Arsenic accumulation, biotransformation and localisation in bertha armyworm moths
Ruwandi Andrahennadi A and Ingrid J. Pickering A BA Department of Geological Sciences, University of Saskatchewan, 114 Science Place, Saskatoon, SK, S7N 5E2, Canada.
B Corresponding author. Email: ingrid.pickering@usask.ca
Environmental Chemistry 5(6) 413-419 https://doi.org/10.1071/EN08065
Submitted: 13 September 2008 Accepted: 27 November 2008 Published: 18 December 2008
Environmental context. Insects play an important role in the impact of environmental pollutants such as arsenic. They may accumulate arsenic to high levels, potentially modifying its chemical form, which affects the insects’ toxicity to predators such as fish and birds. Here we use synchrotron X-ray techniques to determine the distribution and chemical form of arsenic in larva, pupa and adult of the bertha armyworm moth.
Abstract. Insects are important in bioaccumulation and dispersal of environmental contaminants such as arsenic, and biotransformation of arsenic to various chemical forms directly impacts its toxicity to insects and to their predators. In a model study, the toxic effects and biotransformation of arsenic were examined in larvae, pupae and adults of bertha armyworm moth (Mamestra configurata Walker) (Lepidoptera: Noctuidae). A synthetic diet containing 100 μM arsenate caused reduced larval survival and increased pupal stage duration but no effect on pupal weight or larval stage duration. Synchrotron X-ray absorption spectroscopy (XAS) showed that larvae biotransformed dietary arsenate to yield predominantly trivalent arsenic coordinated with three aliphatic sulfurs, modelled as AsIII-tris-glutathione. Similar species were found in pupae and adults. XAS imaging with micro X-ray fluorescence imaging revealed highly localised arsenic species, and zinc and copper within the gut. The implication of these arsenic species in the diets of predators is discussed.
Introduction
Arsenic is widely distributed in the earth via both natural and anthropogenic pathways, but has no confirmed beneficial role for humans or other animals. Excessive arsenic exposure has been associated with increased incidence of skin, lung and possibly liver cancer in humans.[1] Arsenic can be released to the environment through natural weathering, volcanic action, volatilisation, mining and other industrial activities.[2] In several parts of the world, including Bangladesh, inorganic arsenic of geological origin is found in groundwater used for drinking, leading to chronic arsenic poisoning in humans.[1] Anthropogenic sources of arsenic are of great environmental concern. High levels of arsenic are found in copper, lead, gold and uranium mine tailings and the use of pesticides, herbicides and silvicides has increased the levels of arsenic in the environment.[3,4] Arsenic levels range from 56 to 6000 μg g–1 in mine tailings from Rabbit Lake, northern Saskatchewan.[5] In recent years, regulatory and public attention has focussed on the potential risks associated with the use of arsenic in the preservation of timber.[6]
Determining the toxicity, speciation and distribution of arsenic within biological samples is essential for understanding bioavailability, trophic transfer and environmental risks. Insects are very numerous, mobile and food for a variety of other organisms. Thus, they are important components in the functioning of ecosystems and in the dispersal of elements such as arsenic. When life stages of insects occur in different habitats, movement of arsenic to a new habitat may occur. Immature stages living in contaminated terrestrial or aquatic habitats may carry arsenic long distances as they emerge as flying adults. This movement is exemplified by the bioaccumulation of arsenic by herbivorous larvae of bogong moths (Agrotis infusa) (Lepidoptera: Noctuidae) in eastern Australia.[7] Migrating adult bogong moths contain high levels of arsenic and their carcasses caused toxicity to vegetation in mountain regions.[7] Elevated levels of arsenic have been found in other insects and in insectivorous birds in arsenic-contaminated areas.[4] Understanding the effects of arsenic on insects is, therefore, important with regard to assessing the ecological risk posed by arsenic in the food chain. In addition, little is known about the effect of arsenic uptake on the insect life cycle and survival.
Further, the toxicity, behaviour and bioavailability of arsenic in the environment depend critically on the chemical form in which it is presented. Under aerobic conditions, inorganic arsenic often is present predominantly in the pentavalent oxidation state as arsenate; however, reduced trivalent forms such as arsenite can occur and these can be significantly more toxic than arsenate. The dominant arsenic species found in apple orchards in Massachusetts treated with arsenic-based insecticides was found to be arsenate.[3] Marine animals such as fish, crustaceans and molluscs contain predominantly formally trivalent arsonium species such as arsenobetaine, and to a lesser extent arsenocholine,[8] whereas pentavalent arsenosugars are found in marine algae.[8,9] Arsenobetaine found in marine animals is considered to be non-toxic.[10,11] Methylated trivalent arsenic species such as monomethylarsonous acid (MMAIII) and dimethylarsinous acid (DMAIII) are naturally generated in mammals and have recently been reported to be more toxic under some circumstances than inorganic arsenite,[12] but more study is required to confirm this.
