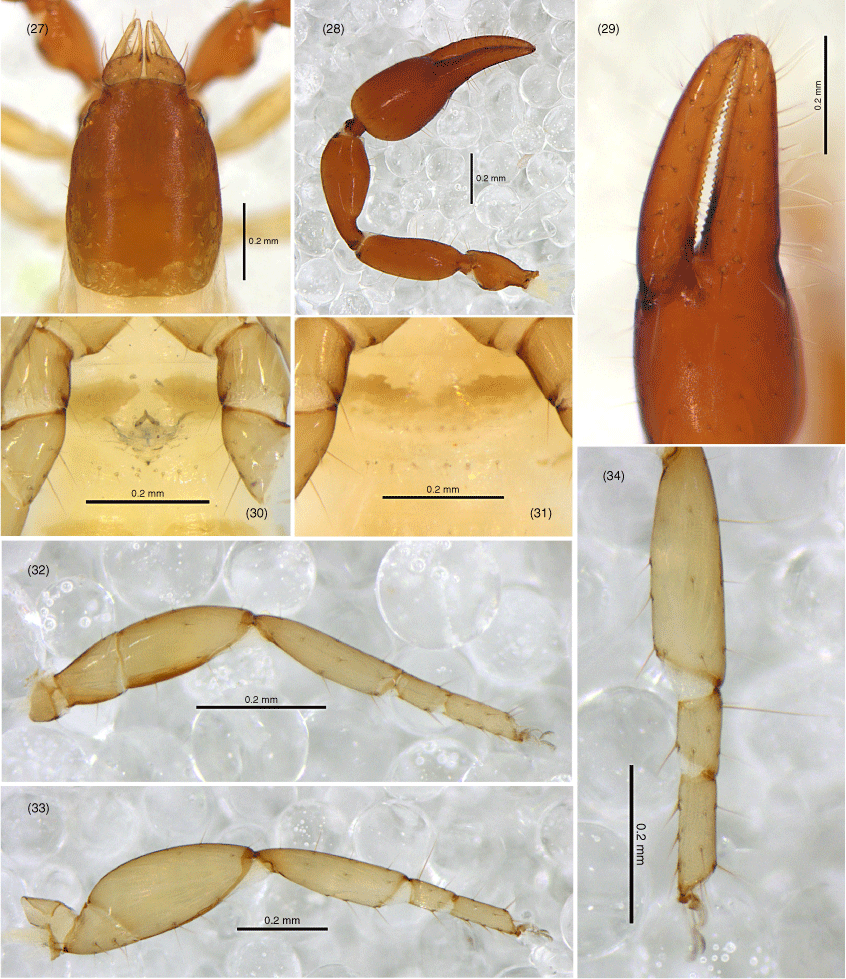
Absensus zhangi sp. nov.: holotype: 35, carapace, position of lyrifissures indicated by black bars, dorsal; 36, right chelicerae, dorsal; 37, right galea; 38, right rallum; 39, left pedipalp (minus chela), dorsal; 40, left chela, retrolateral; 41, left leg I, lateral; 42, left leg IV, lateral; 43, left leg IV, metatarsus and tarsus; 44, sternites VI–VIII, ventral; 45, genital area, ventral. Scale bars: 0.25 mm.
Conflicts of interest
M. Harvey is an editor for Invertebrate Systematics but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. The authors have no further conflicts of interest to declare.
Declaration of funding
This work was supported by Fundamental Research Program of Shanxi Province (202403021211040), the Special Project of ‘1331 Project’ to Wutai Mountain Cultural Ecological Collaborative Innovation Center in 2022, and Xinzhou Science and Technology Plan Project (20230214), all awarded to Zhizhong Gao.
Acknowledgements
Z. Gao and F. Zhang thank Prof. Chao Zhang, Yanmeng Hou, Nana Zhan and Jianzhou Sun (College of Life Science, Hebei University) for collecting the specimens. We express our gratitude to Nana Zhan for the assistance in obtaining the molecular data, and to the editor and anonymous reviewers for providing helpful comments on a draft of the manuscript. M. S. Harvey is grateful to Dr Bruno Buzatto (Flinders University) for supplying the specimen of Amblyolpium from South Australia, and the Western Australian Museum’s Molecular Systematics Unit for supplying some of the new sequence data.
