Antimony leaching from contaminated soil under manganese- and iron-reducing conditions: column experiments
Kerstin Hockmann A C , Susan Tandy A , Markus Lenz B and Rainer Schulin AA Institute of Terrestrial Ecosystems, ETH Zurich, Universitätsstrasse 16, CH-8092 Zurich, Switzerland.
B Institute for Ecopreneurship, School of Life Sciences, University of Applied Sciences and Arts Northwestern Switzerland (FHNW), Gründenstrasse 40, CH-4132 Muttenz, Switzerland.
C Corresponding author. Email: kerstin.hockmann@env.ethz.ch
Environmental Chemistry 11(6) 624-631 https://doi.org/10.1071/EN14123
Submitted: 30 June 2014 Accepted: 13 August 2014 Published: 5 December 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Environmental context. Contamination of shooting range soils by antimony (Sb) released from corroding ammunition has become an issue of public environmental concern. Because many of these sites are subject to waterlogging and consequently limited aeration, we performed column experiments with contaminated shooting range soil to investigate Sb mobility under such conditions. The results are important for our understanding of the risks arising from Sb-contaminated soils, and also for the derivation of appropriate management strategies for such sites.
Abstract. Despite the environmental risks arising from antimony-contaminated sites, critical factors controlling the mobility of Sb in soils have still not been fully identified to date. We performed column experiments to investigate how reducing conditions affect Sb leaching from a calcareous shooting range soil, with a special focus on the relationship between Sb release and mineral dissolution processes. After eluting the columns for 5 days with 15 mM lactate solution at a flow rate of 33 mm day–1, the flow was interrupted for 37 days and then resumed for another 5 days. With the transition to moderately reducing conditions (~300 mV) after 1 day of flow, effluent SbV and manganese (Mn) concentrations showed a concomitant increase, providing evidence that SbV associated to these phases was released by the reductive dissolution of Mn minerals. The release of SbV was counteracted by the reduction to SbIII, which was first scavenged by iron (Fe) (hydr)oxides and then slowly liberated again when the redox potential further decreased to Fe-reducing conditions. Laser ablation–inductively coupled plasma–mass spectrometry revealed the presence of an initial pool of Sb associated with Mn-containing, Fe-free phases, underpinning the important role of the latter in addition to Fe (hydr)oxides as Sb sorbents.
Additional keywords: microbial reduction, redox speciation, Sb mobilisation, Sb transport, shooting range soil.
Introduction
Antimony is an element of increasing industrial use, for example in plastics, lead alloys, pigments or glassware.[1] As a consequence, it is also increasingly released into the environment, potentially leading to soil contamination of more than several thousand milligrams per kilogram in places where it is mined, used and disposed of.[2–4] A major source of Sb input into the environment are shooting activities, as lead bullets contain ~2 to 5 % of metallic Sb as a hardener.[1,5] In Switzerland, for example, 10 to 25 tonnes (~10–25 Mg) of Sb enter the pedosphere every year on more than 2000 shooting ranges[6,7]; and in the United States, shooting activities deposit ~1900 tonnes (~1.9 Gg) of Sb annually[8] on ~12 000 firing ranges.[9] Also in Finland[10,11] and Norway,[12] contamination of shooting range soils by Sb has become a focus of attention. Antimony compounds are considered pollutants of primary concern[13,14] because of their high acute toxicity, chronic health effects, and potential carcinogenicity.[15] Despite the wide distribution of Sb contamination and its well known toxicity, however, the behaviour of Sb in the environment and the risks associated with Sb contamination are still poorly understood.[16,17]
A key factor governing Sb mobility is the soil redox state, which is closely related to the soil water regime. Many soils are subject to strong variations in redox conditions, driven by fluctuations in waterlogging and biological activity. The prevalent Sb redox species in oxic soils is SbV, which primarily exists as Sb(OH)6– (antimonate) in solution, whereas trivalent SbIII in the form of the more toxic neutral species Sb(OH)3 (antimonite) predominates under reducing conditions.[18] Iron (hydr)oxides strongly retain both antimonate and antimonite, and are therefore widely believed to control Sb mobility in soils.[19–21] Because antimonite binds more extensively to Fe (hydr)oxides than antimonate at pH values ~7,[19,20] moderately reducing soil conditions are expected to decrease Sb mobility.[22,23] Batch experiments, however, revealed that such immobilisation may be only transitory and followed by a release of the previously bound antimonite, when Fe minerals become reductively dissolved upon further reduction.[8,22]
In a similar manner as Fe minerals, Mn (hydr)oxides have been shown in studies with pure mineral systems to be strong sorbents for Sb at circumneutral pH.[20,24–26] Little, however, is still known about their role as Sb sorbents in real soils.[17] In an experiment on paddy soil, Mn (hydr)oxides were not considered important for Sb retention,[27] whereas in a study on lake sediments, Sb was found to sorb onto Mn oxides even in preference to Fe oxides.[28] As with Fe (hydr)oxides, antimonite exhibits a much higher sorption affinity to Mn (hydr)oxides than antimonate.[20,24] Once bound to MnIII/IV mineral surfaces, SbIII was found to be quickly oxidised and released as SbV.[20,24,25] Unlike Fe, thermodynamic equilibrium calculations predict that reduction of MnIII/IV occurs at redox potentials above that of SbV.[18,29,30] Both, the decrease in sorption sites as a result of reductive dissolution and the oxidation of SbIII by MnIII/IV are expected to promote Sb mobilisation, thereby increasing the risk of Sb migration from soil into ground or surface waters.
Anaerobic incubation of batch microcosms with shooting range soil indicated that SbV may in fact be released with the reductive dissolution of Mn (hydr)oxides.[22] However, the effect of Mn reduction on Sb mobility was overridden by the initial desorption of SbV and the concomitant reduction of SbV; thus it could not clearly be identified. In contrast, column experiments may provide information on chemical reactions in soil that cannot be obtained from studies under stagnant conditions,[31] as they will take into account effects that are associated with transport processes. Flow-through soil columns may therefore be a powerful tool to separate the different phases of reduction and desorption reactions because of the continuous replacement of soil solution and the possibility to collect samples in high temporal resolution.
The objective of this study was to investigate how changes in soil redox conditions affect Sb release and retention under advective transport, with a special focus on the interplay of reductive Fe and Mn mineral dissolution and Sb (de)sorption processes. Columns packed with contaminated shooting range soil were eluted with 15 mM lactate solution to stimulate microbial activity, interrupted by a stop-flow phase to simulate waterlogging conditions. To our knowledge, this is the first study addressing Sb leaching from reduced soil columns.
Materials and methods
All the chemicals used were of at least analytical grade. All solutions were prepared with ultrapure deionised water (>18 MΩ cm), and all glassware was rinsed with 0.3 M HNO3 (Sigma–Aldrich, Steinheim, Germany) and ultrapure water before use.
