The contributions of Rupert Best to the modern concept of the nature of viruses
Andrew D. W. Geering A *
A *
A
Abstract
Rupert Jethro Best (1903–91), working alone at the Waite Agricultural Research Institute in Adelaide between 1934 and 1937, was among the first to purify tobacco mosaic virus (TMV) and to propose that it was a complex macromolecule containing protein and another reactive group that was responsible for infectivity of the virus. However, his research was completely overshadowed by that of Wendell Stanley and the duo of Frederick Bawden and Norman (aka Bill) Pirie, working in the United States of America and Great Britain, respectively, to the point where Best is not even mentioned in modern histories of TMV. Many factors have contributed to this lack of recognition for Best. Professor James Prescott, a soil scientist and Best’s supervisor at the Waite Institute, failed to appreciate the significance of his research, leading to critical delays in publication that prevented him from claiming precedence for purifying TMV. When Best’s research was eventually published, it was in Australian journals that were not broadly distributed, resulting in minimal international exposure for his research. The plant virology community within which he worked in Australia was very small and entirely focused on plant disease control, and not concerned with fundamental questions about the composition of viruses. Communication with similarly interested scientists in the Northern Hemisphere was hindered by the great distances involved. In this paper, pioneering research done by Best on TMV is reviewed, and placed in context with that undertaken by Stanley and by Bawden and Pirie, who are best remembered for purifying TMV and characterising its physicochemical properties.
Keywords: agriculture, Frederick Bawden, history of virology, microbiology, plants, review, tobamovirus, viruses, Wendell Stanley.
Plant pathologists working in Australia during the first half of the twentieth century faced many challenges. Plant pathologist Walter Mervyn Carne (1885–1952), in his presidential address to the Royal Society of Western Australia in 1928, characterised the research environment of this period as one where there was ‘an absence of colleagues of similar interests with whom to consult, … defects of libraries and equipment, … ignorance of the work going on with other States and the feeling of geographical and mental isolation’.1 The Waite Agricultural Research Institute in the Adelaide suburb of Glen Osmond may have fared slightly better than other agricultural research institutions in Australia,2 but nevertheless the research facilities were still very rudimentary. Tobacco (Nicotiana tabacum and N. glutinosa) plants, so vital for plant virology research at the time, were devastated by outbreaks of blue mould caused by Peronospora tabacina.3 The glasshouse at Waite Institute was not temperature regulated, making it difficult to conduct experiments at the heights of winter and summer. The only local avenues available for publication of plant pathology papers were journals published by the state Departments of Agriculture and the Australian Institute of Agricultural Science, and papers published in these journals were invisible to all but a few scientists in the Northern Hemisphere.4 To obtain exposure for research, Australian plant pathologists had to publish papers in the journals of overseas societies.
It was in this research environment that a young scientist named Rupert Jethro Best (1903–91, Fig. 1) began his scientific career at Waite Institute in 1928, initially as a soil chemist but later as a plant virologist. His discoveries on the physicochemical properties of tobacco mosaic virus (TMV) were revolutionary for the time and helped forge the concept of what a virus is. However, despite a lifetime of achievement, Best is now largely forgotten by the plant pathology community, even in his home country of Australia. This article provides a brief account of Best’s early scientific career, particularly pertaining to TMV (Fig. 2), and provides an explanation as to why he has not received the recognition he richly deserves.
Portrait photograph of Rupert Best in 1936, at the age of 33 (photographer unknown). Rupert Jethro Best—Records, 1929–68, Reference PRG 232, State Library of South Australia, Mortlock Library of South Australiana, Adelaide.

Tobacco plant infected with tobacco mosaic virus. Photograph credit: Bussakan Punlerdmatee/Shutterstock.