Synchrotron-based X-ray absorption spectroscopy (XAS) is a powerful tool that can be used to identify the chemical species of arsenic in complex environmental samples.[13] Its strengths are that it can be applied with minimal pretreatment, and can determine the chemical form in all of the arsenic present in the sample, whether precipitated, aqueous dissolved or in any other form. In the present study, we used XAS to probe arsenic speciation in a model insect, bertha armyworm (Mamestra configurata Walker) (Lepidoptera: Noctuidae), raised in the laboratory on an arsenate-rich diet. Arsenate was chosen as a common arsenic form present in the environment under aerobic conditions and also present in some insecticide residues. It is also likely to undergo biotransformation by the insect. The level of arsenate was chosen to show some impacts on the development of the insect and is commensurate with arsenic concentrations found in some arsenic-rich environments. We examined the toxic effects of arsenate on the development of larvae, pupae and adult stages of the bertha armyworm and used XAS to probe arsenic speciation at each developmental stage.
The elements absorbed into an organism are distributed into different areas including the site where they cause the damage. In insects, the midgut region of the gastrointestinal tract is one of the most important sites where toxicants are absorbed. In an effort to understand the organs responsible for arsenic absorption, biotransformation and toxicity, we also used XAS imaging[14,15] with micro X-ray fluorescence imaging (μ-XRF). This allowed us to determine the microscopic distribution of arsenic chemical forms and other elements within intact larvae.
Materials and methods
Insect rearing
The bertha armyworm (Mamestra configurata Walker) is native to North America and a pest of a wide variety of broadleaved plants including canola, alfalfa and flax.[16] Bertha armyworm is used as a model insect in various physiological and biochemical studies. Its biology and ecology are well known and it can be successfully reared in the laboratory on a synthetic diet. In the current study, bertha armyworm larvae were reared in plastic diet cups containing alfalfa-based modified Bucher and Bracken diet[17] and maintained at a constant temperature of 21 ± 1°C, 60% relative humidity, and a photoperiod of 16 h of light followed by 8 h of darkness (16 : 8, L : D). For the arsenic treatments, sodium arsenate (Na2HAsO4) was dissolved in the prepared diet before pouring into the diet cups. Larvae were introduced to the diet at neonate (newly hatched, first instar) stage, and transferred to new diet cups twice a week.
Preliminary study on dosage and the chemical form of arsenic in the diet
A preliminary study was conducted to determine a level of dietary arsenate that produced low but measurable toxicity and levels of arsenic that were measurable by XAS. Larvae were reared on a diet containing 1 mM, 100 μM or 10 μM of sodium arsenate, or arsenate-free diet (100 larvae per treatment). After 7 days, the mortality of the arsenate-fed larvae was compared. Surviving larvae from the arsenate treatments were used for XAS measurements (as described below). Spectra from the larvae collected from all three treatments showed similar arsenic species (data not shown). Insects fed with 1 mM of arsenate had the highest mortality (52% survival) and therefore this treatment was not included in additional experiments. Larvae reared on 100 μM arsenate showed good survival (86%) and gave a more adequate (stronger) signal in XAS than the larvae reared on 10 μM arsenate (97% survival). Therefore, 100 μM arsenate was used in subsequent studies. X-ray absorption spectroscopy of the surviving larvae from all three concentrations (data not shown) showed similar speciation to each other and to the study described below, indicating that concentration did not have a substantial effect on the biotransformation at these levels.
X-ray absorption spectroscopy of the diet containing 100 μM arsenate, frozen in liquid nitrogen 24 h after preparation, indicated that arsenic in the diet remains as arsenate and the preparation and storage of the diet do not significantly change the chemical form.
Toxicity of arsenate to bertha armyworm
Neonate larvae were reared with 100 larvae fed on 100 μM arsenate, and 100 larvae were also fed on an arsenate-free control diet. There were five replicates, each consisting of five cups with four larvae in each cup, giving 20 larvae per replicate. Larval survival to pupation, time taken to reach pupation, pupal weight and time to reach adult emergence were recorded. The results were analysed by one-way analysis of variance (ANOVA).[18]
Determination of total arsenic using atomic absorption spectroscopy
Larvae in the final stage before pupation (fifth instar), pupae and adults, 10 from each stage, were oven-dried and prepared for analysis according to the method of Wayland and Crosley.[19] Total arsenic concentration was measured using hydride-generation atomic absorption spectrometry. Two independent measurements were made of each sample. Standard solutions were of arsenate at 1, 5, 10, 15 and 20 μg L–1. Blank samples including reagent blanks and digestion blanks were analysed together with insect samples.