Soil characteristics
The soil used in this study was a calcareous floodplain soil (Fluvisol) collected from the surroundings of a stop butt (berm) on a military shooting range (46°51′19′N and 9°30′11′E) near the River Rhine in the vicinity of Chur in Eastern Switzerland. The soil was taken from the surface horizon (0–30 cm), cleaned of roots and sods, air-dried (<2-wt % water content) and passed through a 2-mm sieve. The soil had a silt loam texture (US soil taxonomy[32]), contained 15 % CaCO3, 0.9 % organic carbon (determined by wet oxidation of organic matter using potassium dichromate[33]) and was slightly alkaline (pH 7.8 in 0.01 M CaCl2, liquid/solid ratio = 5). Total Sb, Fe and Mn concentrations measured by energy-dispersive X-ray fluorescence spectroscopy (XRF; X-Laboratory 2000, Spectro, Kleve, Germany) in pressed pellets were 21 mg kg–1 Sb, 29 500 mg kg–1 Fe and 820 mg kg–1 Mn. XRF analysis of WEPAL reference soils 921, 989 and 995 (Wageningen, Netherlands) and Sb-spiked soil samples yielded recoveries in the range of 97 to 101 %. According to XRD analyses (Bruker AXS D8 Advance, Karlsruhe, Germany), the main minerals in the soil were quartz, calcite, muscovite and chlorite.[34] The soil was the same that had been used in a previous study on Sb reduction in a microcosm batch experiment.[22]
Column experiments
The experiments were performed using four chromatographic polypropylene columns (Büchi, Switzerland) with an inner diameter of 4 cm. The columns were uniformly packed with 268 g of dry soil and capped with polyethylene frits (Büchi, Flawil, Switzerland, 70–90-µm pore size). The resulting soil packing was 13.4 cm long and had a bulk density of 1.59 g cm–3. The pore volume (PV), determined as the difference between the weight of the saturated column and its dry weight, was 42 ± 0.8 % (equivalent to 71 ± 1 mL, mean ± s.d.). One of the packed columns was treated with gamma radiation (25–75 Gy), connected to sterilised tubings in a laminar flow hood (B. V. Clean Air Techniek, Woerden, Netherlands) and run under strictly sterile conditions. A peristaltic pump (Ismatec MPC standard, Ismatec, Glattbrugg, Switzerland) was used to feed a heat-sterilised (121 °C, 20 min) and degassed 15 mM sodium lactate (Sigma–Aldrich) solution in up-flow mode through the columns. The effect of lactate on Sb sorption was addressed in a previous study on this soil.[22] Addition of 15-mM lactate was shown to increase SbV equilibrium concentrations by competitive adsorption and complexation, but did not have an effect on Sb redox speciation. The feeding solution had a pH of 8.2 and a redox potential (Eh) of ~270 mV. It was prepared freshly at least every 48 h. Except for the phase of flow interruption, the flow rate was kept at 33.2 ± 2.0 mm day–1 (mean ± s.d.), equivalent to ~6 PV per day, during the entire experiment starting with the saturation (also with 15 mM Na lactate) of the initially dry columns. For the sterilised column, the feeding solution was passed through a sterile 0.22-µm filter (Millex GS, Millipore, Billerica, MA, USA) before entering the column. All columns were operated in darkness at 26 ± 1 °C (mean ± s.d.) in an incubator (Labtherm, Kühner, Birsfelden, Switzerland).
The start of the experiment (t = 0 days) was defined as the time when effluent started to flow out of the column. Effluent solution was collected in fractions of ~0.3 PV (sample size 24 mL) using a fraction collector (Büchi). Samples used for the determination of total Sb, Mn and Fe concentrations were collected in plastic vials containing 250 µL of concentrated HNO3 (Merck, Darmstadt, Germany) to prevent Fe (hydr)oxide precipitation (pre-acidified samples). For Sb speciation, pH and Eh measurements, selected effluent samples (non-acidified samples) were collected in vials without acid under argon atmosphere to avoid contact with atmospheric oxygen.
After 5 days (~30 PV, first flow phase) the flow was stopped for 37 days (flow interruption) and resumed again for another 4 days (~23 PV, second flow phase) after SbIII concentrations had reached a plateau. At different time points during the flow interruption phase (i.e. 1, 2, 3, 5, 7, 10, 14, 19, 26 and 37 days after interruption), 20 mL of soil solution were eluted by resuming the flow at a rate of ~100 mm day–1 for 20 min using the same, freshly prepared feeding solution as before. The first 5 mL of each of these samples were discarded to remove dead water in the sampling system. The extraction of solution during this phase was equivalent to an average flow rate of 1 PV in 5 to 19 days, depending on the frequency of sampling (compared to 4 h for the replacement of 1 PV soil solution during the flow phase). Samples were collected under Ar and treated in the same way as the non-acidified samples.
At the end of the experiment, a bromide tracer test was carried out to determine the physical transport characteristics of the packings (Supplementary material Fig. S1).
Solution sample analysis
The pre-acidified samples were passed through 0.2-µm nylon filters (WICOM, Heppenheim, Germany) and analysed by inductively coupled plasma–mass spectrometry (ICP-MS; Varian ICP-MS 810, Middelburg, Netherlands) for total dissolved Sb and by ICP-optical emission spectroscopy (ICP-OES; Vista-MPX CCS simultaneous, Varian) for dissolved Fe and Mn.
Eh and pH were measured under Ar flow in the non-acidified samples using a pH meter (Metrohm713, Zofingen, Switzerland) equipped with a Pt redox electrode (reference electrode Ag/AgCl, 6.0451.100, Metrohm) or with a pH electrode (6.0259.100, Metrohm). Redox electrode readings are reported as potential differences to a standard hydrogen electrode (Eh). For Sb speciation, the solution samples were passed through 0.2-µm nylon filters (WICOM), stabilised with degassed 0.05 M ethylenediaminetetraacetic acid (EDTA) (Titriplex III, Merck) and kept in 1-mL polyethylene vials under Ar atmosphere until analysis. Antimony species were analysed using a slightly modified version of the method described by Lintschinger et al.[35] Briefly, a strong anion exchange column (Hamilton PRP-X100, Bonaduz, Switzerland) was connected to the ICP-MS described above. A mobile phase of 10 mM EDTA, 2 mM potassium hydrogen phthalate (Fluka, Buchs, Switzerland) and 2 % methanol (Biosolve, Valkenswaard, Netherlands) at pH 4.3, was used for chromatographic separation. The flow rate was 1.25 mL min–1. Mixed calibration standards of SbV and SbIII in 0.05 M EDTA were freshly prepared before measurement using 1000 mg L–1 stock solutions of SbV (KSb(OH)6 dissolved in water; Merck) and SbIII (Sb2O3 in 2 M HCl; Merck). The quantification limits, determined as ten times the standard deviation (σ) of a 0.5 μg L–1 sample (n = 10), were 0.3 and 0.5 µg L–1 for SbV and SbIII respectively. The Sb recovery, obtained by dividing the sum of measured SbIII and SbV concentrations by the measured total Sb concentrations in the soil solution, ranged from 61 to 101 %. All Sb speciation analyses were completed within 5 h of sampling.