Best was born in the Adelaide Hills (Birdwood) and attended Adelaide High School from 1917 to 1921.5 His father, Jethro, was a contract miner who was tragically killed by a fall of earth in a subway trench at the Mile-End Railway Station in Adelaide in 1927.6 In 1922, Best commenced as a cadet in the chemistry department of the University of Adelaide under Professor Edward Henry Rennie (1852–1927), and in 1926, he graduated with a Bachelor of Science (Honours II), majoring in physical and organic chemistry.7 Best immediately continued onto higher degree education, earning a Master of Science degree in 1927 for his research on the nature and properties of metal colloids, especially platinum hydrosols.8
After completing his university studies, Best was briefly employed in junior teaching positions within the chemistry department before being appointed as an assistant chemist under Professor James Arthur Prescott (1890–1987) at the Waite Agricultural Research Institute in the Adelaide suburb of Glen Osmond on 1 December 1928.9 Under the direction of Prescott, a renowned soil scientist,10 Best turned his attention to measuring the chemical properties of soils. He developed methods of electrometric determination of soil pH, chloride content and total soluble salts.11 Of note was a portable field device he developed for measuring the chloride content of soils, which was subsequently adopted for soil salinity surveys throughout Australia and elsewhere in the world. Best was awarded the first ever Rennie Memorial Medal by the Royal Australian Chemical Institute, an honour that celebrates an early career scientist with less than eight years of professional experience and who has contributed most towards the development of a branch of chemical science.12 It is apt that Best received this award, as he was the last Honours student of Professor Rennie before he died in January 1927.13
Best’s introduction to plant virology followed a discussion with Geoffrey Samuel (1898–1985), who was the senior plant pathologist at Waite Institute and was leading a long-running project on tomato spotted wilt virus (TSWV).14 One of the impediments to research on TSWV was the rapid loss of infectivity of the virus when a sap extract was exposed to air. Best counselled Samuel to investigate the relationships between pH values, redox potentials, and the activity of the virus, and to use TMV as a control, as it was at the opposite end of the spectrum to TSWV in terms of stability. Upon hearing this advice, Samuel declared the subject area to be a closed book to him and invited Best to design and conduct experiments following the chemical approach he suggested.15 Thenceforth began a four-month-long collaboration between the two, beginning in December 1933, which resulted in three publications.16 Unfortunately, this highly productive partnership was short-lived, with Samuel departing for Great Britain in April 1934 to take up a research position at Rothamsted Experimental Station.17
Best was so encouraged by the success of the early experiments on TSWV and TMV that he decided to continue the research program alone after the departure of Samuel:
I (Best) changed my approach and concentrated first on TMV with a view to isolating it in as pure form as possible so that (a) it could be stored in either wet or dry form to use at any time in a standard condition and (b) to build up stocks of pure virus to examine chemically and to determine its nature and properties.18
By the end of 1934, Best ‘was satisfied from qualitative tests and nitrogen assays that TMV was predominantly protein, but a complex one with at least two active parts’. He approached Prescott for endorsement to publish a short paper on his discoveries about the protein nature of TMV and its isoelectric point. However, Prescott took a firm stand that ‘the results (he) claimed were so revolutionary that he should publish nothing until (he) had water-tight proof’.19 Nevertheless, Best took the opportunity to insert a footnote in a paper he wrote in the latter part of 1935 on the effect of the environment on the production of primary lesions by plant viruses, which stated that: ‘The inocula were prepared from a sample of virus purified by precipitating it from clarified plant juice at the isoelectric point of the virus or associated protein (pH 3.4 ± 0.2)’.20
Unbeknownst to Best, a biochemist called Wendell Meredith Stanley (1904–71) was working in parallel with him at the Princeton Laboratories of the Rockefeller Institute for Medical Research, New Jersey, to characterise TMV.21 The Rockefeller Institute where Stanley worked was well-resourced and brimming with eminent scientists, who had access to the most technologically advanced equipment of the time. Simon Flexner, the institute director, encouraged interdisciplinary collaboration and advocated a physiochemical approach to the study of viruses. Housing and a clubhouse were provided to institute staff on the 800-acre-estate, which helped to create a close-knit social and professional community. Stanley worked within the Division of Plant Pathology as part of a large plant virology team including Louis O. Kunkel (division head), Francis O. Holmes, William C. Price, and Philip R. White as principal investigators. The division was housed in a three-story laboratory complex that was connected to eight spacious greenhouses. Importantly, Stanley’s laboratory was located 100 yards (~90 m) from that of Northrop, who had perfected salt fractionation techniques for purifying digestive enzymes such as pepsin and was pursuing the idea that bacteriophages were also autocatalytic enzymes.22 Northrop provided technical and academic guidance to Stanley during the initial stages of his virology career.