Arsenic speciation using X-ray absorption spectroscopy
Arsenic-treated insects were taken for XAS studies as follows: larva (ten fifth instar larvae, 2 larvae per replicate); pupa (ten pupae, 2 pupae per replicate); and adult (five males and five females of newly emerged moths, 1 moth per replicate). Samples were rapidly frozen and stored in liquid nitrogen. They were then finely ground under liquid nitrogen, packed in 2-mm pathlength sample cuvettes, and kept frozen in liquid nitrogen until data collection.
X-ray absorption spectroscopy data were collected at the Stanford Synchrotron Radiation Laboratory (SSRL) with the storage ring SPEAR-3 operating at 3 GeV and with maximum storage ring currents of 100 mA. Arsenic K-edge spectra were recorded on beamline 9–3 with an upstream Rh-coated collimating mirror, a Si(220) double-crystal monochromator and a downstream Rh-coated focussing mirror. The incident X-ray intensity was monitored using a N2-filled ionisation chamber. Near-edge spectra were recorded by monitoring the arsenic Kα fluorescence using a 30-element germanium detector equipped with Soller slits and germanium filters. During data collection, samples were maintained at ~10K in a liquid helium-flow cryostat in order to minimise photoreduction or other beam-induced changes in the arsenic chemical form. Energies were calibrated with reference to the spectrum of an elemental arsenic foil, collected in transmittance simultaneously with the spectra of the samples, the first energy inflection of which was assumed to be 11 867.0 eV.[20]
Background subtraction, normalisation and data analysis were carried out according to standard methods using the EXAFSPAK program suite (http://www-ssrl.slac.stanford.edu/exafspak.html, accessed 2 December 2008). Principal component analysis was used to determine the number of standards required in the subsequent analyses, and the most likely standards were identified with target transformation. Near-edge spectra were quantitatively fitted to the sum of spectra of arsenic standards. The fractional contribution of a standard spectrum to the sum is equal to the fraction of total arsenic present in that chemical form. Spectra of standards were collected in fluorescence of dilute aqueous solutions (1–5 mM) of arsenic species, including the inorganic arsenic forms, arsenate at pH 9 and arsenite at pH 7, as well as the aqueous thiolate-coordinated arsenic AsIII-tris-glutathione, which was synthesised as previously described.[13] In addition, spectra of methylated arsenic compounds including monomethylarsonic acid (MMAV), dimethylarsinic acid (DMAV) and arsenobetaine were initially included in the fits and later rejected as they were not significant components in the sample spectra.
Micro X-ray fluorescence imaging and X-ray absorption spectroscopy imaging
Micro X-ray fluorescence imaging (μ-XRF) with XAS imaging was conducted on arsenate (100 μM)-fed third instar bertha armyworm larvae using the methods previously described by Pickering and coworkers.[14,15,21] In μ-XRF, the sample is rastered in the beam using a fixed energy above the excitation energy of the elements of interest. In XAS (or chemically specific) imaging, μ-XRF data are collected at two or more energies chosen to be spectroscopically sensitive to chemical forms of interest. Energies for chemically specific imaging were chosen based on the dietary arsenic and speciation results obtained from bulk XAS of larvae. These energies correspond to the maximum in absorption of the standard solutions of arsenate at 11 874.9 eV and AsIII-tris-glutathione at 11 869.7 eV.
Data were collected on beamline 9–3 at the SSRL, where microfocus beams were provided by a tapered glass monocapillary optic (X-Ray Optical Systems, Inc., East Greenbush, NY, USA). A 10-μm diameter beam spot was achieved at the focal point of the optic and the sample was moved downstream to a slightly defocussed position to achieve a 20-μm diameter spot. The spot sizes were characterised by scanning a knife-edge through the beam. The beamline energy was calibrated with reference to an arsenic metal foil before each image was recorded.
Each bertha armyworm larva was mounted live, and was positioned vertically in a thin-walled quartz capillary at room temperature. Larvae were immobilised by flowing nitrogen gas through the capillary, which acted as an anaesthetic; offline tests showed that larvae could survive many hours under these conditions. Arsenic Kα fluorescence and scatter intensities were monitored using a 50-mm2 silicon drift detector (Vortex SII Radiant Technologies, Northridge, CA, USA). The distributions of other metals such as copper and zinc were also monitored simultaneously with those of arsenic, to observe possible co-association of these metals with arsenic. The sample was spatially rastered in the microbeam using a PM500 stage (Newport Corporation, Irvine, CA, USA). A horizontal raster scan was collected at both energies and then the sample was moved vertically, thus ensuring that data were collected on a given pixel in the shortest amount of time.[21] Images of arsenic chemical forms were generated from the maps at different energies using methods described by Pickering and coworkers.[14,21] Following the collection of arsenic species maps, arsenic K-edge micro X-ray absorption spectroscopy (μ-XAS) was performed at selected pixels to corroborate the arsenic chemical speciation.