A filtered aliquot of each sample collected for Sb speciation was acidified to 10 % HNO3 (Merck) and analysed (without EDTA addition) for the same parameters as the pre-acidified samples.
Elemental association of Sb in the study soil
Laser ablation (LA) ICP-MS was used for qualitative assessment of elemental associations in the contaminated soil. Samples of air-dried soil were embedded in epoxy resin, thin-sectioned, polished and analysed for spatial distributions of elements using an ESI NRW213 laser ablation system (Munich, Germany) (Nd:YAG 213 nm), interfaced with an ICP-MS (Agilent Technologies, Agilent 7500cx, Basel, Switzerland). The ICP-MS octopole reaction system was pressurised with 5 mL min–1 helium. The laser was operated at an energy level of 70 % with a fluence 20.96 J cm–2, a pulse repetition rate of 20 Hz and a scan speed of 10 µm s–1. Laser ablation was conducted along 120 lines with a spot size of 5 μm, resulting in a mapped area of 0.6 mm × 2.4 mm. By determing intensities instead of absolute concentrations, potential problems in interpretation and calibration as a result of differential ablation effects were avoided. Scan data were processed and analysed using the software package Iolite.[36]
Statistical data treatment
A linear regression to a significance level of P < 0.01 was employed to compare the soluble Fe and Mn concentrations by using the software R version 2.13.0 (The R Foundation for Statistical Computing, Vienna, Austria, see http://www.r-project.org, accessed 11 November 2014).
Results
Analysis of column effluent before flow interruption
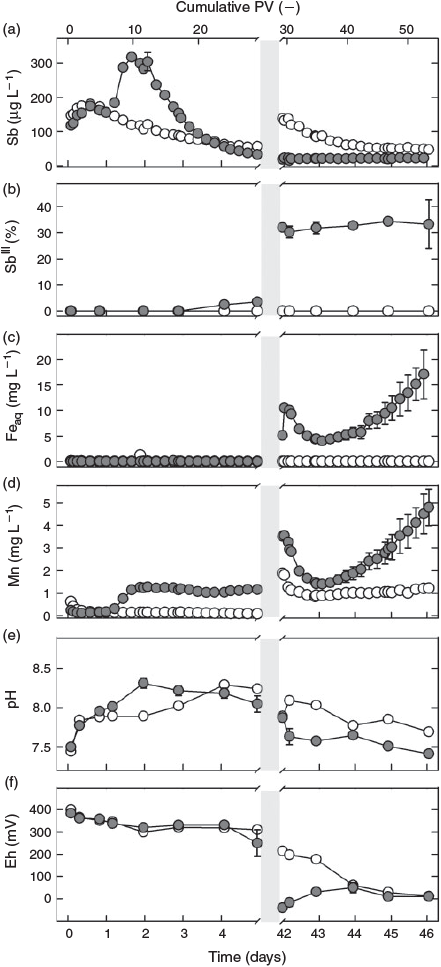
During the first day of flow, effluent pH, redox potential and metal(oid) concentrations did not differ between the two treatments: the pH increased from 7.5 to 8.0 (Fig. 1e), whereas the redox potential remained stable at ~350 mV in the effluents of both the sterilised and non-sterilised columns (Fig. 1f). In both treatments, the total Sb concentration of the effluent increased within 18 h from ~130 µg L–1 to a maximum of ~180 µg L–1 and then slightly decreased again (Fig. 1a). Antimony(III), Fe and Mn concentrations (Fig. 1b–d) remained below or close to their respective quantification limits (0.5 µg L–1, 0.1 mg L–1 and 0.08 mg L–1). Whereas total Sb concentrations tailed off to ~60 µg L–1 in effluent from the sterilised column at the end of the first flow phase, Sb effluent concentrations from the non-sterilised columns steeply increased, reaching a peak of >300 µg L–1 after 10 PV (1.8 days). This increase was paralleled by an increase in Mn concentrations and a slight rise in pH to 8.3 on day 2. Thereafter, total Sb concentrations gradually decreased to <35 µg L–1 on day 5, approaching and then even slightly dropping lower than total Sb in the effluent from the sterilised column, whereas Mn concentrations remained almost constant at 1.3 mg L–1 until flow interruption. In contrast, Mn remained low (<0.15 mg L–1) in leachate from the sterilised column, and pH required 4 days to increase to a similar level as in the effluent from the non-sterilised columns. Whereas SbIII concentrations remained below the quantification level in the effluent from the sterilised column, they started to slightly increase after day 3 in the effluent from the non-sterilised columns, reaching ~1 µg L–1 SbIII at the onset of flow interruption and thereby contributing 1–4 % to total Sb leaching (Fig. 1b). At almost the same time, the redox potential started to drop in the non-sterilised columns to reach 250 mV by day 5 before the flow was interrupted, whereas it did not change in the effluent of the sterilised column. No Fe (<0.1 mg L–1) was detected in the effluent of either treatment.
