Enrichment of saccharides at the air–water interface: a quantitative comparison of sea surface microlayer and foam
Thilina Jayarathne A D , Dilini Kirindigoda Gamage A , Kimberly A. Prather B C and Elizabeth A. Stone A *
A *
A Department of Chemistry, University of Iowa, Iowa City, IA 52242, USA.
B Department of Chemistry and Biochemistry, University of California, San Diego, La Jolla, CA 92093, USA.
C Scripps Institution of Oceanography, University of California, San Diego, La Jolla, CA 92093, USA.
D Present address: Bristol Myers Squibb, 1 Squibb Drive, New Brunswick, NJ 08901, USA.
Environmental Chemistry 19(8) 506-516 https://doi.org/10.1071/EN22094
Submitted: 12 August 2022 Accepted: 31 January 2023 Published: 3 March 2023
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Environmental context. Saccharides contribute substantially to dissolved organic carbon in the ocean and are enriched at the ocean surface. In this study, we demonstrate that saccharides are more enriched in persistent whitecap foam compared to the sea surface. The maturation of bubbles at the air–water interface is thus expected to enhance the enrichment of organic matter at the ocean surface and ultimately in the sea spray aerosol that forms when bubbles burst at the ocean surface.
Rationale. Organic matter accumulates at the ocean surface. Herein, we provide the first quantitative assessment of the enrichment of dissolved saccharides in persistent whitecap foam and compare this enrichment to the sea surface microlayer (SSML) during a 9 day mesocosm experiment involving a phytoplankton bloom generated in a Marine Aerosol Reference Tank (MART).
Methodology. Free monosaccharides were quantified directly, total saccharides were determined following mild acid hydrolysis and the oligo/polysaccharide component was determined as the difference between total and free monosaccharides.
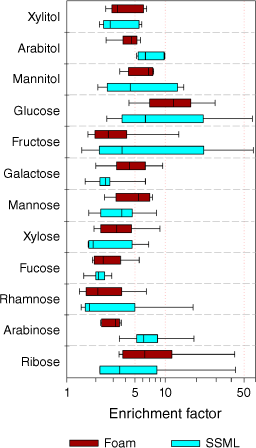
Results. Total saccharides contributed a significant fraction of dissolved organic carbon (DOC), accounting for 13% of DOC in seawater, 27% in SSML and 31% in foam. Median enrichment factors (EFs), calculated as the ratio of the concentrations of saccharides relative to sodium in SSML or foam to that of seawater, ranged from 1.7 to 6.4 in SSML and 2.1–12.1 in foam. Based on median EFs, xylitol, mannitol, glucose, galactose, mannose, xylose, fucose, rhamnose and ribose were more enriched in foam than SSML.
Discussion. The greatest EFs for saccharides coincided with high chlorophyll levels, indicating increasing ocean surface enrichment of saccharides during phytoplankton blooms. Higher enrichments of organic matter in sea foam over the SSML indicate that surface active organic compounds become increasingly enriched on persistent bubble film surfaces. These findings help to explain how marine organic matter becomes highly enriched in sea spray aerosol that is generated by bursting bubbles at the ocean surface.
Keywords: carbohydrates, dissolved organic carbon, enrichment factor, phytoplankton bloom, sea surface microlayer, sugar alcohols, ultrafiltration, whitecap foam.
Introduction
Seawater, in addition to salt, contains complex matrix organic compounds, with saccharides (carbohydrates), proteins and lipids being the predominant compound classes (Larsson et al. 1974; Henrichs and Williams 1985; Garabetian et al. 1993; Aluwihare et al. 1997). Saccharides, in particular, are present in a variety of chemical forms, including sugar alcohols (e.g. arabitol, mannitol), free monosaccharides (e.g. glucose, galactose), oligo/polysaccharides (e.g. glucan, transparent exopolysaccharides or TEP) and saccharides complexed with other molecules such as lipids and protein (e.g. lipopolysaccharides, glycoproteins) (Borch and Kirchman 1997; Verdugo et al. 2004; van Pinxteren et al. 2012). These saccharides serve as substrates for energy storage and the formation of structural materials (e.g. cell walls) of marine micro-organisms (Haug and Myklestad 1976; Dean Pakulski and Benner 1992; Hung et al. 2001; Verdugo et al. 2004). They have been estimated to contribute up to 40% of dissolved organic carbon (DOC) in the ocean with levels dependent on biological activity (Dean Pakulski and Benner 1992; Skoog and Benner 1997; Biersmith and Benner 1998; Engbrodt and Kattner 2005). During a phytoplankton bloom, the molecular composition of saccharides is altered by phytoplankton and bacteria (Ittekkot 1982). Glucose and fructose are the monomers of major energy-related polysaccharides in phytoplankton (e.g. glucan, fructan) and are released in large quantities following the peak of the bloom due to phytoplankton lysis and can also be released under stressed conditions such as low nutrients, elevated temperature and high light exposure (Mopper et al. 1980; Ittekkot 1982; Compiano et al. 1993; Thornton 2014). Galactose, mannose, xylose, fucose, rhamnose and arabinose comprise less labile, structurally related polysaccharides that are released by bacterial breakdown of phytoplankton cellular materials. Meanwhile, fucose, arabinose and rhamnose-containing polysaccharides are synthesised by stressed phytoplankton under nutrient deficiency, and elevated levels of these carbohydrate monomers are observed during phytoplankton bloom decay (Ittekkot 1982; Ittekkot et al. 1982; Compiano et al. 1993). In addition, polysaccharides containing rhamnose and arabinose are associated with bacterial secretions and are elevated in areas of the ocean with relatively high bacterial activity (Liebezeit et al. 1980; Ittekkot 1982; Mühlenbruch et al. 2018; Hasenecz et al. 2020). Hence, the changes in the concentrations and molecular distributions of saccharides can provide insights to biochemical processes controlling DOC in the ocean (Ittekkot 1982).