Results and discussion
Toxicity to arsenate
The toxicity of arsenate to bertha armyworm was examined by rearing larvae on a diet of 100 μM arsenate throughout their larval stages. Observations on development of the arsenate-fed larvae, through their metamorphosis to pupae, and their eventual emergence as adults were made in comparison with larvae fed on an arsenic-free diet. Days to pupation, larval survival, pupal weight and days to adult emergence for bertha armyworm are shown in Table 1. Development times of arsenate-treated and untreated larvae were not significantly different. When compared with untreated larvae, survival of arsenate-fed bertha armyworm larvae was significantly reduced, although a survival of 79% was still achieved (Table 1). Pupal weight of surviving larvae was not significantly affected by the arsenate treatment, whereas duration of pupal stage was significantly longer for the arsenate-treated larvae. Interestingly, larvae fed on the same concentration of selenate showed significantly greater effects on development than arsenate in all criteria except for pupal weight (R. Andrahennadi and I. J. Pickering, unpubl. data), indicating that arsenate is less toxic than selenate at the level of 100 μM.
Total arsenic analysis
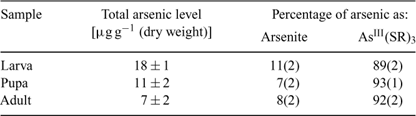
Atomic absorption spectroscopy results showed that the larvae fed with arsenate accumulated 18 ± 1 μg g–1 (dry weight) of arsenic and that elimination subsequently occurred during larval development, resulting in arsenic levels of 11 ± 2 and 7 ± 2 μg g–1 (dry weight) in pupae and adults, respectively (Table 2). In an earlier study,[22] larvae of chironomids (Diptera, Chironomidae) exposed to zinc, cadmium and copper ions showed a decrease in these elements during metamorphosis. Loss of metals and metalloids by shedding of the exoskeleton is the most likely explanation for reduction in levels during metamorphosis of insects.[22] However, the presence of arsenic in the flying adults, albeit at a lower level than in the larvae, may pose a risk for insectivorous predators living away from the point sources and deserves more attention.
|
Arsenic speciation using X-ray absorption spectroscopy
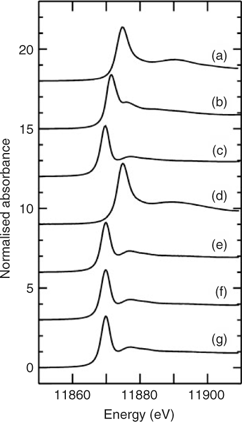
Fig. 1a–c shows the X-ray absorption near-edge spectra of selected aqueous arsenic standards. Arsenic K-edge spectra are dominated by dipole-allowed transitions of the core 1s electron to unoccupied bound states with a lot of 4p orbital character, and the spectra thus are very sensitive not only to valence but to geometry and nature of the ligand.[13] Fig. 1 also shows the arsenic near-edge spectra of larval, pupal and adult Mamestra configurata in comparison with the arsenic in the diet. The spectrum of the diet confirms that arsenate is not transformed before consumption, and the spectrum of the larvae is quite different from that of the diet, indicating that arsenate supplied in the diet is biotransformed by the larva. In contrast, the spectra of pupa and adult are very similar to that of the larva, indicating that the majority of arsenic retained within the insect body is not further modified during subsequent life stages. Indeed, principal component analysis showed that the three spectra from whole larvae, pupae and adults require one predominant component, with possibly a small contribution from a second component. The major component shows good correspondence with the spectrum of AsIII-tris-glutathione (AsIII(GS)3), in which reduced arsenic (AsIII) is bound to three thiolate sulfur atoms from the cysteine peptide in glutathione.[13] However, XAS is not generally sensitive to very long-range structure and AsIII(GS)3 is used as a model for trivalent arsenic coordinated to three aliphatic thiols, AsIII(SR)3, in general. Such species have been observed spectroscopically in Brassica juncea treated with arsenate.[13] When spectra of the insect samples were fitted to the sum of spectra of standard species (Table 2), larvae, pupae and adult samples showed a small percentage of arsenite (ranging from 7 to 11%) in addition to the major component of AsIII(SR)3. Although some uncomplexed arsenite may indeed be present, the fact that its proportion is the same within the error of the analysis for each of these samples could suggest that this is instead attributable to minor differences in the spectrum due to coordination by thiolates other than glutathione. Extended X-ray absorption fine structure (EXAFS) data of arsenic-fed larvae was consistent with predominantly three-fold As–S coordination (data not shown).