|
Analysis of pore water samples collected during flow interruption
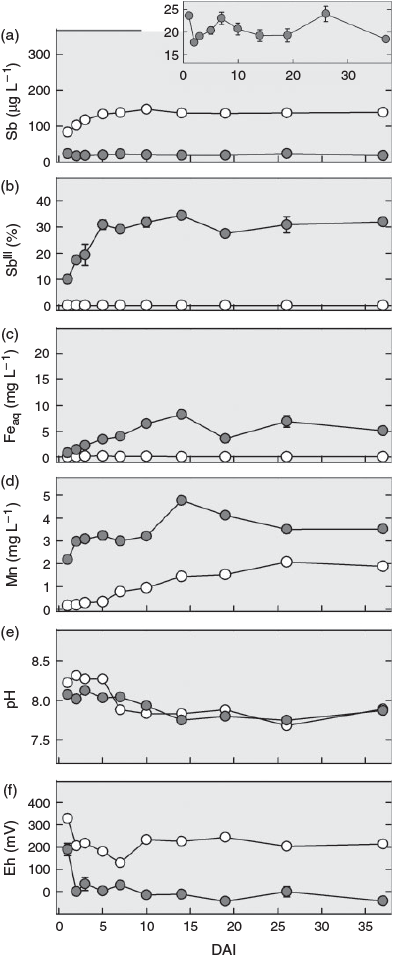
During the first ~3 days after interrupting the flow (DAI), the redox potential decreased from 250 to 0 mV in the pore water from the non-sterilised columns and from 350 mV to values ~100–200 mV in the sterilised column, remaining approximately stable thereafter (Fig. 2f). The pH also remained fairly stable in both treatments during this time. Following a slight decline after 5 DAI in the sterilised column, the pH values became almost identical (~8) to those in the samples from the non-sterilised columns (Fig. 2e). After interrupting the flow, total Sb concentrations in the pore water of the sterilised column increased steadily to ~140 µg L–1 until DAI 5 and then remained at this level, whereas concentrations in the non-sterilised columns fluctuated between 17 and 25 µg L–1 (Fig. 2a). Whereas SbIII in the sterilised column remained below the quantification limit (0.5 µg L–1), the percentage of SbIII in the solution samples from the non-sterilised columns gradually increased from 10 % on DAI 1 to 30 % on DAI 5 (equivalent to 1.5 and ~4 µg L–1 respectively, Fig. 2b). Soluble Fe concentrations also increased in the non-sterilised columns, but over a longer time. After a steady increase, they reached a maximum of 8 mg L–1 on DAI 14 and then fluctuated between 4 and 7 mg L–1 (Fig. 2c). Manganese showed a bi-phasic pattern in the non-sterilised columns: A rapid increase from 2 to 3 mg L–1 in the first 2 to 3 DAI and then, after a stable period, another increase between DAI 10 and 15 to a maximum of 4.9 mg L–1, before gradually decreasing again to 3.5 mg L–1 by DAI 37 (Fig. 2d). In contrast to SbIII and Fe, Mn concentrations also increased in the sterilised column. This increase started right after the flow was stopped and continued over the entire duration of flow interruption reaching a level of 2 mg L–1 at the end.
|
Analysis of column effluent after flow interruption
After resuming flow, the redox potential in the effluent from the sterilised column dropped with a short initial delay of 1 day from 200 to 0 mV at the end of the second flow phase (Fig. 1f). In contrast, Eh values in effluent from the non-sterilised columns showed an increase, starting from ~–50 mV and gradually reaching a similar redox potential to the sterile column at the end of the experiment. During the same time, the pH decreased from pH 7.9 to 7.4 in the non-sterilised and, after a slight initial increase, from 8.1 to 7.7 in the sterilised column (Fig. 1e). Total Sb concentrations in the effluent from the sterilised column gradually decreased from ~120 to 47 µg L–1 at the end of the experiment, while they remained stable at ~20 µg L–1 in effluent of the non-sterilised columns (Fig. 1a). Concentrations of SbIII remained stable at ~5 µg L–1 (and thus also its percentage of total Sb) in the effluent from the non-sterilised columns and below the quantification limit in the effluent from the sterilised column (Fig. 1b). No Fe was released from the sterilised column also during the last phase of the experiment. In contrast, Fe concentrations in effluent from the non-sterilised columns peaked at 10 mg L–1 immediately after re-initiation of flow (42 days), then dropped to 4 mg L–1 within one day and subsequently increased again at an accelerating rate, reaching a value of 17 mg L–1 at the end of the experiment without tendency of a stop in this trend (Fig. 1c). Except for the sharp initial peak, the rate of Mn leaching from the non-sterilised columns paralleled that of Fe, with effluents reaching Mn concentrations of ~5 mg L–1 at the end of the experiment (Fig. 1d). In the effluent from the sterilised column, the Mn concentration decreased from 1.9 mg L–1 at the beginning of the second flow phase to ~1.1 mg L–1 within a few hours and then remained at this level until the end of the experiment.
Elemental associations of Sb in the soil
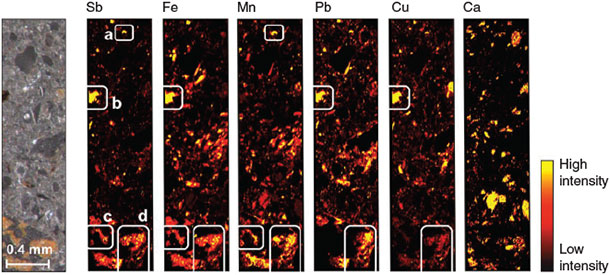
The LA-ICP-MS analysis of the contaminated soil samples revealed that Sb was distributed in patches (Fig. 3). Four types of Sb-rich ablated sample regions (ASR) could be distinguished with respect to their elemental associations: (a) ASR exclusively containing Sb and Mn; (b) ARS in which Sb was associated with Fe, Pb and Cu, but not with Mn; (c) ASR in which Sb was associated with Fe and Mn (but not with Pb or Cu) and (d) ASR in which Sb was associated with Fe, Mn and Pb and, to a lesser extent, also with Cu. We found no clear signals of associations between Sb and Ca.
|
Discussion
SbV release upon Mn (hydr)oxide reduction
The fact that the sterilisation treatment showed no effect on effluent pH, redox potential and metal(oid) concentrations at the beginning of the experiment (<1 day) reveals that Sb leaching in both treatments was not influenced by microbial processes during this phase. After 1 day, however, strong differences started to emerge in Mn concentrations (Fig. 1d), because lack of oxygen and continuous supply of lactate for microbial respiration led to Mn-reducing conditions in the non-sterilised columns. The parallel increase in eluted Sb and Mn during the second day of the experiment (Fig. 1a,d), which did not occur in the sterilisation treatment, strongly suggests that these two processes were coupled. During this phase of increased Sb leaching from the non-sterilised columns, Sb was exclusively present in the form of SbV, in agreement with X-ray absorption near edge structure (XANES) data obtained in a previous experiment with the same soil that revealed SbV as the dominant species in the solid phase of the oxic soil.[22] Thus, the additional peak of SbV in the effluent from the non-sterilised columns was attributable to the release of sorbed SbV from dissolving Mn phases. In fact, Mn (hydr)oxides are known to be effective sorbents for SbV at circumneutral pH in pure-mineral systems.[20,24–26] This explanation is also in agreement with our findings of Sb associations with Mn in the laser ablation analysis. The simultaneous presence of Pb, Cu and Fe with Mn in ASR types c and d (Fig. 3) suggests that these two ASR directly originated from weathered bullets.[37] Reductive dissolution of Sb-loaded Mn minerals provides the only plausible explanation for the difference in Sb leaching between the two sterilisation treatments in the first phase of the experiment, as there were still no differences in other parameters that could explain this effect.[38] Although the increase in Sb concentration during the first 18 h of the experiment may have been caused by the initial increase in pH, the slight increase in pH (Fig. 1e) on the second day (which was probably a result of the consumption of protons with the reductive dissolution of Mn (hydr)oxides) was too small to be the dominant cause of the large Sb peak by itself. Sorption of SbV to Fe (hydr)oxides is known to decrease with increasing pH above pH 7, but this can explain only a very small part of the observed increase in SbV that occurred parallel with the release of Mn in the non-sterilised columns.[19] Other abiotic processes that have also been suggested in the literature to mobilise Sb, such as rate-limited desorption of SbV from Fe (hydr)oxides,[22] chemical dissolution of Sb-bearing phases[7,39] or competitive desorption,[40] do not provide plausible explanations for the difference in Sb release at the initial stages of the experiment between columns with and without sterilisation treatment, although they may have contributed to the release of Sb that occurred independent of these treatments.