Breaking waves entrain air in sub-surface seawater. As tiny bubbles rise to the ocean surface, they scavenge surface-active materials in marine DOC (e.g. lipopolysaccharides), leading to their accumulation at the ocean surface (Mopper et al. 1995; Zhou et al. 1998; Cunliffe et al. 2013; Burrows et al. 2014). In general, bubbles that reach the surface may either burst instantly (<1 s) or persist for an extended period of time (10–100 s), producing a persistent layer of whitecap foam (Callaghan et al. 2012; Modini et al. 2013). Elevated levels of either DOC or surface active material in seawater increases bubble lifetime, leading to more persistent bubbles (Callaghan et al. 2013; Modini et al. 2013; Collins et al. 2014). As bubbles age, bubble films decrease in thickness (1 µm–100 nm) as less surface-active materials drain to the base of the bubble creating a highly organic-enriched bubble film (Modini et al. 2013; Burrows et al. 2014). Surface active materials reside at the interior and exterior surfaces of the bubble film and become enriched (Burrows et al. 2014). The drainage creates a thin (20–400 µm), chemically distinct film at the ocean surface referred to as the sea surface microlayer (SSML) (Cunliffe et al. 2013; Burrows et al. 2014). Bubble films have been predicted to exhibit higher enrichment than the SSML because of their larger surface area to volume ratio and this drainage process (Burrows et al. 2014).
The extent of the accumulation of material at the ocean surface is often quantitatively evaluated by enrichment factors (EFs). An EF for species x (saccharides) relative to sodium (Na+) in phase i (either SSML or foam) over seawater can be calculated by Eqn 1.
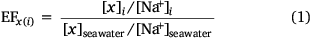
An EF greater than one indicates enrichment of species x relative to sodium in phase i, while an EF less than one indicates depletion. Prior studies have demonstrated enrichment of DOC, organic and inorganic species in the SSML (Compiano et al. 1993; Gao et al. 2012; van Pinxteren et al. 2012). In particular, DOC in the SSML has been reported to be enriched 0.8–1.6 times than in seawater (García-Flor et al. 2005; Wurl and Holmes 2008). Furthermore, enrichment of total saccharides has been observed in the SSML with EFs ranging from 0.7 to 12.1 across field and laboratory studies, indicating a selective enrichment of saccharides over bulk DOC (Compiano et al. 1993; Gao et al. 2012; van Pinxteren et al. 2012; Jayarathne et al. 2016). It has been proposed that water soluble saccharides may chemically adsorb to organic surfactants that have more affinity towards bubble films and be co-transported to the ocean surface (Burrows et al. 2016; Schill et al. 2018; Vazquez de Vasquez et al. 2022). Such an association may be even stronger with oligo/polysaccharides due to the presence of multiple binding sites (–OH) able to interact with anionic head groups (–COOH) of the surfactant molecules facilitated by multivalent cations (e.g. Mg2+, Ca2+) (Kundu et al. 2008; Li and McClements 2014; Vazquez de Vasquez et al. 2022). Furthermore, phenolic (Ar–OH), aromatic and other hydroxy (R–OH) functional groups within extracellular polymeric substances (EPS) that are produced by marine micro-organisms can self-assemble by chelation with multivalent cations (Xu et al. 2016). These macromolecules form TEP or larger DOC colloids and selectively transfer to the ocean surface via scavenging on rising bubbles and/or by positive buoyancy (Garabetian et al. 1993; Dai et al. 1998; Verdugo et al. 2004). Similarly, aggregation of colloidal dissolved organic matter via multivalent metal ions may provide a conduit from the dissolved to particulate carbon pool, and eventually form marine snow that sinks in the ocean and removes DOC from seawater (Verdugo et al. 2004).
Enrichments of saccharides have not been quantitatively evaluated for persistent bubble films. Meanwhile, it has been suggested that extremely high organic enrichment (102–103) in sea spray aerosol (SSA) may result from organic matter being selectively enriched on bubble film surfaces prior to bursting (Russell et al. 2010; Burrows et al. 2014). Collins et al. (2014) demonstrated a preferential enrichment of organic matter in SSA in the presence of foam compared to free bubble bursting at the ocean surface. Furthermore, fine SSA (with particle diameters <2.5 µm) has been demonstrated to be more enriched in saccharides than coarse SSA (with particle diameters 2.5–10 µm), which is proposed to result from fine SSA generation by the bursting of bubble films, called film drops, that have the greatest carbohydrate enrichment (Jayarathne et al. 2016). Such size-dependent saccharide enrichment in SSA has been demonstrated by several complementary techniques in numerous studies (Russell et al. 2010; Quinn et al. 2014; Jayarathne et al. 2016; Aller et al. 2017; Rastelli et al. 2017). Furthermore, it was reported that dissolved and particulate saccharides selectively transfer to fine and coarse SSA particles, respectively, which was proposed to result from the inclusion of particulate organic carbon comprised of cell wall materials into coarse particles (Jayarathne et al. 2016). On a molecular level, surface activity, chelation of divalent cations and the seawater matrix have been demonstrated to impact saccharide enrichment (Hasenecz et al. 2019). In addition, phytoplankton blooms and the enzymatic activity of heterotrophic bacteria are associated with the largest saccharide enrichments in SSA (Hasenecz et al. 2020). As the air–water interface is involved in SSA production, it is essential to understand its chemical composition in order to predict the chemical composition of SSA and its potential to influence atmospheric processes and the climate.