|
The observed arsenic speciation has implications for the mobility, toxicity and bioavailability of arsenic within insects. AsV from the diet was reduced to AsIII. Inorganic AsIII as arsenite and some methylated species are generally accepted as the most harmful metabolite responsible for toxic and carcinogenic effects. However, methylated arsenic forms were not found to be significant in insects, and the predominant form was AsIII(SR)3, modelled as AsIII(GS)3. It has been shown that, in the presence of excess glutathione, arsenate is reduced by oxidation of glutathione and the resulting AsIII is bound by additional glutathione molecules to form AsIII(GS)3.[23] Glutathione is a prevalent biological thiol and plays a role in protecting the mammalian kidney from arsenite-induced toxicity.[24] A similar protection mechanism may occur in insects, resulting in lower toxicity in arsenate-fed insects. The present study showed that AsIII(SR)3 is the the main chemical form of arsenic in larvae, pupae and emerging adult insects. These insects may transfer arsenic in the form of AsIII(SR)3 to their predators and toxicity to predators may depend on their ability to tolerate AsIII(SR)3.
Arsenic species localisation using X-ray absorption spectroscopy imaging
X-ray absorption spectroscopy imaging is used to produce maps of the location of arsenic species within a complex structure such as an intact organism.[14,15,21] It is related to μ-XRF imaging, in which the incident X-ray energy is set well above the absorption edge of the elements of interest; by rastering the sample in the beam and measuring the fluorescence, μ-XRF generates a projection map of the elements. In XAS imaging, the fine structure of the absorption edge is exploited to derive maps of the particular chemical forms of the element. In essence, the incident X-ray energy is sequentially set to the intensity maximum in the near-edge spectrum of n chemical species of interest in the sample, resulting in n fluorescence maps of the element of interest (here arsenic). By using the intensities from the arsenic near-edge spectra of standards, the maps can be deconvolved to obtain quantitative concentration maps of arsenic species. This method has been described in detail previously.[14,15,21] The major strengths of XAS imaging are three-fold. First, it is essentially non-destructive, as the X-rays can interrogate intact tissues of a live organism. This means that there is no risk of the chemical form being modified as a function of pretreatments such as extractions. Second, XAS imaging detects all occurrences of the element of concern, whether dissolved, precipitated or in any other form; thus, there are no ‘hidden’ components. Third, it can produce microscopic maps of chemical species. To our knowledge, XAS imaging has not been carried out on insects, and relatively little on organisms in general, although μ-XRF imaging of selenium localisation was carried out on high-concentration selenium-fed diamondback moth larvae (Plutella xylostella) inhabiting selenium hyperaccumulating plants.[25]
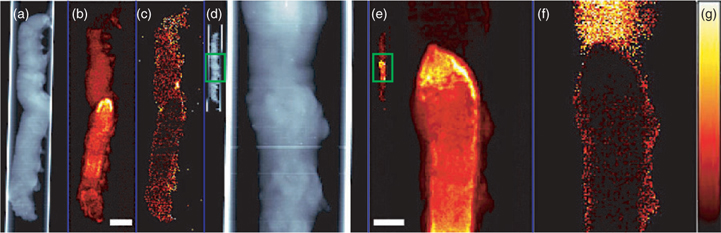
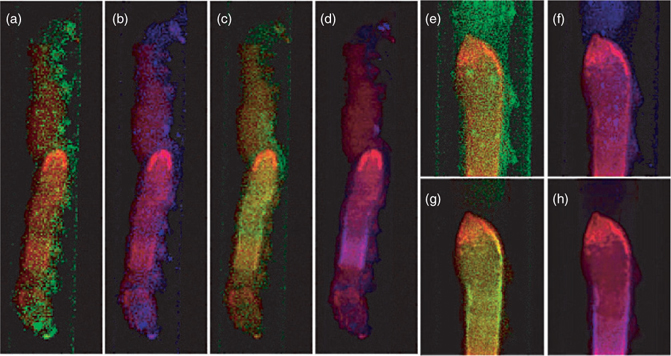
Fig. 2 shows images of two different bertha armyworm larvae. First, an intact larva was imaged at 20-μm resolution to give an overview of the entire organism (Fig. 2a–d). A second larva was then imaged at 10-μm resolution (Fig. 2e–h) to provide more details on the region of the midgut, as described below. The larvae are both in lateral orientation and Fig. 2a, e shows the X-ray transmittance of both larvae, which gives an indication of the amount of material in the projection.