Although no consumption of lactate by soil microbial communities was found before flow interruption in the sterilised column (Supplementary material, Fig. S4b), the decrease in lactate from ~540 mg C L–1 (equivalent to 15 mM lactate in the feeding solution) to 90 mg C L–1 and the production of the metabolites acetate and propionate after flow was interrupted (Fig. S4b–d) shows that the sterilisation treatment did not completely eliminate all microorganisms and that microbial activity recovered with time in the sterilised column. For soil sterilisation by gamma-radiation, we chose a moderate dose of 25–70 Gy to minimise inadvertent changes in soil chemical properties, being aware that some radiation-resistant bacteria are able to survive this dose.[41] Gradual recovery of microbial activity also explains the increasing release of Mn and decrease in Eh during flow interruption in the sterilisation treatment (Fig. 2d,f). As in the non-sterilised columns, the leaching of Mn was associated with an increased release of SbV (Fig. 2a), suggesting that the same sequence of biogeochemical processes was taking place in both treatments, just at very different rates because of the initial suppression and only very slow recovery of microbial activity in the case of the gamma-irradiation treatment.
The relationship between Mn and SbV concentration in the leachate in both treatments did not follow a simple proportionality. Although Mn soon reached a plateau in the non-sterilised columns before flow interruption, Sb concentrations continually decreased after an initial peak; and, although Mn concentrations continued to increase in the pore water of the sterilised columns during and again after flow interruption, the concentration of Sb remained unchanged after an initial increase. This means in both cases that the more Mn became dissolved, the less Sb was released. This agrees with the view that Sb was primarily released from surface sites of dissolving mineral Mn phases and that part of it was re-adsorbed to freshly exposed surfaces temporarily until these new surface sites were also eliminated, as dissolution continued.
Sb immobilisation induced by reduction of SbV
The release of SbV from the non-sterilised columns was counteracted by the transformation of SbV to SbIII, as indicated by the slight increase in SbIII concentration that started ~3 to 4 days after the beginning of the experiment. The conversion of SbV into SbIII was microbially induced,[42,43] and may have been enhanced by the oxidation of FeII[44] or sulfur[45] as soon as Fe- and sulfate-reducing conditions developed (Fig. 2c and Supplementary material, Fig. S3c). The delay of SbV reduction relative to the reductive dissolution of Mn is in line with the lower redox potential at thermodynamic equilibrium of SbV reduction (~400 mV for MnIII/IV[29] and ~100 mV for SbV[18] at pH 8). Antimonite's large sorption affinity to Fe (hydr)oxides at alkaline pH[19,20,23] explains why SbIII concentrations still remained low as long as no Fe reduction occurred, in close agreement with results from recent studies on reduced mine and paddy soil.[27,46]
Even under SbV reducing conditions, SbIII never exceeded more than a third of the total concentration of Sb in solution (Figs 1b, 2b). Again, this could be explained by Sb sorption to Mn (hydr)oxides. In a similar manner to Fe (hydr)oxides, Mn (hydr)oxides also have a much greater sorption affinity to antimonite than antimonate,[20,24] but with a higher equilibrium redox potential of MnIII/IV, this association is thermodynamically labile. Therefore, SbIII sorbed to Mn (hydro)xides was likely to be oxidised within hours to SbV and then liberated into solution as a result of the lower sorption affinity of Mn (hydr)oxides for antimonate.[20,24] In contrast, SbIII sorbed to Fe (hydr)oxides is thermodynamically stable at neutral or higher pH,[30] suggesting that the dissolution of SbIII in soil environments is much more controlled by Fe (hydr)oxides than by Mn minerals.
Sb dynamics under Fe-reducing conditions
The important role of Fe (hydr)oxides for the retention of antimonite was reflected by the increasing release of SbIII into soil solution[22] of the non-sterilised columns during flow interruption. Concentrations of SbIII continued to increase after soluble Mn had already reached a plateau, whereas Fe concentrations kept rising (Fig. 2d), indicating that Fe-bound antimonite was released into solution at this stage of the experiment. The liberation of SbIII during the 37 days of flow interruption amounted to <0.01 % of the total Sb initially present in the soil and was thus much lower than in a recent microcosm study with the same soil,[22] in which 1 % (~20 µg L–1) of the total Sb in the soil was released as SbIII within 21 days of anaerobic incubation. We attribute this difference to the much larger amounts of Fe that were reduced in the microcosm experiment, in which the molar ratio of lactate to total Fe was 1.4, whereas it was ~100 times smaller in the columns during the phase of flow interruption. As a result, 2 % of the total Fe in the soil was reductively dissolved in the microcosm experiment, but not even 0.01 % was dissolved in the columns investigated here. The closer lactate-to-Fe ratio led to a depletion of lactate during the flow interruption (Supplementary material, Fig. S5). However, with the re-supply of lactate and flush-out of metabolites in the second flow phase (Fig. S4), microbial Fe reduction was reinitiated after a short period of microbial acclimation to the new conditions in the non-sterilised columns. As a consequence, Fe reduction reached rates (6.5 mg Fe day–1 from day 44 onwards; Fig. 1c) greatly exceeding those during flow interruption, when only 0.5 mg of Fe were reduced within 37 days (Fig. 2c). Yet, summarised over the whole experiment, less than 0.2 % of the total Fe was eluted, and reductive Fe dissolution did not result in a significant loss of sorption capacity. Thus, the soil kept its potential to retain SbIII on its way through the column. As a consequence, maximum SbIII concentrations remained low (~5 µg L–1) and did not result in an overall increase in Sb concentrations.
As MnIV/III is known to substitute for FeIII in goethite and other commonly found Fe (hydr)oxides in soils,[47,48] the correlation between Mn and Fe may indicate release of Mn that was entrapped or incorporated in Fe (hydr)oxide phases following the reductive dissolution of these minerals.
Sequestration of Sb into stibnite-like phases, which was shown to occur in pure sulfidic solutions at pH ≤ 6,[45] is not likely to have controlled soluble Sb concentrations even though sulfate-reducing conditions existed (Fig. S3c): First, at neutral to alkaline pH, soluble thioantimonite species should predominate[18]; second, in the above-mentioned microcosm experiment,[22] Sb K-edge XANES analysis of the reduced soil did not indicate the presence of stibnite even under sulfate-reducing conditions. However, Sb speciation and fate in sulfidic solutions is still poorly understood and stibnite formation may govern Sb solubility in soils with a lower pH. Effluent Fe concentrations were closely correlated with Mn concentrations during the second flow phase (R2 = 0.85, P < 0.01).