The central objective of this study is the quantitative assessment of saccharide enrichment in SSML and sea foam over a full phytoplankton bloom cycle. A Marine Aerosol Reference Tank (MART) was used for foam production providing an accurate mimic for wave breaking, air entrainment and bubble generation that occurs in the marine environment (Stokes et al. 2013). This study provides insight to the enrichment of saccharides in sea foam compared to SSML that is relevant to the formation of SSA at the air–water interface.
Experimental
Sample collection and preparation
A mesocosm was created in a MART following the method described by Lee et al. (2015) during the Investigation into Marine Particle Chemistry and Transfer Science (IMPACTS) laboratory study. The experiment was conducted under natural sun light using natural sea water collected at the end of Scripps Pier (La Jolla, CA; 32°52′00″N, 117°15′21″W; 275 m offshore). Guillard’s f medium diluted by a factor of two (f/2) including Na2SiO3 was used as nutrients to stimulate the growth of phytoplankton. A full list of nutrient components, their concentrations and microorganisms present in a parallel MART experiment are described in detail by Lee et al. (2015). The phytoplankton biomass of the MART was monitored immediately before the sample collection by chlorophyll-a (chl-a) levels using a Wetlabs ECO BBFL2 sensor and Turner AquaFluor handheld unit, with further details and calibration procedures provided by Wang et al. (2015).
Seawater samples were collected from 15 to 20 cm below the air–water interface of the MART using a disposable plastic pipette and SSML samples were collected from the upper 35–42 μm of the air–water interface following the membrane filter method for nine consecutive days (Cunliffe et al. 2013). Foam samples were collected from day 3 to 9 using an auto-pipette by rastering the pipette tip over the foam layer (Supplementary Fig. S1). The foam was generated by plunging the MART acheived by impacting a water sheet on the water surface to mimic the plunging jet of water from a breaking wave crest (Stokes et al. 2013; Collins et al. 2014). The MART was plunged for 5 min and accumulated foam was immediately collected. Several plunging cycles (10–15 cycles) were carried out to collect sufficient volume of foam for the chemical analysis. All samples were stored in polypropylene bottles in the dark and frozen (−20°C). Prior to chemical analysis, samples were filtered (450 nm PTFE filters, Whatman) to isolate DOC, operationally defined as solutes and colloids smaller than 450 nm. A subset of seawater, SSML and foam samples (corresponding to days 1–3, 5, 7 and 9) were subjected to ultrafiltration for fractionation of material <3 kDa and <100 kDa using Amicon centrifugal ultracel membrane filters (Sigma-Aldrich). These cutoff diameters are referenced to globular proteins and in general ~1 kDa refers to a globular protein of ~1 nm in diameter (Ogura 1974; McCarthy et al. 1996; Erickson 2009). Based on that approximation DOC smaller than 450 nm corresponds to solutes and colloids smaller than ~450 kDa.
Saccharide and DOC analysis
Instrumental analysis of saccharides was performed by high performance anion exchange chromatography (HPAEC) (Dionex-ICS 5000) with pulsed amperometric detection (PAD) following the conditions described by Jayarathne et al. (2016). Samples were analysed in four ways: (1) direct analysis to quantify free monosaccharides, (2) hydrolysis using trifluoroacetic acid (0.1 M) at 100°C for 12 h for total saccharides, (3) 3 kDa ultrafiltration and then hydrolysis and (4) 100 kDa ultrafiltration and then hydrolysis. This allowed for saccharides to be quantified in four size bins: free monosaccharides, low molecular weight (LMW) oligo- and polysaccharides less than 3 kDa, high molecular weight (HMW) polysaccharides ranging from 3 to 100 kDa and colloidal polysaccharides ranging from 100 kDa to 450 nm. Analytical uncertainties were propagated from the method detection limits and 10% of the saccharide concentration based on spike recovery samples.
DOC was analysed using a Sievers 5310C Laboratory Total Organic Carbon Analyzer (GE Instruments) and inorganic ion concentrations were analysed by ion-exchange chromatography coupled with conductivity detection as described in detail elsewhere (Jayarathne et al. 2014). The enrichment of saccharide relative to Na+ in SSML and foam with respect to seawater was calculated using Eqn 1. Equal volumes of day 1–4 and day 5–9 seawater, SSML and foam samples were composited together to represent time periods of high chl-a and bacteria, respectively.