Incentivised by the opportunity for a promotion from assistant to associate member at the Rockefeller Institute, Stanley set about the task of purifying TMV. In the purification protocol he devised, Stanley combined elements of the ammonium sulfate fractionation techniques for proteins developed by Northrop,23 with the lead subacetate precipitation step utilised by Carl Vinson.24 Using this protocol, he obtained 0.03-mm-long, needle-shaped crystals from the ‘juice’ of infected tobacco plants, which contained all the properties of the native virus, even when the crystals were dissolved and diluted a billion-fold. These crystals contained 20% nitrogen (N) and reacted positively in a variety of protein assays but the two assays for carbohydrate gave negative results. Stanley published his ground-breaking discoveries in the June 1935 issue of the journal Science.25 He concluded that the crystals were composed entirely of protein, and that the viral activity was an intrinsic property of the protein as infectivity declined following pepsin digestion of the protein. In the final sentence of his paper, Stanley proposed that TMV was an autocatalytic protein, thus drawing the analogy between a virus and an enzyme such as trypsin, which is generated by self-cleavage from an inactive precursor, trypsinogen.26
The day Stanley’s paper in Science appeared in print, the New York Times covered his report with a front-page story entitled ‘Crystals Isolated at Princeton Believed Unseen Disease Virus’, and more articles followed in other newspapers. Despite the excitement generated in America, Stanley’s research findings did not immediately filter through to Best in Adelaide until Stanley himself wrote to Best stating that he thought they were working on the same thing.27 Best was later to comment that:
In those days I read a wide range of specialist scientific journals but confined my reading of popular journals to 'Nature', and did not read 'Science', which in those days was not highly regarded.28
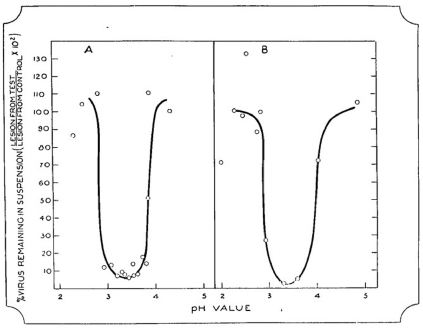
Best’s paper on the ‘Precipitation of the tobacco mosaic virus at its isoelectric point’ was eventually published in the March 1936 issue of the Australian Journal of Experimental Biology and Medical Science,29 some nine months after that of Stanley. The experiments described in this paper had been conducted in the second half of 1934.30 Best drew on previous observations by Vinson and Petre31 of the protein-like behaviour of TMV to hypothesise that the virus would have an isoelectric point, at which point it would fall out of solution due to the net neutral charge of the molecule. To test this hypothesis, Best prepared a range of phthalate-phosphate-borate buffers of varying pH but constant salt concentration, and each buffer was added to a clarified sap extract from an infected tobacco or tomato plant, and the precipitate collected after a thirty-minute incubation by low-speed centrifugation. The precipitates were then dissolved in pH 7 buffer and compared with equivalent dilutions of infected plant sap or supernatant left after precipitation, using local lesion assays to measure relative virus concentrations. Maximum virus precipitation occurred at pH 3.4, where, under suitable conditions, more than 99% of the virus activity was precipitated (Fig. 3). This fraction of virus gave positive biochemical test results for protein, and desiccator-dried samples contained 14% N, which was within the normal range expected for proteins. Best concluded that ‘the virus itself is something in the nature of an altered protein, in which another reactive group (not normal to the protein) has been produced through some agency at present unknown, and that such a protein can produce a similar change in other proteins having a similar structure; and so the process of proliferation goes on. On account of this new reactive group the altered protein (virus or virus-complex) enters into reactions which are not normal to the metabolic processes’.
Reproduction of graph produced by Rupert Best showing the precipitation of tobacco mosaic virus at various pH values. Panel (a) from clarified juice of infected tobacco plants; panel (b) from a suspension of purified virus complex. From the Australian Journal of Experimental Biology and Medical Science, vol. 14, 1936.