Conclusions and practical implications
Although some studies have indicated the importance of Mn (hydr)oxides as sorbent phases for Sb in pure mineral systems,[24–26] in river deposits[49] and lake sediments,[28] their role in Sb mobility in soils has not been fully elucidated to date. This study demonstrates that Mn (hydr)oxides can play a key role in the mobility of Sb in natural soils. Our column experiments showed that SbV leaching was closely linked to that of Mn, indicating a direct cause–effect relationship to the reductive dissolution of these minerals. In addition, the LA-ICP-MS analyses revealed that Sb was directly associated with Mn-containing phases. The results suggest that despite their generally much lower concentrations in soils in comparison to Fe minerals (~1/50 of the concentration of Fe oxides),[50] Mn (hydr)oxides can exert a major control on the mobility of Sb in contaminated soils, which has not been adequately recognised up to now. With the transition from oxic to anaerobic conditions, a situation often observed in poorly drained soils and typical for water-logged soils with fluctuating water table,[51] SbV can be mobilised as soon as reduction of MnIV and MnIII leads to the dissolution of Mn (hydr)oxides. As a moderate decrease in redox potential (generally by <200 mV[29]) may be sufficient to result in MnIII/IV reduction as compared to FeIII reduction, it is important to include the possibility of uncontrolled Sb release into the environment from redox-variable soils in future risk assessment studies. This risk has been considered rather low in the past as it was assumed that under reducing conditions, the reduction of SbV to SbIII would effectively immobilise Sb because of the much stronger retention of antimonite compared to antimonate by FeIII phases,[23,46] at least in soils with high pH and as long as these Fe phases are not reductively dissolved.[22]
Supplementary material
Additional information on the bromide tracer experiment and calcium, nitrate, sulfate, dissolved organic carbon and fatty acid concentrations during the column experiment (Figs S1–S5 cited in the text) is given in the Supplementary material, which is available from the journal online.
Acknowledgements
The authors thank Björn Studer for his help with the experiment, in particular for the lactate measurements, and Peter Nastold for the VFA analysis. Funding of this research by the Swiss National Science Foundation (Grant 200021-130250) and the supply of the study soil by armasuisse Immobilien is gratefully acknowledged.
References
[1] W. C. Butterman, J. F. Carlin, US Geological Survery Mineral Commodity Profiles: Antimony. Open-File Report 03-019 2004 (US Department of the Interior & US Geological Survey). Available at http://pubs.usgs.gov/of/2003/of03-019/of03-019.pdf [Verified 9 October 2014].[2] F. Baroni, A. Boscagli, G. Protano, F. Riccobono, Antimony accumulation in Achillea ageratum, Plantago lanceolata and Silene vulgaris growing in an old Sb-mining area. Environ. Pollut. 2000, 109, 347.
| Antimony accumulation in Achillea ageratum, Plantago lanceolata and Silene vulgaris growing in an old Sb-mining area.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXktFSrtbo%3D&md5=2d93d60f8d68baa454e23b96f8dd3bd6CAS | 15092905PubMed |
[3] B. H. Robinson, S. Bischofberger, A. Stoll, D. Schroer, G. Furrer, S. Roulier, A. Gruenwald, W. Attinger, R. Schulin, Plant uptake of trace elements on a Swiss military shooting range: uptake pathways and land management implications. Environ. Pollut. 2008, 153, 668.
| Plant uptake of trace elements on a Swiss military shooting range: uptake pathways and land management implications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmtVagtLc%3D&md5=dfa887f921ec2bc021b414b2765c9945CAS | 17949872PubMed |
[4] J. Clausen, N. Korte, The distribution of metals in soils and pore water at three US Military training facilities. Soil Sediment Contam. 2009, 18, 546.
| The distribution of metals in soils and pore water at three US Military training facilities.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ehtbvM&md5=86d60cfa2aa62b839b46fd3a8104951bCAS |
[5] M. Filella, N. Belzile, Y. W. Chen, Antimony in the environment: a review focused on natural waters I. Occurrence. Earth Sci. Rev. 2002, 57, 125.
| Antimony in the environment: a review focused on natural waters I. Occurrence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXos1Wgsr4%3D&md5=f86f9ed3fb613780dd470c9cfbcf69dcCAS |
[6] R. Mathys, J. Dittmar, A. Johnson, Antimony in Switzerland: A Substance Flow Analysis 2007 (Federal Office for the Environment: Bern, Switzerland).
[7] C. A. Johnson, H. Moench, P. Wersin, P. Kugler, C. Wenger, Solubility of antimony and other elements in samples taken from shooting ranges. J. Environ. Qual. 2005, 34, 248.
| 1:CAS:528:DC%2BD2MXotlShsA%3D%3D&md5=e35473c245ea69a8a80c0073e636158fCAS | 15647555PubMed |
[8] X.-m. Wan, S. Tandy, K. Hockmann, R. Schulin, Changes in Sb speciation with waterlogging of shooting range soils and impacts on plant uptake. Environ. Pollut. 2013, 172, 53.
| Changes in Sb speciation with waterlogging of shooting range soils and impacts on plant uptake.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs12itb7L&md5=2efabb0b229b0eca37b2ad5d17b8ab5dCAS | 22982553PubMed |
[9] D. I. Bannon, J. W. Drexler, G. M. Fent, S. W. Casteel, P. J. Hunter, W. J. Brattin, M. A. Major, Evaluation of small arms range soils for metal contamination and lead bioavailability. Environ. Sci. Technol. 2009, 43, 9071.
| Evaluation of small arms range soils for metal contamination and lead bioavailability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVahtrfE&md5=6bc831afb9088cdc234f6eb80cbbe459CAS | 20000496PubMed |
[10] J. Sorvari, Environmental risks at Finnish shooting ranges – a case study. Hum. Ecol. Risk Assess. 2007, 13, 1111.
| Environmental risks at Finnish shooting ranges – a case study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtVOrtLnK&md5=1491aac93b5f57dbc3ef1be84fd59434CAS |
[11] J. Sorvari, R. Antikainen, O. Pyy, Environmental contamination at Finnish shooting ranges – the scope of the problem and management options. Sci. Total Environ. 2006, 366, 21.
| Environmental contamination at Finnish shooting ranges – the scope of the problem and management options.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmvValsrY%3D&md5=7941c7e543367b81f0b1b28a2de5b333CAS | 16458952PubMed |
[12] A. E. Strømseng, M. Ljones, L. Bakka, E. Mariussen, Episodic discharge of lead, copper and antimony from a Norwegian small arm shooting range. J. Environ. Monit. 2009, 11, 1259.
| Episodic discharge of lead, copper and antimony from a Norwegian small arm shooting range.Crossref | GoogleScholarGoogle Scholar | 19513458PubMed |
[13] Water Related Fate of the 129 Priority Pollutants, Vol. 1 1979 (United States Environmental Protection Agency: Washington, DC).