Results and discussion
Biological activity of the seawater
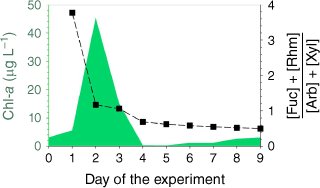
The initial chl-a concentration of the seawater was 3.1 µg L−1 (day 0) indicating a mild phytoplankton bloom was occurring in the coastal ocean (Quinn et al. 2014; Lee et al. 2015). Addition of nutrients and exposure to natural sunlight triggered the growth of phytoplankton, increasing to a maximum chl-a level of 45.4 µg L−1 on day 2 (Fig. 1). Natural levels of chl-a in the open and coastal ocean have been observed in the range of 0.03–40 µg L−1 (Cloern 1996; Quinn et al. 2014). Chl-a levels rapidly declined to a minimum of 0.24 µg L−1 on day 5. Potential reasons for this decline include nutrient deficiency, growth of heterotrophic bacteria and viruses and mechanical breakdown of phytoplankton due to plunging of the MART (Azam and Malfatti 2007; Lee et al. 2015). After day 5, chl-a levels steadily increased up to 3.1 µg L−1 by the end of experiment on day 9. The growth of phytoplankton after day 5 was likely promoted by nutrients released to the seawater by the crash of the previous phytoplankton bloom, a process that is commonly observed in the ocean (Ittekkot 1982; Norrman et al. 1995; Azam and Malfatti 2007).
|
The ratio of the sum of fucose and rhamnose concentrations to the sum of arabinose and xylose concentrations in seawater was used as an indicator for the bacterial activity in the tank (Fig. 1). Typically, ratios <1 indicate the presence of less-labile organic matter which is indicative of higher bacterial concentration (Frimmel 1998; Engbrodt and Kattner 2005; Jiao et al. 2010). In this experiment, this ratio decreased below one (0.69) on day 4, with a consistent decrease to 0.50 on day 9 indicating the growth of marine bacteria after the phytoplankton bloom (Frimmel 1998; Engbrodt and Kattner 2005).
Contribution of saccharides to DOC
The total quantified saccharide concentration (<450 nm) in seawater ranged from 0.9 to 5.1 µM and averaged 3 ± 1 µM. These concentration levels agree well with previously reported saccharide concentrations in seawater (Amon and Benner 2003; Engbrodt and Kattner 2005). The total saccharide concentration in SSML ranged from 1.4 to 51 µM, averaging 18 ± 15 µM. In foam, saccharide concentrations ranged from 11 to 32 µM and averaged 22 ± 7 µM. Total measured saccharides comprised a considerable fraction of DOC in seawater (13%), SSML (27%) and foam (31%).
Sugar alcohols
Concentrations of three sugar alcohols – xylitol, arabitol and mannitol – varied from below method detection limits to 674 ± 67 nM for mannitol on day 9 in the SSML (Supplementary Table S1). Sugar alcohol contributions to quantified total saccharides were small, contributing an average of 2.1 ± 0.8% in seawater, 2.5 ± 1.9% in SSML and 2.4 ± 0.5% in foam with a maximum contribution of 5.8% on day 9 in SSML. From this, we conclude that xylitol, arabitol and mannitol have minor contributions to the total saccharide pool in the ocean. The sugar alcohol concentration significantly increased at the latter part of the mesocosm suggesting a probable bacterial origin (Fig. 2a–c) (Pramanik et al. 2011; Dai et al. 2015). Sugar alcohols were primarily (>43%) in the form of free monosaccharides (Fig. 3a), likely due to direct release of free alditols by bacteria from lignocellulosic material breakdown (Pérez-Bibbins et al. 2016). Furthermore, a systematic distribution of sugar alcohols in different size fractions was not observed (Fig. 3), suggesting that the transfer of sugar alcohols to the ocean surface was relatively independent of particle size.

|

|
Energy-related saccharides
Concentrations of energy-related saccharides were elevated during the phytoplankton bloom, declined with bloom crashing and consistently increased thereafter (Fig. 2d, e). The high concentrations of energy-related saccharides as the phytoplankton bloom progressed were likely due to the synthesis of energy storage products (e.g. glucans, fructans) and their release to surrounding water upon cell lysis (Ittekkot 1982; Compiano et al. 1993; Mopper et al. 1995). Bacteria rapidly utilise these labile polysaccharides as energy substrates, leading to their sharp decline in concentration after the bloom crashed (Handa and Yanagi 1969). In this process, either in situ enzymatic hydrolysis or extra-cellular hydrolysis by bacterial enzymes could release free monosaccharides, producing free glucose and fructose (Mopper et al. 1980), which was observed on day 3 following the peak of the phytoplankton bloom. The steady increase of total saccharide from day 4 was likely related to breakdown of more stable structural saccharides (e.g. cellulose, hemicellulose) by bacterial hydrolysis (Hecky et al. 1973; Haug and Myklestad 1976). The dominant form of energy-related saccharides was the LMW fraction that accounted for 34–65% by mass (Fig. 3b). The colloidal fraction considerably increased in SSML and foam (27–36%) relative to seawater, likely due to enrichment of cellular materials at the air–water interface.