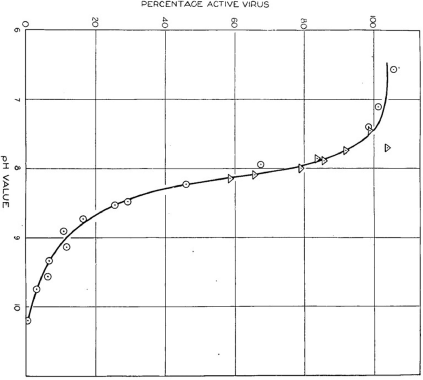
In the December 1936 issue of the Australian Journal of Experimental Biology and Medical Science, Best published a second paper that expanded upon his hypothesis that TMV was a complex macromolecule containing protein and another reactive group.32 He observed that solutions of purified virus in a pH neutral buffer could be kept for over a year without any apparent loss in viral activity, allowing a large stockpile of the virus to be created for use in multiple comparable experiments. Aliquots of purified virus or clarified sap extracts were added to different buffer solutions ranging from pH 5 to 10.2 and incubated at room temperature for twelve hours to allow the solutions to reach a steady state. The pH of the solutions was then adjusted back to neutrality and viral activity quantified using local lesion counts on N. glutinosa. Using this experimental design, Best showed that infectivity of the virus progressively declined as pH increased above 7 and that this alkali treatment caused irreversible inactivation of the virus, suggesting intra-molecular changes to the virus. The largest decline in infectivity of the virus occurred between pH 8.0–8.9 (Fig. 4), within which range the ratio of hydrion concentration to virus activity was constant, as would be observed if inactivation of the virus was associated with neutralisation of an acidic group.33 He concluded that TMV could be regarded as a ‘protein complex with a prosthetic group or groups’, with the activity of the virus residing within this prosthetic group. He made a point of distinguishing between this prosthetic group and the bulk of the virus that was composed of protein and was primarily responsible for determining the isoelectric point of the virus. Best did not define what he meant by ‘prosthetic group’, but this term was commonly used in the 1930s to describe a molecule other than a polypeptide that was conjugated to a protein moiety, and which was essential for the activity of the protein.34
Reproduction of graph produced by Rupert Best showing the percentage of tobacco mosaic virus remaining active after twelve hours storage at various pH values. Triangles represent values obtained with purified virus, and circles represent values obtained with clarified infective juice. From the Australian Journal of Experimental Biology and Medical Science, vol. 14, 1936.
An answer as to what this prosthetic group might be was revealed in a matter of a few weeks in a letter to Nature by Frederick Charles Bawden, Norman (aka Bill) Wingate Pirie, John Desmond Bernal and Isidor Frankuchen.35 Bawden, a recently appointed plant pathologist at Rothamsted Experimental Station, and Pirie, a biochemist at Cambridge University, attempted to recreate Stanley’s purification of TMV but the composition of the substance they obtained was different—it contained less protein (16.7% N) and had trace elements of sulfur (0.2–0.7%), phosphorus (0.5%) and carbohydrate (2.5%), which were contained within ribonucleic acid (RNA) that was released from the protein upon heat-denaturation. No comment was made as to the significance of the detection of RNA. Bernal, a friend, and colleague of Pirie from Cambridge University,36 contributed X-ray crystallography results to the paper which showed that the purified substance was not truly crystalline in nature as proposed by Stanley because the arrangement of molecules was only regular in cross-section. It was postulated that TMV was a rod-shaped molecule with a minimum length of at least ten times the width and a length greater than 100 nm.
Best was quick to recognise the significance of the results obtained by Bawden and his coworkers. In April 1937, he published a letter to Nature describing the formation of visible mesomorphic fibres in clarified sap extracts from diseased plants that had been stored at 1°C for several months.37 These fibres could be lifted as a clump out of the test tube using the tip of a pipette.38 Best suggested that the fibres were primarily composed of virus as they contained 97% of the viral activity within the extract. Moreover, they most likely were aggregates of virus particles as the fibres could easily be disrupted by agitation, then re-form after the sap extract was left to stand still. At 90°C, the fibres collapsed and formed irregular clumps that no longer were infective. When viewed under a polarising light microscope, the fibres showed spontaneous double refraction with straight extinction, providing evidence of the paracrystalline nature of the virus. He concluded that the needle-shaped crystals observed by Stanley may have been similar structures to those observed by him.