Fig. 2b, f shows the localisation of AsIII(SR)3, which is the predominant form of arsenic in the larvae. Arsenic as AsIII(SR)3 was detectable in the midgut area of the larvae, with distinctly high localisation towards the middle area of the midgut (Fig. 2b, f). Relatively low levels of arsenic as AsIII(SR)3 were measured in the foregut and anterior part of the midgut region. The presence of As–S complexes in the intestine has been well documented in earthworms.[26] Similarly, previous studies have shown that some metals tend to be concentrated in the midgut where they are stored in the form of granules[27,28] or in lysosomes.[29] Depuration of metals from the gut may occur when these storage structures are excreted from the body.[30] The presence of metals and metalloids in the gut may also be favoured by their binding to the insect peritrophic membrane, which is a protective chitinous sheet lining the midgut. In Lepidoptera, the peritrophic membrane is produced continuously by the midgut and excreted from the anus.[31,32]
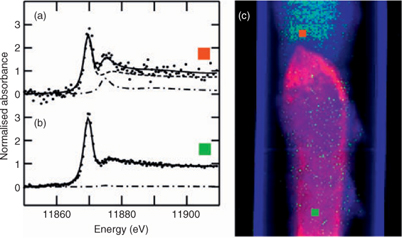
In contrast to AsIII(SR)3, arsenic as arsenate was found only diffusely distributed throughout the larval body (Fig. 2d, h), possibly on the exoskeleton. Similarly, arsenic appeared to be in the exoskeleton of aquatic nymphs belonging to the genus Hexagenia.[33] Arsenate is additionally found, albeit at low levels, in the anterior foregut (Fig. 2h), a region that is relatively low in AsIII(SR)3. The same area is shown in Fig. 3, in which a single μ-XAS dataset from this region shows a small but significant fraction of arsenate (Fig. 3a, c), and may correspond to undigested arsenate in the diet. This arsenate region is located directly anterior to the region that shows highest AsIII(SR)3 and essentially no arsenate (Fig. 2g, h; Fig. 3b, c). This may suggest that the high AsIII(SR)3 region is the site of reduction and complexation.
|
Fig. 4 shows the distribution of manganese, iron, copper and zinc, each superimposed on the map of total arsenic. In insects, a variety of metals including zinc, manganese, iron and copper are found concentrated within the mandibles, ovipositors and various body parts.[34–36] Insects also need small amounts of metals and metalloids as enzyme cofactors and as constituents for metalloenzymes. Both zinc and copper are found to be associated with the gut. Zinc is concentrated in regions of the midgut (Fig. 4d, h). The observed profile of two parallel lines is expected for the projection image of a cylindrical structure and is consistent with the zinc being localised at the gut wall or lining. The arsenic shows a similar profile in this region of the gut except that arsenic is concentrated more anteriorly, zinc more posteriorly. Copper is also localised in the gut area of the larvae with the highest level in the midgut region. In contrast to zinc, copper is present at lower levels and is not associated with the gut wall but instead is diffusely distributed. Slightly anterior to the midgut, another copper localised area is present on the ventral side of the larval body (Fig. 4c). Copper accumulation in the intestinal cells has been observed in larval Drosophila.[29]
|
Zinc (Fig. 4d) is observed highly concentrated in the mandibles (which appear as two bright spots near the top of the image) in addition to the midgut. The presence of zinc in mandibles of herbivorous insects has been known for some time[34,35] and reduces the abrasive wear that mandibles would suffer through chewing hard plant material. Enhanced levels of zinc have also been observed in grasshoppers and leaf-cutter ants where high levels of zinc are linked with greater hardness of the cuticle.[34,35] Manganese is also commonly found in insect mandibles. However, in the case of bertha armyworm, we find that manganese is not concentrated in the mandibles of the larvae, but is observed on the cuticle (Fig. 4a, e), possibly in association with pigment spots that are characteristic for this larvae. Potassium and calcium were seen in oral secretions (data not shown) but the low energy fluorescence of these elements combined with the fact that the larva was in a quartz capillary precluded additional detailed observations.
Iron was diffusely distributed, but additionally appeared in small areas of high concentration throughout the body (Fig. 4b, f). In general, insects acquire only a trace amount of iron, except for species that employ haemoglobin in respiration. Iron is involved in the formation of insect cuticle.[36] Iron containing magnetite (Fe3O4) has been found in the head and thorax of adult monarch butterflies, suggesting that the butterflies might use Earth’s magnetic fields to navigate in their migratory flights.[37]
Conclusion and future direction
In summary, the present model study provides new insights into toxicity and biotransformation of arsenate in different life stages of an insect. XAS at the arsenic K edge showed the role of sulfur coordination following arsenic biotransformation in bertha armyworm larvae. During metamorphosis, arsenic speciation varied only slightly between pupal and adult stages of the insect. XAS imaging directly visualised the distribution of arsenic as arsenate and sulfur-coordinated arsenic forms in the larvae, indicating possible target organs during arsenic biotransformation. Zinc is seen closely associated with arsenic in the periphery of the midgut. Better understanding on how these animals regulate arsenic exposure is needed to predict the potential risk of arsenic contamination. The role of various excretory pathways on the tolerance of bertha armyworm to arsenate, and the consequent impact on the environment, will be the subject of a future study (R. Andrahennadi, G. N. George, I. J. Pickering, unpubl. data).