Structure-related saccharides
Concentrations of structural saccharides differed from the previously discussed energy-related saccharides in that they were not elevated during the phytoplankton bloom and instead steadily increased throughout the experiment (Fig. 2f–k). This is likely due to gradual bacterial degradation of more stable cellular materials, such as marine particulate organic carbon (POC) or high molecular weight DOC (Coombs and Volcani 1968; Handa and Yanagi 1969; Haug and Myklestad 1976; Liebezeit et al. 1980; Mopper et al. 1980). The saccharide composition in the ocean was observed to shift towards structural polysaccharides at the end of a mesocosm as a result of preferential bacterial uptake of LMW algal exopolymers during the initial stage, followed by feeding on less labile structural components (Chrost and Faust 1983; Gershey 1983). In this process bacteria reduce detrital organic matter to soluble foams and convert algal exopolysaccharides into bacterial polysaccharides as evident by elevated levels of structural and bacterial-related saccharides at the end of the experiment (Mopper et al. 1995). Galactose has some characteristics of energy-related saccharides such as elevated concentrations during the phytoplankton bloom and release of free galactose monosaccharide after bloom crashing. This trend is probably due to galactan, the polysaccharide form of galactose that is used as an energy storage material by some phytoplankton species (Ittekkot et al. 1982; Biersmith and Benner 1998). Ribose also showed a distinct variation pattern over the experiment (Fig. 2i) relative to other structure-related saccharides. The lysis of phytoplankton nucleotides during the phytoplankton bloom and the lysis of bacterial nucleotides at the latter part of the bloom is likely the source of ribose oligo/polysaccharides (Cowie and Hedges 1984; McCarthy et al. 1996). Unlike sugar alcohols and energy-related saccharides, structure-related saccharides showed a distinct distribution pattern in different compartments. The LMW saccharide (free–3 kDa) fraction dominated (44–59%) the seawater saccharide pool while the HMW saccharide (3–100 kDa) fraction dominated the SSML and foam (45–88%) saccharide pool (Fig. 3c). This is attributed to selective transfer of higher molecular weight oligo/polysaccharides towards ocean surface likely due to scavenging on rising bubbles due their high surface activity. Furthermore, smaller surface active oligo/polysaccharides can coagulate to form HMW polysaccharides and these could rise to the ocean surface due to positive buoyancy (Ogura 1977; McCarthy et al. 1996).
Enrichment of saccharides in foam and SSML over seawater
Total saccharides (<450 nm) were significantly enriched (P < 0.05) in SSML and foam with individual median EFs ranging from 1.7–6.4 to 2.1–12, respectively (Fig. 4). This enrichment provides evidence for scavenging of the saccharides on bubble surfaces and their accumulation at the ocean surface where SSA is generated. Median EFs for xylitol, mannitol, glucose, galactose, mannose, xylose, fucose, rhamnose and ribose demonstrated greater enrichment in foam compared to SSML. The relatively higher EFs in the foam layer provide the first quantitative evidence of further enrichment of saccharides on bubble films compared to SSML. This is attributed to thinning the bubble film by draining the seawater constituents keeping the surface active materials on the bubble film (Mopper et al. 1995; Zhou et al. 1998; Modini et al. 2013; Collins et al. 2014). This phenomenon could lead to further enrichment on the bubble film with bubble ageing and could result in an extremely enriched bubble film at the time of bursting to generate SSA that is highly enriched in organic matter and saccharides.
|
The enrichment process was dynamic with the greatest EFs occurring on days 2–4 with peak chl-a levels (Supplementary Fig. S2) indicating higher accumulation of saccharides in SSML and foam during the active bloom period. This observation demonstrates that the saccharide enrichment at the ocean surface is influenced by the biological activity of the seawater.
The size of the saccharide played a significant role in the enrichment process. LMW oligo/polysaccharides (free–3 kDa) were the major enriched species for sugar alcohols (Fig. 5a, b) and showed 2–15 times greater EFs than those for total dissolved (<450 nm) saccharides. The enrichment of energy-related saccharides was dominated by colloidal (100 kDa–450 nm) polysaccharides (Fig. 5c, d). This is likely due to bulky glucan and fructan polysaccharides and TEP which are found to be effectively transported to the ocean surface by scavenging on bubble surfaces due to their larger radius and/or positive buoyancy (Marty et al. 1988; Verdugo et al. 2004; Kuznetsova et al. 2005; Burrows et al. 2014; Dai et al. 2015). Furthermore, these bulky colloids could drain back to the SSML during the bubble ageing, thus resulting in higher EFs in SSML than foam (Supplementary Fig. S2d, e). HMW (3–100 kDa) saccharides dominated the enrichment of structure-related saccharides (Fig. 5e, f) showing a 2–4 times higher enrichment than total dissolved (<450 nm) saccharides. This enrichment was more prominent in foam than SSML. This is likely due to a higher surface activity of these saccharides than other saccharide types, and coating of interior and exterior surfaces of the bubble film by these highly surface-active saccharides (Burrows et al. 2014). In addition this could be due to the co-adsorption of HMW saccharides into organic surfactants such as fatty acids and effective transfer of them into the SSML and greater enrichment in foam (Vazquez de Vasquez et al. 2022). Interestingly, LMW structure-related oligo/polysaccharides were depleted in both SSML and foam. According to the competitive Langmuir adsorption model, there is a competition within molecules for surface area at the air–water interface, therefore the most surface-active compounds are retained at the air–water interface, while less surface-active molecules tend to be in the bulk sea water. The depletion of LMW polysaccharides in both SSML and foam could result from their low surface activity compared to HMW saccharides and colloidal polysaccharides, which show higher EFs in SSML (Burrows et al. 2014).