In September 1937, Best published a second letter to Nature providing results to support his previous conclusion that the mesomorphic or paracrystalline fibres were viral in nature.39 The fibres spontaneously formed when the pH of an aqueous solution of purified virus was adjusted to pH 5 and ammonium sulfate was added to a final concentration of 0.4 M. Dried preparations of these fibres contained 16.6% nitrogen and 0.5% phosphorous, confirming the results of the British researchers on the chemical composition of TMV. Best left no doubt as to his opinion as to the composition of TMV by describing it as a ‘nucleoprotein’ in the title of the paper. He concluded that ‘there is evidence to associate the acid prosthetic groups deduced by me on the basis of the pH activity curve for this virus, with the nucleic acid demonstrated by Bawden and Pirie’.
In 1936, Stanley exchanged TMV preparations with Bawden and Pirie and privately reached agreement with them as to the composition of the virus,40 but publicly he held steadfast to his belief that the active portion of the virus was protein.41 Stanley also provided samples of TMV to Ralph Wyckoff, an expert in analytical ultracentrifugation working at the Rockefeller Institute, who using this technique, provided strong evidence that the viral protein was homogenous in size and had a molecular weight that was probably greater than 10 million Da.42 However, in a blow to the autocatalytic protein hypothesis for viruses, candidate protein precursors (proteins with a higher molecular weight than the viral protein) could not be identified in the juice of healthy plants. This result, combined with the observation that TMV was unable to propagate in a test tube in the same manner as trypsin in the presence of trypsinogen, forced Stanley to modify his autocatalytic protein hypothesis for viruses in 1937.43 Instead, he proposed that the TMV protein attracted intermediate compounds in the cell protoplasm such as amino acids or polypeptides and these arranged in positions directed by the existing template to form identical protein molecules, analogous to crystal growth in a seeded solution of chemical components.
Best’s theory on the nature of viruses, as described in a conference paper presented to the Australian Chemical Institute in May 1937,44 differed in several significant ways from that of Stanley, and came much closer to describing the true essence of viruses:
It has been shown that it is possible to destroy virus activity by relatively mild chemical treatments without in any way changing the power of the virus to produce antibodies or to enter into the precipitation reaction. It follows that in these cases the chemical groupings which are responsible for the serological behaviour are not the same as those which are responsible for virus activity in the ordinary way … Although virus activity is a property of the whole molecule, there seems to be little doubt that the main effects are produced through the agency of specific chemical groups – prosthetic groups. Slight differences in the architecture of the main molecule are probably responsible for the differences shown by different strains of the same virus, and different virus types probably owe their characters to differences in the nature and number of the prosthetic groups … Viruses may be regarded as complex chemical structures, built on a protein base with a large number of and variety of prosthetic groups, through which they enter into those reactions by which they become evident and by which they multiply—reactions we have come to associate with life and living … Viruses may be regarded as living molecules of graded complexity and organisation covering the transition between the architecture of the larger non-living chemical molecules and the architecture of the simplest living cell.45
Best clearly considered viruses to be heterogeneous molecules composed of smaller constituents, and that they were living, able to govern their own reproduction through the action of prosthetic groups.
In a second paper presented at the January 1939 meeting of the Australian & New Zealand Association for the Advancement of Science in Canberra,46 Best offered alternative theories for the reproduction of viruses, the first closely resembling that of Stanley’s, as he suggested that the virus acted as a template with a weak surface charge that attracted the essential building blocks for a new virus molecule. Best also speculated that the virus had ‘analytic as well as synthetic powers, and in this sense it would be in part be producing some of its own raw materials and environment’.