Acknowledgements
The present research is supported by the Province of Saskatchewan and a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) (to I. J. Pickering). I. J. Pickering is a Canada Research Chair. We thank Agriculture and Agri-Food Canada (Saskatoon Research Centre) for insects and rearing facilities. We also thank Graham George and Helen Nichol for helpful discussions and Pickering/George group members for assistance in data collection. Portions of this research were carried out at the Stanford Synchrotron Radiation Laboratory (SSRL), a national user facility operated by Stanford University on behalf of the US Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program.
[1]
M. G. M. Alam ,
G. Allinson ,
F. Stagnitti ,
A. Tanaka ,
M. Westbrooke ,
Arsenic contamination in Bangladesh groundwater: a major environmental and social disaster.
Int. J. Environ. Health Res. 2002
, 12, 235.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[2]
S. Wang ,
C. N. Mulligan ,
Occurrence of arsenic contamination in Canada: sources, behavior and distribution.
Sci. Total Environ. 2006
, 366, 701.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[3]
K. Newton ,
D. Amarasiriwardana ,
B. Xing ,
Distribution of soil arsenic species, lead and arsenic bound to humic acid molar mass fractions in a contaminated apple orchard.
Environ. Pollut. 2006
, 143, 197.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[4]
C. A. Morrissey ,
C. A. Albert ,
P. L. Dods ,
W. R. Cullen ,
V. W.-M. Lai ,
J. E. Elliott ,
Arsenic accumulation in bark beetles and forest birds occupying mountain pine beetle-infested stands treated with monosodium methanearsonate.
Environ. Sci. Technol. 2007
, 41, 1494.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[5]
B. J. Moldovan ,
D.-T. Jiang ,
M. J. Hendry ,
Mineralogical characterization of arsenic in uranium mine tailings precipitated from iron-rich hydrometallurgical solutions.
Environ. Sci. Technol. 2003
, 37, 873.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[6]
G. J. Zagury ,
S. Dobran ,
S. Estrela ,
L. Deschenes ,
Inorganic arsenic speciation in soil and groundwater near in-service chromated copper arsenate-treated wood poles.
Environ. Toxicol. Chem. 2008
, 27, 799.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[7]
K. Green ,
Migratory bogong moths (Agrotis infusa) transport arsenic and concentrate it to lethal effect by estivating gregariously in alpine regions of the Snowy Mountains of Australia.
Arct. Antarct. Alp. Res. 2008
, 40, 74.
| Crossref | GoogleScholarGoogle Scholar |

[8]
K. A. Francesconi ,
J. S. Edmonds ,
Arsenic in the sea.
Oceanogr. Mar. Biol. 1993
, 31, 111.

[9]
[10]
M. A. Lopez-Gonzalvez ,
M. Milagros-Gomez ,
C. Camara ,
M. A. Palacios ,
Determination of toxic and non-toxic arsenic species in urine by microwave-assisted mineralization and hydride generation atomic absorption spectrometry.
Mikrochim. Acta 1995
, 120, 301.
|
CAS |

[11]
P. Andrewes ,
D. M. DeMarini ,
K. Funasaka ,
K. Wallace ,
V. W. M. Lai ,
H. Sun ,
W. R. Cullen ,
K. T. Kitchin ,
Do arsenosugars pose a risk to human health? The comparative toxicities of a trivalent and pentavalent arsenosugar.
Environ. Sci. Technol. 2004
, 38, 4140.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[12]
S. Hirano ,
Y. Kobayashi ,
X. Cui ,
S. Kanno ,
T. Hayakawa ,
A. Shraim ,
The accumulation and toxicity of methylated arsenicals in endothelial cells: important roles of thiol compounds.
Toxicol. Appl. Pharmacol. 2004
, 198, 458.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[13]
I. J. Pickering ,
R. C. Prince ,
M. J. George ,
R. D. Smith ,
G. N. George ,
D. E. Salt ,
Reduction and coordination of arsenic in indian mustard.
Plant Physiol. 2000
, 122, 1171.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[14]
I. J. Pickering ,
R. C. Prince ,
D. E. Salt ,
G. N. George ,
Quantitative chemically specific imaging of selenium transformation in plants.
Proc. Natl. Acad. Sci. USA 2000
, 97, 10717.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[15]
I. J. Pickering ,
L. Gumaelius ,
H. H. Harris ,
R. C. Prince ,
G. Hirsch ,
J. A. Banks ,
D. E. Salt ,
G. N. George ,
Localizing the biochemical transformations of arsenate in a hyperaccumulating fern.