|
Interestingly, free monosaccharide fractions of glucose, galactose, fructose, xylitol, arabitol and mannitol were significantly enriched in SSML and foam with EFs ranging from 3 to 18 despite their complete solubility in water. This suggests a preferential movement of these water-soluble saccharides towards the ocean surface and provides experimental evidence to support the theoretical concept of the OCEANFILM-2 model that describes co-adsorption of water soluble saccharides on organic surfactants and their enrichment at the air–water interface (Burrows et al. 2016). Furthermore, these EFs of monosaccharides at the SSML is also supported by the work of Vazquez de Vasquez et al. (2022) which showed co-adsorption interactions of glucuronate which is the representative monomer of alginate at a proxy SSML (Vazquez de Vasquez et al. 2022). In addition, the enrichment of free monosaccharides at the ocean surface may also result from faster enzymatic hydrolysis rates at the air–water interface due to favourable conditions (e.g. higher temperature and dissolved oxygen), even though there are other conditions (e.g. high UV radiation) that could decrease rates of enzymatic hydrolysis (Whatley et al. 1951; Levinson 1968; Mopper et al. 1980; Compiano et al. 1993).
The EF of glucose measured in this study is compared to prior studies of its EF in SSML and SSA to gain a better understanding of the enrichment process (Table 1). The EFs of free glucose in SSML in the field study by van Pinxteren et al. (2012) and the laboratory model study by Hasenecz et al. (2019) indicate a slight depletion or no enrichment. The EF for free glucose in the SSML indicates enrichment, which could be influenced by the observed high biological activity within the active bloom period compared to these prior studies. Total glucose demonstrates enrichment in the SSML in this study and that of Jayarathne et al. (2016), with different ranges of EF likely a result of different biological systems. These increased EFs for total glucose relative to free glucose reflect the selective transfer of oligomers or polysaccharides into the SSML. In the aerosol phase, the EF further increased. Jayarathne et al. (2016) showed that the EFs of total glucose in SSA particles were greater than those in the SSML, by factors of 50–90 for PM2.5 and 15–40 for PM10. The EFs can be further magnified in smaller SSA particles, such as PM1, because of size-dependent enrichment and the presence of heterotrophic bacteria as shown in Hasenecz et al. (2020).

|
The comparison of EFs across all seawater, SSML, foam and SSA implies a connection between the enrichment of organic matter in foam and the larger EFs in SSA. With EFs for saccharides in foam exceeding those of SSML, it indicates that the maturation of bubble films at the ocean surface leads to greater organic enrichment. The process of this enrichment is likely due to the drainage of less surface-active molecules at the air–water interface, which leads to greater enrichment of highly surface-active molecules on the thin bubble film. Hence, ultimately those molecules on the thin bubble film are those that are transferred into the SSA.
Conclusions
Here we provide the first quantitative enrichment of saccharides on bubble film surfaces where SSA is generated. We observed higher saccharide enrichment in persistent foam compared to SSML, which suggests that maturation of bubbles at the air–water interface contributes to enrichment of organic matter at the ocean surface and ultimately in SSA. Structure-related saccharides, particularly those with HMW, are selectively enriched at the ocean surface. Because of the size of HMW structure-related saccharides, they are unlikely to be transferred to sub-micrometre sized particles, and are more likely to be in super-micron SSA. The quantitative results obtained from this study support the theoretical concept of free monosaccharide enrichment at the ocean surface (Burrows et al. 2014, 2016) and field observations of saccharide enrichment in SSA.
Supplementary material
The supplementary material includes a table of saccharide and sodium ion concentrations in bulk sea water, sea surface microlayer and foam (Supplementary Table S1), a picture of the marine aerosol reference tank (MART) before and after plunging (Supplementary Fig. S1), a figure of the daily variation of total saccharide (<450 nm) enrichment factors (Supplementary Fig. S2) and the daily variation of DOC (<450 nm) enrichment factors (Supplementary Fig. S3). Supplementary material is available online.
Data availability
The data that support this study are available in University of California San Diego (UCSD) Library Digital Collections (Jayarathne et al. 2022).
Conflicts of interests
The authors declare no conflicts of interest
Declaration of funding
This work was funded by the National Science Foundation through the Center of Chemical Innovation Program under grant CHE 1801971.
Acknowledgements
For their contributions to IMPACTS, the authors thank Prof. Kimberly Prather’s research group members Christopher Lee, Camille Sultana, Josh Cox, Matthew Pendergraft, Grace Irumva and Hosiana Abewe at the University of California-San Diego. The authors thank Prof. Christopher D. Cappa at the University of California-Davis, Prof. Timothy H. Bertram at the University of Wisconsin-Madison and Prof. Vicki H. Grassian at the University of California-San Diego for their contributions to designing the IMPACTS experiment. They also thank Zehra Khan and Josh Kettler at the University of Iowa for assistance with sample preparation. This paper forms part of the PhD thesis of Thilina Jayarathne entitled Chemical characterization of biomass burning and sea spray aerosol (2017) (Jayarathne 2017).
References
Aller JY, Radway JC, Kilthau WP, Bothe DW, Wilson TW, Vaillancourt RD, Quinn PK, Coffman DJ, Murray BJ, Knopf DA (2017). Size-resolved characterization of the polysaccharidic and proteinaceous components of sea spray aerosol. Atmospheric Environment 154, 331–347.| Size-resolved characterization of the polysaccharidic and proteinaceous components of sea spray aerosol.Crossref | GoogleScholarGoogle Scholar |
Aluwihare LI, Repeta DJ, Chen RF (1997). A major biopolymeric component to dissolved organic carbon in surface sea water. Nature 387, 166–169.
| A major biopolymeric component to dissolved organic carbon in surface sea water.Crossref | GoogleScholarGoogle Scholar |
Amon RMW, Benner R (2003). Combined neutral sugars as indicators of the diagenetic state of dissolved organic matter in the arctic ocean. Deep Sea Research Part I: Oceanographic Research Papers 50, 151–169.