Best published two more papers on TMV in 1940, the first notable as it showed that this virus followed the phase rule, with it precipitating out of solution as the concentration of electrolyte increased.47 Best had planned to take a year of sabbatical leave at Rothamsted Experimental Station in England where Bawden was based, but the advent of World War 2 curtailed these plans.48 After the war finished, Best virtually ceased working on TMV, instead focussing his attention on TSWV, which was the more economically important pathogen of the two. Among the more significant findings of the latter part of his career was demonstrating the exchange of character determinants between TWSV strains in a mixed infection, which now would be recognised as evidence for pseudorecombination.49
It is now etched into history that Stanley was jointly awarded the Nobel Prize for Chemistry in 1946 with John Northrop for ‘preparation of enzymes and virus proteins in a pure form’.50 This prize was principally awarded to Stanley for his isolation of TMV in a pure crystalline state as described in the Science paper of 1935. Although this research was beset by technical errors and misconceptions, it had a profound impact on the way that scientists studied viruses by treating them as chemical compounds. It is indisputable that Stanley had publishing precedence for purifying TMV, although Best appears to have achieved this technical feat at the same time as Stanley, but his publication plans were hindered by his overly cautious supervisor at Waite Institute.51 Stanley cultivated the popular media to publicise his research, thereby gaining widespread fame, whereas Best confined discussion of his research to academic circles. Stanley’s credentials were also promoted by his colleague Arne Tiselius, a biochemist at the University of Uppsala, whose opinions carried significant weight with the Nobel Committee.52
Best missed out on any major international recognition through awards for his research, and his name does not even rate a mention in modern histories of TMV. However, as elegantly stated by prominent plant virologist Richard Francki (1930–1990), a successor to Best at Waite Institute, there seems little doubt that ‘he contributed very significantly to the development of modern concepts of the nature of viruses. His achievements are all the more praiseworthy as they were made by a lone worker in a very isolated academic environment at a time when communication between researchers was far from that which is taken for granted today’.53 The only other scientist in Australia working on the basic biology of viruses at the time when Best purified TMV was Frank Macfarlane Burnet, who worked on bacteriophages and animal viruses at the Walter and Eliza Hall Institute of Medical Research in Melbourne.54 Emphasising the difficulties an Australian scientist had making traction in the American and European research scenes, Burnet’s conceptual advances on the identity of bacteriophages were largely ignored by Max Delbrück at the California Institute of Technology in Pasadena.55 It took until 1956 before it was proven beyond doubt by Heinz Fraenkel-Conrat, Alfred Gierer and Gerhard Schramm that infectivity of TMV was imparted by the RNA component of the virus and not the protein.56
Acknowledgements
I thank surviving members of Rupert Best’s family for permission to publish his 1977 letter to Professor Quirk. The assistance of staff at the State Library of South Australia and the Bancroft Library at the University of California, Berkeley in retrieving original documents during research for this paper is also gratefully acknowledged.
References
Bawden, F. C., Pirie, N. W., Bernal, J. D., and Fankuchen, I. (1936) Liquid crystalline substances from virus-infected plants, Nature, 138, 1051-1052.
| Crossref | Google Scholar |
Best, R. J. (1929a) A rapid electrometric method for determining the chloride content of soils, The Journal of Agricultural Science, 19, 533-540.
| Crossref | Google Scholar |
Best, R. J. (1929b) The acid reaction and CO2 content of conductivity water, Australian Journal of Experimental Biology and Medical Science, 6, 107-110.
| Crossref | Google Scholar |
Best, R. J. (1935) The effect of environment of the production of primary lesions by plant viruses, Journal of the Australian Institute of Agricultural Science, 1, 159-161.
| Google Scholar |
Best, R. J. (1936a) Precipitation of the tobacco mosaic virus complex at its isoelectric point, Australian Journal of Experimental Biology and Medical Science, 14, 1-13.
| Google Scholar |
Best, R. J. (1936b) The relationship between the activity of tobacco mosaic virus suspensions and hydrion concentration over the pH range 5 to 10, Australian Journal of Experimental Biology and Medical Science, 14, 323-328.
| Crossref | Google Scholar |
Best, R. J. (1937a) Visible mesomorphic fibres of tobacco mosaic virus in juice from diseased plants, Nature, 139, 628-629.
| Crossref | Google Scholar |
Best, R. J. (1937b) Artificially prepared visible paracrystalline fibres of tobacco mosaic virus nucleoprotein, Nature, 140, 547-548.
| Crossref | Google Scholar |
Best, R. J. (1937c) The chemistry of some plant viruses, The Australian Chemical Institute Journal and Proceedings, 4, 375-392.