[14] Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption, Official Journal L 330/32 1998 (Council of the European Communities). Available at https://www.fsai.ie/uploadedFiles/Legislation/Food_Legisation_Links/Water/EU_Directive_98_83_EC.pdf [Verified 10 October 2014].
[15] Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Antimony and Compounds 1992 (US Department of Health and Human Services: Georgia, USA).
[16] M. Filella, N. Belzile, Y. W. Chen, Antimony in the environment: a review focused on natural waters II. Relevant solution chemistry. Earth Sci. Rev. 2002, 59, 265.
| Antimony in the environment: a review focused on natural waters II. Relevant solution chemistry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XoslCmsrw%3D&md5=a1b9af669f3b9ca2196a725673656953CAS |
[17] S. C. Wilson, P. V. Lockwood, P. M. Ashley, M. Tighe, The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: a critical review. Environ. Pollut. 2010, 158, 1169.
| The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: a critical review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmt1Knt7g%3D&md5=67c846c71d555e25e02166d0d66ecfbcCAS | 19914753PubMed |
[18] K. Hockmann, R. Schulin, Leaching of antimony from contaminated soils, in Competitive Sorption and Transport of Heavy Metals in Soils (Ed. H. M. Selim) 2013, pp. 119–145 (CRC Press: Boca Raton, FL).
[19] A. K. Leuz, H. Monch, C. A. Johnson, Sorption of Sb(III) and Sb(V) to goethite: influence on Sb(III) oxidation and mobilization. Environ. Sci. Technol. 2006, 40, 7277.
| Sorption of Sb(III) and Sb(V) to goethite: influence on Sb(III) oxidation and mobilization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XoslOqsb8%3D&md5=d0d3cbbaff0948c94d1bb1db6c62c1f6CAS | 17180978PubMed |
[20] K. Blay, Sorption wässriger Antimon-Spezies an bodenbildende Festphasen und Remobilisierung durch natürliche Komplexbildner 1999, Ph.D thesis, Technische Universität.
[21] A. C. Scheinost, A. Rossberg, D. Vantelon, I. Xifra, R. Kretzschmar, A. K. Leuz, H. Funke, C. A. Johnson, Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochim. Cosmochim. Acta 2006, 70, 3299.
| Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XlvFarsbw%3D&md5=3f9642bd436390543a872e8559218cfcCAS |
[22] K. Hockmann, S. Tandy, M. Lenz, M. Nachtegaal, M. Janousch, R. Schulin, Release of antimony from contaminated soil induced by redox changes. J. Hazard. Mater. 2014, 275, 215.
| Release of antimony from contaminated soil induced by redox changes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXps12lsbw%3D&md5=bd914ba803fa1cc6f03e1ebb17362fe3CAS | 24862348PubMed |
[23] S. Mitsunobu, Y. Takahashi, Y. Terada, μ-XANES evidence for the reduction of Sb(V) to Sb(III) in soil from Sb mine tailing. Environ. Sci. Technol. 2010, 44, 1281.
| μ-XANES evidence for the reduction of Sb(V) to Sb(III) in soil from Sb mine tailing.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnt1Wjsg%3D%3D&md5=aaafad914387b62df38935bd73a005caCAS | 20085342PubMed |
[24] N. Belzile, Y. W. Chen, Z. J. Wang, Oxidation of antimony(III) by amorphous iron and manganese oxyhydroxides. Chem. Geol. 2001, 174, 379.
| Oxidation of antimony(III) by amorphous iron and manganese oxyhydroxides.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhsVens78%3D&md5=ac2bcbda9ce560ab7c269d6966329d17CAS |
[25] X. Wang, M. He, C. Lin, Y. Gao, L. Zheng, Antimony(III) oxidation and antimony(V) adsorption reactions on synthetic manganite. Chemie der Erde – Geochemistry 2012, 72, 41.
| Antimony(III) oxidation and antimony(V) adsorption reactions on synthetic manganite.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjs1Oru7Y%3D&md5=82976c189382e3a98c7dec2c246183bfCAS |
[26] P. Thanabalasingam, W. F. Pickering, Specific sorption of antimony(III) by the hydrous oxides of Mn, Fe, and Al. Water Air Soil Pollut. 1990, 49, 175.
| Specific sorption of antimony(III) by the hydrous oxides of Mn, Fe, and Al.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXit1aqt78%3D&md5=005e1166135cb4acba0d66618f1a94ebCAS |
[27] S. Mitsunobu, T. Harada, Y. Takahashi, Comparison of antimony behavior with that of arsenic under various soil redox conditions. Environ. Sci. Technol. 2006, 40, 7270.
| Comparison of antimony behavior with that of arsenic under various soil redox conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVaqs7vI&md5=6099d007e1cd44eeef5f411d7869d25dCAS | 17180977PubMed |
[28] B. Müller, L. Granina, T. Schaller, A. Ulrich, B. Wehrli, P, As, Sb, Mo, and other elements in sedimentary Fe/Mn layers of Lake Baikal. Environ. Sci. Technol. 2002, 36, 411.
| P, As, Sb, Mo, and other elements in sedimentary Fe/Mn layers of Lake Baikal.Crossref | GoogleScholarGoogle Scholar | 11871556PubMed |
[29] C. A. J. Appelo, D. Postma, Geochemistry, Groundwater and Pollution, Second Edition. 2005 (Taylor & Francis: Amsterdam).
[30] G. Cornelis, C. A. Johnson, T. Van Gerven, C. Vandecasteele, Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: a review. Appl. Geochem. 2008, 23, 955.
| Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXkvVGrsbo%3D&md5=429a29c96a39d5b747307f142cf4f994CAS |
[31] D. R. Jackson, B. C. Garrett, T. A. Bishop, Comparison of batch and column methods for assessing leachability of hazardous waste. Environ. Sci. Technol. 1984, 18, 668.
| Comparison of batch and column methods for assessing leachability of hazardous waste.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXkslygs7k%3D&md5=cfa56480d98243eadc8a5c658b5ce241CAS |
[32] Soil Survey Division Staff, Soil Survey Manual. Handbook 18 1993 (US Department of Agriculture). Available at http://soils.usda.gov/technical/manual/ [Verified 11 November 2014].
[33] Bestimmung des organisch gebundenen Kohlenstoffs (Corg), Schweizerische Referenzmethoden der Eidgenössischen landwirtschaftlichen Forschungsanstalten 1996 (Agroscope FAL Reckenholz, Agroscope RAC Changins, Agroscope FAW Wädenswil).