| Combined neutral sugars as indicators of the diagenetic state of dissolved organic matter in the arctic ocean.Crossref | GoogleScholarGoogle Scholar |
Azam F, Malfatti F (2007). Microbial structuring of marine ecosystems. Nature Reviews Microbiology 5, 782–791.
| Microbial structuring of marine ecosystems.Crossref | GoogleScholarGoogle Scholar |
Biersmith A, Benner R (1998). Carbohydrates in phytoplankton and freshly produced dissolved organic matter. Marine Chemistry 63, 131–144.
| Carbohydrates in phytoplankton and freshly produced dissolved organic matter.Crossref | GoogleScholarGoogle Scholar |
Borch NH, Kirchman DL (1997). Concentration and composition of dissolved combined neutral sugars (polysaccharides) in seawater determined by hplc-pad. Marine Chemistry 57, 85–95.
| Concentration and composition of dissolved combined neutral sugars (polysaccharides) in seawater determined by hplc-pad.Crossref | GoogleScholarGoogle Scholar |
Burrows SM, Ogunro O, Frossard AA, Russell LM, Rasch PJ, Elliott SM (2014). A physically based framework for modeling the organic fractionation of sea spray aerosol from bubble film langmuir equilibria. Atmospheric Chemistry and Physics 14, 13601–13629.
| A physically based framework for modeling the organic fractionation of sea spray aerosol from bubble film langmuir equilibria.Crossref | GoogleScholarGoogle Scholar |
Burrows SM, Gobrogge E, Fu L, Link K, Elliott SM, Wang H, Walker R (2016). Oceanfilms-2: Representing coadsorption of saccharides in marine films and potential impacts on modeled marine aerosol chemistry. Geophysical Research Letters 43, 8306–8313.
| Oceanfilms-2: Representing coadsorption of saccharides in marine films and potential impacts on modeled marine aerosol chemistry.Crossref | GoogleScholarGoogle Scholar |
Callaghan AH, Deane GB, Stokes MD, Ward B (2012). Observed variation in the decay time of oceanic whitecap foam. Journal of Geophysical Research: Oceans 117, C09015
| Observed variation in the decay time of oceanic whitecap foam.Crossref | GoogleScholarGoogle Scholar |
Callaghan AH, Deane GB, Stokes MD (2013). Two regimes of laboratory whitecap foam decay: Bubble-plume controlled and surfactant stabilized. Journal of Physical Oceanography 43, 1114–1126.
| Two regimes of laboratory whitecap foam decay: Bubble-plume controlled and surfactant stabilized.Crossref | GoogleScholarGoogle Scholar |
Chrost RH, Faust MA (1983). Organic carbon release by phytoplankton: Its composition and utilization by bacterioplankton. Journal of Plankton Research 5, 477–493.
| Organic carbon release by phytoplankton: Its composition and utilization by bacterioplankton.Crossref | GoogleScholarGoogle Scholar |
Cloern JE (1996). Phytoplankton bloom dynamics in coastal ecosystems: A review with some general lessons from sustained investigation of san francisco bay, california. Reviews of Geophysics 34, 127–168.
| Phytoplankton bloom dynamics in coastal ecosystems: A review with some general lessons from sustained investigation of san francisco bay, california.Crossref | GoogleScholarGoogle Scholar |
Collins DB, Zhao DF, Ruppel MJ, Laskina O, Grandquist JR, Modini RL, Stokes MD, Russell LM, Bertram TH, Grassian VH, Deane GB, Prather KA (2014). Direct aerosol chemical composition measurements to evaluate the physicochemical differences between controlled sea spray aerosol generation schemes. Atmospheric Measurement Techniques 7, 3667–3683.
| Direct aerosol chemical composition measurements to evaluate the physicochemical differences between controlled sea spray aerosol generation schemes.Crossref | GoogleScholarGoogle Scholar |
Compiano A-M, Romano J-C, Garabetian F, Laborde P, De La Giraudièrea I (1993). Monosaccharide composition of particulate hydrolysable sugar fraction in surface microlayers from brackish and marine waters. Marine Chemistry 42, 237–251.
| Monosaccharide composition of particulate hydrolysable sugar fraction in surface microlayers from brackish and marine waters.Crossref | GoogleScholarGoogle Scholar |
Coombs J, Volcani BE (1968). Studies on the biochemistry and fine structure of silica shell formation in diatoms. Planta 80, 264–279.
| Studies on the biochemistry and fine structure of silica shell formation in diatoms.Crossref | GoogleScholarGoogle Scholar |
Cowie GL, Hedges JI (1984). Carbohydrate sources in a coastal marine environment. Geochimica et Cosmochimica Acta 48, 2075–2087.
| Carbohydrate sources in a coastal marine environment.Crossref | GoogleScholarGoogle Scholar |
Cunliffe M, Engel A, Frka S, Gašparović B, Guitart C, Murrell JC, Salter M, Stolle C, Upstill-Goddard R, Wurl O (2013). Sea surface microlayers: A unified physicochemical and biological perspective of the air–ocean interface. Progress in Oceanography 109, 104–116.
| Sea surface microlayers: A unified physicochemical and biological perspective of the air–ocean interface.Crossref | GoogleScholarGoogle Scholar |
Dai Z, Dukhin S, Fornasiero D, Ralston J (1998). The inertial hydrodynamic interaction of particles and rising bubbles with mobile surfaces. Journal of Colloid and Interface Science 197, 275–292.