References
Arabi J, Judson ML, Deharveng L, Lourenço WR, Cruaud C, Hassanin A (2012) Nucleotide composition of CO1 sequences in Chelicerata (Arthropoda): detecting new mitogenomic rearrangements. Journal of Molecular Evolution 74, 81-95.
| Crossref | Google Scholar | PubMed |
Beier M (1932) ‘Pseudoscorpionidea I. Subord. Chthoniinea et Neobisiinea, Vol. 57’. (Walter de Gruyter) 10.1515/9783111435107
Benavides LR, Cosgrove JG, Harvey MS, Giribet G (2019) Phylogenomic interrogation resolves the backbone of the Pseudoscorpiones Tree of Life. Molecular Phylogenetics and Evolution 139, 106509.
| Crossref | Google Scholar | PubMed |
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972-1973.
| Crossref | Google Scholar | PubMed |
Chamberlin JC (1930a) I.—A synoptic classification of the false scorpions or chela-spinners, with a report on a cosmopolitan collection of the same.—Part II. The Diplosphyronida (Arachnida–Chelonethida). Annals and Magazine of Natural History 5(10), 1-48.
| Crossref | Google Scholar |
Chamberlin JC (1930b) LXI.—A synoptic classification of the false scorpions or chela-spinners, with a report on a cosmopolitan collection of the same.—Part II. The Diplosphyronida (Arachnida–Chelonethida). Annals and Magazine of Natural History 5(30), 585-620.
| Crossref | Google Scholar |
Chamberlin JC (1931) The arachnid order Chelonethida. Stanford University Publications, Biological Sciences 7(1), 1-284.
| Google Scholar |
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek RC (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3, 294-299.
| Google Scholar | PubMed |
Gao Z, Zhang F (2019) First record of the genus Orochernes (Pseudoscorpiones: Chernetidae) from China, with description of a new species. Zootaxa 4612, 126-132.
| Crossref | Google Scholar | PubMed |
Giribet G, Carranza S, Baguñà J, Riutort M, Ribera C (1996) First molecular evidence for the existence of a Tardigrada+Arthropoda clade. Molecular Biology and Evolution 13, 76-84.
| Crossref | Google Scholar | PubMed |
Harvey MS (1988) Pseudoscorpions from the Krakatau Islands and adjacent regions, Indonesia (Chelicerata: Pseudoscorpionida). Memoirs of the Museum of Victoria 49, 309-353.
| Crossref | Google Scholar |
Harvey MS (1992) The phylogeny and classification of the Pseudoscorpionida (Chelicerata: Arachnida). Invertebrate Taxonomy 6, 1373-1435.
| Crossref | Google Scholar |
Harvey MS (2023) A preliminary phylogeny for the pseudoscorpion family Garypinidae (Pseudoscorpiones: Garypinoidea), with new taxa and remarks on the Australasian fauna. Invertebrate Systematics 37, 623-676.
| Crossref | Google Scholar |
Harvey MS, Harms D (2022) The pseudoscorpion genus Centrochthonius (Pseudoscorpiones: Pseudotyrannochthoniidae) from central Asia and description of a new species from Nepal. Journal of Arachnology 50, 158-174.
| Crossref | Google Scholar |
Harvey MS, Wynne JJ (2014) Troglomorphic pseudoscorpions (Arachnida: Pseudoscorpiones) of northern Arizona, with the description of two new short-range endemic species. Journal of Arachnology 42, 205-219.
| Crossref | Google Scholar |
Harvey MS, Ratnaweera PB, Udagama PV, Wijesinghe MR (2012) A new species of the pseudoscorpion genus Megachernes (Pseudoscorpiones: Chernetidae) associated with a threatened Sri Lankan rainforest rodent, with a review of host associations of Megachernes. Journal of Natural History 46, 2519-2535.
| Crossref | Google Scholar |
Harvey MS, Lopes PC, Goldsmith GR, Halajian A, Hillyer MJ, Huey JA (2015) A novel symbiotic relationship between sociable weaver birds (Philetairus socius) and a new cheliferid pseudoscorpion (Pseudoscorpiones: Cheliferidae) in southern Africa. Invertebrate Systematics 29, 444-456.