[34] H. M. Conesa, M. Wieser, M. Gasser, K. Hockmann, M. W. H. Evangelou, B. Studer, R. Schulin, Effects of three amendments on extractability and fractionation of Pb, Cu, Ni and Sb in two shooting range soils. J. Hazard. Mater. 2010, 181, 845.
| Effects of three amendments on extractability and fractionation of Pb, Cu, Ni and Sb in two shooting range soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXosF2nsbs%3D&md5=892e908b9b9bb10695baed63ca9fe1d3CAS | 20542377PubMed |
[35] J. Lintschinger, B. Michalke, S. Schulte-Hostede, P. Schramel, Studies on speciation of antimony in soil contaminated by industrial activity. Int. J. Environ. Anal. Chem. 1998, 72, 11.
| Studies on speciation of antimony in soil contaminated by industrial activity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjsFygs74%3D&md5=19d1d4a5a1d74ba3e65ce78e23dc4fb8CAS |
[36] J. Hellström, C. Paton, J. Hergt, Iolite: software for spatially resolved LA-(QUAD and MC) ICP-MS analysis, in Laser-Ablation-ICP-MS in the Earth Sciences (Ed. P. Sylvester) 2008, short course 40, pp. 343–348 (Mineralogical Association of Canada: Quebec City, QC).
[37] S. Ackermann, R. Gieré, M. Newville, J. Majzlan, Antimony sinks in the weathering crust of bullets from Swiss shooting ranges. Sci. Total Environ. 2009, 407, 1669.
| Antimony sinks in the weathering crust of bullets from Swiss shooting ranges.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1GnsrY%3D&md5=70306e9dce8c091967bae8db2ccd5a3fCAS | 19117594PubMed |
[38] S. Fendorf, B. D. Kocar, Biogeochemical processes controlling the fate and transport of arsenic: implications for South and Southeast Asia. Adv. Agron. 2009, 104, 137.
| Biogeochemical processes controlling the fate and transport of arsenic: implications for South and Southeast Asia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1aitLnK&md5=c08f129bba75bd48aec7583c4f33943eCAS |
[39] G. Okkenhaug, Y.-G. Zhu, L. Luo, M. Lei, J. Mulder, Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ. Pollut. 2011, 159, 2427.
| Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFeht7bF&md5=b6cc215c798955332f5d8af087e68a6dCAS | 21767897PubMed |
[40] M. Biver, M. Krachler, W. Shotyk, The desorption of antimony(V) from sediments, hydrous oxides, and clay minerals by carbonate, phosphate, sulfate, nitrate, and chloride. J. Environ. Qual. 2011, 40, 1143.
| The desorption of antimony(V) from sediments, hydrous oxides, and clay minerals by carbonate, phosphate, sulfate, nitrate, and chloride.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFKitbs%3D&md5=fb1a6ed1238d7cd749f2602a2f1ff562CAS | 21712584PubMed |
[41] N. P. McNamara, H. I. J. Black, N. A. Beresford, N. R. Parekh, Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl. Soil Ecol. 2003, 24, 117.
| Effects of acute gamma irradiation on chemical, physical and biological properties of soils.Crossref | GoogleScholarGoogle Scholar |
[42] C. A. Abin, J. T. Hollibaugh, Dissimilatory antimonate reduction and production of antimony trioxide microcrystals by a novel microorganism. Environ. Sci. Technol. 2014, 48, 681.
| Dissimilatory antimonate reduction and production of antimony trioxide microcrystals by a novel microorganism.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvV2mt7vE&md5=5134c81cfdd4baedd673f006408e3d32CAS | 24319985PubMed |
[43] T. R. Kulp, L. G. Miller, F. Braiotta, S. M. Webb, B. D. Kocar, J. S. Blum, R. S. Oremland, Microbiological reduction of Sb(V) in anoxic freshwater sediments. Environ. Sci. Technol. 2014, 48, 218.
| Microbiological reduction of Sb(V) in anoxic freshwater sediments.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVCis7rN&md5=949dfd696c17ad1d2c3df98b4f4b8d62CAS | 24274659PubMed |
[44] R. Kirsch, A. C. Scheinost, A. Rossberg, D. Banerjee, L. Charlet, Reduction of antimony by nano-particulate magnetite and mackinawite. Mineral. Mag. 2008, 72, 185.
| Reduction of antimony by nano-particulate magnetite and mackinawite.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtV2rtrbJ&md5=7fc282c0a86559c9e29a836f2a9fa8a6CAS |
[45] R. Polack, Y.-W. Chen, N. Belzile, Behaviour of Sb(V) in the presence of dissolved sulfide under controlled anoxic aqueous conditions. Chem. Geol. 2009, 262, 179.
| Behaviour of Sb(V) in the presence of dissolved sulfide under controlled anoxic aqueous conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlt12ktbg%3D&md5=fa121228df8d69375545e1a1edd6b96aCAS |
[46] G. Okkenhaug, Y. G. Zhu, J. W. He, X. Li, L. Luo, J. Mulder, Antimony (Sb) and arsenic (As) in Sb mining impacted paddy soil from Xikuangshan, China: differences in mechanisms controlling soil sequestration and uptake in rice. Environ. Sci. Technol. 2012, 46, 3155.
| Antimony (Sb) and arsenic (As) in Sb mining impacted paddy soil from Xikuangshan, China: differences in mechanisms controlling soil sequestration and uptake in rice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVSrsb4%3D&md5=53467a6ce86ee61e594283b186bd55a4CAS | 22309044PubMed |
[47] R. M. Cornell, U. Schwertmann, The Iron Oxides 2004 (Wiley-VCH: Weinheim).
[48] R. Gilkes, R. McKenzie, Geochemistry and mineralogy of manganese in soils, in Manganese in Soils and Plants (Eds D. R. Graham, R.J. Hannam, N. C. Uren) 1988 pp 23–35 (Kluwer Academic Publishers: Dordrecht, Netherlands).
[49] M. Filella, S. Philippo, N. Belzile, Y. Chen, F. Quentel, Natural attenuation processes applying to antimony: a study in the abandoned antimony mine in Goesdorf, Luxembourg. Sci. Total Environ. 2009, 407, 6205.
| Natural attenuation processes applying to antimony: a study in the abandoned antimony mine in Goesdorf, Luxembourg.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlCmt77M&md5=3f4fddc1c6ddc891807709c6fcb628e9CAS | 19775729PubMed |
[50] A. C. Scheinost, Metal oxides, in Encyclopedia of Soils in the Environment (Ed. D. Hillel) 2005, pp. 428–438 (Elsevier: Amsterdam, Netherlands).
[51] G. Kirk, The Biogeochemistry of Submerged Soils 2004 (Wiley: Chichester, UK).