| Google Scholar |
Best, R. J. (1939b) Virus activity as a property of some protein molecules, The Journal of the Australian Institute of Agricultural Science, 5, 94-102.
| Google Scholar |
Best, R. J. (1940b) The action of electrolytes on solutions of tobacco mosaic virus nucleoprotein (Marmor tabaci var. vulgare Holmes), Australian Journal of Experimental Biology, 18, 307-312.
| Crossref | Google Scholar |
Best, R. J. (1940c) Methods for the preparation of pure tobacco mosaic virus nucleoprotein (Marmor tabaci var. vulgare Holmes), Australian Journal of Experimental Biology, 18, 401-403.
| Crossref | Google Scholar |
Best, R. J. (1961) Recombination experiments with strains A and E of tomato spotted wilt virus, Virology, 15, 327-339.
| Crossref | Google Scholar | PubMed |
Best, R. J. (1968) Tomato spotted wilt virus, Advances in Virus Research, 13, 65-146.
| Crossref | Google Scholar | PubMed |
Best, R. J., and Samuel, G. (1936a) The effect of various chemical treatments on the activity of the viruses of tomato spotted wilt and tobacco mosaic, Annals of Applied Biology, 23, 759-780.
| Crossref | Google Scholar |
Best, R. J., and Samuel, G. (1936b) The reaction of the viruses of tomato spotted wilt and tobacco mosaic to the pH value of media containing them, Annals of Applied Biology, 23, 509-537.
| Crossref | Google Scholar |
Carne, W. M. (1928) An outline of the history of phytopathology with special reference to its development in Australia, Journal of the Royal Society of Western Australia, 14, 24-36.
| Google Scholar |
Fenner, F. J. (1987) Frank Macfarlane Burnet, 3 September 1899 - 31 August 1985, Biographical memoirs of fellows of the Royal Society. Royal Society (Great Britain), 33, 101-162.
| Google Scholar | PubMed |
Fraenkel-Conrat, H. (1956) The role of the nucleic acid in the reconstitution of active tobacco mosaic virus, Journal of the American Chemical Society, 78, 882-883.
| Crossref | Google Scholar |
Geering, A. D. W. (in press) The discovery of tomato spotted wilt virus, Historical Records of Australian Science, published online: 5 September 2023.
| Crossref | Google Scholar |
Gierer, A., and Schramm, G. (1956) Infectivity of ribonucleic acid from tobacco mosaic virus, Nature, 177, 702-703.
| Crossref | Google Scholar | PubMed |
Kay, L. E. (1986) W. M. Stanley’s crystallization of the tobacco mosaic virus, 1930–1940, Isis, 77, 450-472.
| Crossref | Google Scholar | PubMed |
Kunitz, M., and Northrop, J. H. (1935) Crystalline chymo-trypsin and chymo-trypsinogen: I. isolation, chrystallization, and general properties of a new proteolytic enzyme and its precursor, Journal of General Physiology, 18, 433-458.
| Crossref | Google Scholar | PubMed |
Lavin, G. I., and Stanley, W. M. (1937) The ultraviolet absorption spectrum of crystalline tobacco mosaic virus protein, Journal of Biological Chemistry, 118, 269-274.
| Crossref | Google Scholar |
Marshall, T. J. (2012) Prescott, James Arthur (1890–1987). Australian Dictionary of Biography, National Centre of Biography. Australian National University, https://adb.anu.edu.au/biography/prescott-james-arthur-15530/text26743, published first in hardcopy 2012, accessed online 27 September 2023.
Ross, A. F., and Stanley, W. M. (1938) The partial reactivation of formolized tobacco mosaic virus protein, Journal of General Physiology, 22, 165-191.
| Crossref | Google Scholar | PubMed |
Samuel, G., Best, R. J., and Bald, J. G. (1935) Further studies on quantitative methods with two plant viruses, Annals of Applied Biology, 22, 508-524.
| Crossref | Google Scholar |
Sankaran, N. (2008) Stepping-stones to one-step growth: Frank Macfarlane Burnet’s role in elucidating the viral nature of the bacteriophages, Historical Records of Australian Science, 19, 83-100.