| Crossref | Google Scholar |
Harvey MS, Abrams KM, Beavis AS, Hillyer MJ, Huey JA (2016) Pseudo-scorpions of the family Feaellidae (Pseudoscorpiones: Feaelloidea) from the Pilbara region of Western Australia show extreme short-range endemism. Invertebrate Systematics 30, 491-508.
| Crossref | Google Scholar |
Harvey MS, Hillyer MJ, Carvajal JI, Huey JA (2020) Supralittoral pseudoscorpions of the genus Garypus (Pseudoscorpiones: Garypidae) from the Indo-West Pacific region, with a review of the subfamily classification of Garypidae. Invertebrate Systematics 34, 34-87.
| Crossref | Google Scholar |
Judson MLI (2007) A new and endangered species of the pseudoscorpion genus Lagynochthonius from a cave in Vietnam, with notes on chelal morphology and the composition of the Tyrannochthoniini (Arachnida, Chelonethi, Chthoniidae). Zootaxa 1627, 53-68.
| Crossref | Google Scholar |
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772-780.
| Crossref | Google Scholar | PubMed |
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30, 3059-3066.
| Crossref | Google Scholar | PubMed |
Kearse M, Moir R, Wilson AC, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond AJ (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12), 1647-1649.
| Crossref | Google Scholar | PubMed |
Kolesnikov VB, Mikhailov KG, Turbanov IS (2023) A new species of the genus Centrochthonius Beier, 1931 (Pseudoscorpiones: Pseudotyrannochthoniidae) from Kyrgyzstan. Arthropoda Selecta 32, 444-454.
| Crossref | Google Scholar |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37, 1530-1534.
| Crossref | Google Scholar | PubMed |
Morikawa K (1960) Systematic studies of Japanese pseudoscorpions. Memoirs of Ehime University (2B) 4, 85-172.
| Google Scholar |
Murienne J, Harvey MS, Giribet G (2008) First molecular phylogeny of the major clades of Pseudoscorpiones (Arthropoda: Chelicerata). Molecular Phylogenetics and Evolution 49, 170-184.
| Crossref | Google Scholar | PubMed |
Nassirkhani M, Doustaresharaf MM (2019) Description of a new pseudoscorpion species of the genus Amblyolpium Simon (Pseudo-scorpiones: Garypinidae) from north-western Iran. Zoology in the Middle East 65, 268-273.
| Crossref | Google Scholar |
Nassirkhani M, Shoushtari RV, Abadi MR (2016) A new pseudoscorpion species in Amblyolpium (Pseudoscorpiones: Garypinidae) from a house in Kermanshah Province, Iran. Arachnologische Mitteilungen 52, 1-3.
| Crossref | Google Scholar |
Novák J, Harvey MS, Szabó M, Hammel J, Harms D, Kotthoff U, Hörweg C, Brazidec M, Ősi A (2023) A new Mesozoic record of the pseudoscorpion family Garypinidae from Upper Cretaceous (Santonian) Ajkaite amber, Ajka area, Hungary. Cretaceous Research 153, 105709.
| Crossref | Google Scholar |
Schwarze D, Harms D, Hammel J, Kotthoff U (2022) The first fossils of the most basal pseudoscorpion family (Arachnida: Pseudoscorpiones: Pseudotyrannochthoniidae): Evidence for major biogeographical shifts in the European paleofauna. PalZ 96, 11-27.
| Crossref | Google Scholar |
Stanczak N, Harvey MS, Harms D, Hammel JU, Kotthoff U, Loria SF (2023) A new pseudoscorpion genus (Garypinoidea: Garypinidae) from the Eocene supports extinction and range contraction in the European paleobiota. PeerJ 11, e15989.
| Crossref | Google Scholar |
Trifinopoulos J, Nguyen L-T, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44(W1), W232-235.
| Crossref | Google Scholar | PubMed |
Whiting MF, Carpenter JC, Wheeler QD, Wheeler WC (1997) The Strepsiptera problem: phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Systematic Biology 46, 1-68.
| Crossref | Google Scholar | PubMed |