Environ. Sci. Technol. 2006
, 40, 5010.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[16]
W. J. Turnock ,
Developmental, survival and reproductive parameters of bertha armyworm, Mamestra configurata (Lepidoptera: Noctuidae) on four plant species.
Can. Entomol. 1985
, 117, 1267.

[17]
G. E. Bucher ,
G. K. Bracken ,
The bertha armyworm, Mamestra configurata (Lepidoptera: Noctuidae). Artificial diet and rearing technique.
Can. Entomol. 1976
, 108, 1327.

[18]
[19]
M. Wayland ,
R. Crosley ,
Selenium and other trace elements in aquatic insects in coal-mine affected streams in the rocky mountains of Alberta, Canada.
Arch. Environ. Contam. Toxicol. 2006
, 50, 511.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[20]
[21]
I. J. Pickering ,
G. N. George ,
X-ray absorption spectroscopy imaging of biological tissues.
AIP Conf. Proc. 2007
, 882, 311.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[22]
K. R. Timmermans ,
P. A. Walker ,
The fate of trace metals during the metamorphosis of chironomids (Diptera, Chironomidae).
Environ. Pollut. 1989
, 62, 73.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[23]
N. Scott ,
K. M. Hartlelid ,
N. E. Mackenzie ,
D. E. Carter ,
Reactions of arsenic(III) and arsenic(V) species with glutathione.
Chem. Res. Toxicol. 1993
, 6, 102.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[24]
M. Hirata ,
A. Hisanaga ,
A. Tanaka ,
N. Ishinishi ,
Glutathione and methylation of inorganic arsenate in hamsters.
Appl. Organomet. Chem. 1988
, 2, 315.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[25]
J. L. Freeman ,
C. F. Quinn ,
M. A. Marcus ,
S. Fakra ,
E. A. H. Pilon-Smits ,
Selenium-tolerant diamondback moth disarms hyperaccumulator plant defense.
Curr. Biol. 2006
, 16, 2181.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[26]
C. J. Langdon ,
A. A. Meharg ,
J. Feldmann ,
T. Balgar ,
J. Charnock ,
M. Farquhar ,
T. G. Piearce ,
K. T. Semple ,
J. Cotter-Howells ,
Arsenic speciation in arsenate-resistant and non-resistant populations of the earthworm, Lumbricus rubellus.
J. Environ. Monit. 2002
, 4, 603.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[27]
A. Y. Jeantet ,
C. Ballan-Dufrançais ,
R. Martoja ,
Insects resistance to mineral pollution. Importance of spherocrystal in ionic regulation.
Rev. Ecol. Biol. Sol. 1977
, 14, 563.
|
CAS |

[28]
W. Humbert ,
Cytochemistry and X-ray microprobe analysis of the midgut of Tomocerus minor Lubbock (Insecta: Collembola) with special reference to the physiological significance of the mineral concretions.
Cell Tissue Res. 1978
, 187, 397.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[29]
R. L. Tapp ,
A. Hockaday ,
Combined histochemical and X-ray microanalytical studies on the copper-accumulating granules in the midgut of larval Drosophila.
J. Cell Sci. 1977
, 26, 201.
|
CAS |
PubMed |

[30]
W. Humbert ,
The mineral concretions in the midgut of Tomocerus minor (Collembola): microprobe analysis and physiological significance.
Rev. Ecol. Biol. Sol. 1977
, 14, 71–80.

[31]
D. F. Waterhouse ,
Occurrence and endodermal origin of the peritrophic membrane in some insects.
Nature 1953
, 172, 676.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[32]
M. J. Lehane ,
Peritrophic matrix structure and function.
Annu. Rev. Entomol. 1997
, 42, 525.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[33]
L. Hare ,
A. Tessier ,
P. G. C. Campbell ,
Trace element distributions in aquatic insects: variations among genera, elements and lakes.
Can. J. Fish. Aquat. Sci. 1991
, 48, 1481.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[34]
B. W. Cribb ,
A. Stewart ,
H. Huang ,
R. Truss ,
B. Noller ,
R. Rasch ,
M. P. Zalucki ,
Insect mandibles – comparative mechanical properties and links with metal incorporation.
Naturwissenschaften 2007
, 95, 17.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[35]
D. L. J. Quicke ,
P. Wyeth ,
J. D. Fawke ,
H. H. Basibuyuk ,
J. F. V. Vincent ,
Manganese and zinc in the ovipositors and mandibles of hymenopterous insects.
Zool. J. Linn. Soc. 1998
, 124, 387.
| Crossref | GoogleScholarGoogle Scholar |

[36]
M. Locke ,
H. Nichol ,
Iron economy in insects: transport, metabolism and storage.
Annu. Rev. Entomol. 1992
, 37, 195.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[37]