| Crossref | Google Scholar |
Stanley, W. M. (1935) Isolation of a crystalline protein possessing the properties of tobacco-mosaic virus, Science, 81, 644-645.
| Crossref | Google Scholar | PubMed |
Stanley, W. M. (1936) Chemical studies on the virus of tobacco mosaic VI: the isolation from diseased turkish tobacco plants of a crystalline protein possessing the properties of tobacco-mosaic virus, Phytopathology, 26, 305-320.
| Google Scholar |
Stanley, W. M. (1937) Crystalline tobacco-mosaic virus protein, American Journal of Botany, 24, 59-68.
| Crossref | Google Scholar |
Stanley, W. M. (1938) The reproduction of virus proteins, The American Naturalist, 72, 110-123.
| Crossref | Google Scholar |
Stanley, W. M. (2002) Virus proteins—a new group of macromolecules, The Journal of Physical Chemistry, 42, 55-70.
| Crossref | Google Scholar |
Stanley, W. M. (1941) Some chemical, medical and philosophical aspects of viruses, Science, 93, 145-151.
| Crossref | Google Scholar | PubMed |
Stanley, W. M., and Loring, H. S. (1936) The isolation of crystalline tobacco mosaic virus protein from diseased tomato plants, Science, 83, 85.
| Crossref | Google Scholar | PubMed |
Stern, K. G. (1938) The relationship between prosthetic group and protein carrier in certain enzymes and biological pigments, Cold Spring Harbor Symposia on Quantitative Biology, 6, 286-300.
| Crossref | Google Scholar |
van Helvoort, T. (1991) What is a virus? The case of tobacco mosaic disease, Studies in History and Philosophy of Science Part A, 22, 557-588.
| Crossref | Google Scholar | PubMed |
van Helvoort, T. (1992) The controversy between John H. Northrop and Max Delbrück on the formation of bacteriophage: bacterial synthesis or autonomous multiplication? Annals of Science, 49, 545-575.
| Crossref | Google Scholar | PubMed |
Vinson, C. G., and Petre, A. W. (1929) Mosaic disease of tobacco, Botanical Gazette, 87, 14-38.
| Crossref | Google Scholar |
Wyckoff, R. W. G., Biscoe, J., and Stanley, W. M. (1937) An ultracentrifugal analysis of the crystalline virus protein isolated from plants diseased with different strains of tobacco mosaic virus, Journal of Biological Chemistry, 117, 57-71.
| Crossref | Google Scholar |
Footnotes
1 Carne (1928).
2 White (1981).
4 White (1981).
5 Best (n.d.).
8 Best (1927).
9 Best (n.d.).
10 Marshall (2012).
11 Best (1929a, 1929b).
12 https://raci.org.au/RACI/Web/Awards/National-Awards/Rennie-Memorial-Medal.aspx, viewed July 2023.
13 Anonymous (1931).
15 Best (1977).
18 Best (1977).
19 Best (1977).
20 Best (1935).
21 Much information on Wendell Stanley and the Princeton Laboratories of the Rockefeller Institute for Medical Research is sourced from Kay (1986), Creager (2002)—chapters 2 and 3, Stanley (1941) and van Helvoort (1991).
25 Stanley (1935).
26 Stanley (1936).
28 Best (1977).
27 Best (1927).
29 Best (1936b).
30 Best (1936a).
32 Best (1936c).
33 A modern interpretation of the pH-activity curve of TMV would be that the alkali treatment had initiated disassembly of the viral capsid and exposed the genomic RNA to hydrolysis and RNase degradation. Nevertheless, Best’s conclusion that viral activity was a function of a prosthetic group was still valid.
34 Stern (1938).
37 Best (1937a).
38 Best (1937c).
39 Best (1937b).
40 Creager (2002).
45 Best (1937c).
44 Best (1937c).
46 Best (1939b).
47 Best (1940b, 1940c).
48 Best (1940a).
49 Best (1961, 1968).
50 https://www.nobelprize.org/prizes/chemistry/1946/summary/, accessed 15 August 2023.
51 Best (1977). This letter by Rupert Best giving his own account of his research on TMV, is presented as Supplementary Document S1.
52 Kay (1986).
53 Francki (1977).
55 Sankaran (2008).