| The inertial hydrodynamic interaction of particles and rising bubbles with mobile surfaces.Crossref | GoogleScholarGoogle Scholar |
Dai XF, Shi XC, Gao X, Liang J, Zhang X-H (2015). Salipiger nanhaiensis sp. nov., a bacterium isolated from deep sea water. International Journal of Systematic and Evolutionary Microbiology 65, 1122–1126.
| Salipiger nanhaiensis sp. nov., a bacterium isolated from deep sea water.Crossref | GoogleScholarGoogle Scholar |
Dean Pakulski J, Benner R (1992). An improved method for the hydrolysis and mbth analysis of dissolved and particulate carbohydrates in seawater. Marine Chemistry 40, 143–160.
| An improved method for the hydrolysis and mbth analysis of dissolved and particulate carbohydrates in seawater.Crossref | GoogleScholarGoogle Scholar |
Engbrodt R, Kattner G (2005). On the biogeochemistry of dissolved carbohydrates in the greenland sea (arctic). Organic Geochemistry 36, 937–948.
| On the biogeochemistry of dissolved carbohydrates in the greenland sea (arctic).Crossref | GoogleScholarGoogle Scholar |
Erickson HP (2009). Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biological Procedures Online 11, 32–51.
| Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy.Crossref | GoogleScholarGoogle Scholar |
Frimmel FH (1998). Characterization of natural organic matter as major constituents in aquatic systems. Journal of Contaminant Hydrology 35, 201–216.
| Characterization of natural organic matter as major constituents in aquatic systems.Crossref | GoogleScholarGoogle Scholar |
Gao Q, Leck C, Rauschenberg C, Matrai PA (2012). On the chemical dynamics of extracellular polysaccharides in the high arctic surface microlayer. Ocean Science 8, 401–418.
| On the chemical dynamics of extracellular polysaccharides in the high arctic surface microlayer.Crossref | GoogleScholarGoogle Scholar |
Garabetian F, Romano J-C, Paul R, Sigoillot J-C (1993). Organic matter composition and pollutant enrichment of sea surface microlayer inside and outside slicks. Marine Environmental Research 35, 323–339.
| Organic matter composition and pollutant enrichment of sea surface microlayer inside and outside slicks.Crossref | GoogleScholarGoogle Scholar |
García-Flor N, Guitart C, Ábalos M, Dachs J, Bayona JM, Albaigés J (2005). Enrichment of organochlorine contaminants in the sea surface microlayer: An organic carbon-driven process. Marine Chemistry 96, 331–345.
| Enrichment of organochlorine contaminants in the sea surface microlayer: An organic carbon-driven process.Crossref | GoogleScholarGoogle Scholar |
Gershey RM (1983). Characterization of seawater organic matter carried by bubble-generated aerosols. Limnology and Oceanography 28, 309–319.
| Characterization of seawater organic matter carried by bubble-generated aerosols.Crossref | GoogleScholarGoogle Scholar |
Handa N, Yanagi K (1969). Studies on water-extractable carbohydrates of the particulate matter from the northwest pacific ocean. Marine Biology 4, 197–207.
| Studies on water-extractable carbohydrates of the particulate matter from the northwest pacific ocean.Crossref | GoogleScholarGoogle Scholar |
Hasenecz ES, Kaluarachchi CP, Lee HD, Tivanski AV, Stone EA (2019). Saccharide transfer to sea spray aerosol enhanced by surface activity, calcium, and protein interactions. ACS Earth and Space Chemistry 3, 2539–2548.
| Saccharide transfer to sea spray aerosol enhanced by surface activity, calcium, and protein interactions.Crossref | GoogleScholarGoogle Scholar |
Hasenecz ES, Jayarathne T, Pendergraft MA, Santander MV, Mayer KJ, Sauer J, Lee C, Gibson WS, Kruse SM, Malfatti F, Prather KA, Stone EA (2020). Marine bacteria affect saccharide enrichment in sea spray aerosol during a phytoplankton bloom. ACS Earth and Space Chemistry 4, 1638–1649.
| Marine bacteria affect saccharide enrichment in sea spray aerosol during a phytoplankton bloom.Crossref | GoogleScholarGoogle Scholar |
Haug A, Myklestad S (1976). Polysaccharides of marine diatoms with special reference to Chaetoceros species. Marine Biology 34, 217–222.
| Polysaccharides of marine diatoms with special reference to Chaetoceros species.Crossref | GoogleScholarGoogle Scholar |
Hecky RE, Mopper K, Kilham P, Degens ET (1973). The amino acid and sugar composition of diatom cell-walls. Marine Biology 19, 323–331.
| The amino acid and sugar composition of diatom cell-walls.Crossref | GoogleScholarGoogle Scholar |
Henrichs SM, Williams PM (1985). Dissolved and particulate amino acids and carbohydrates in the sea surface microlayer. Marine Chemistry 17, 141–163.
| Dissolved and particulate amino acids and carbohydrates in the sea surface microlayer.Crossref | GoogleScholarGoogle Scholar |
Hung C-C, Tang D, Warnken KW, Santschi PH (2001). Distributions of carbohydrates, including uronic acids, in estuarine waters of galveston bay. Marine Chemistry 73, 305–318.
| Distributions of carbohydrates, including uronic acids, in estuarine waters of galveston bay.Crossref | GoogleScholarGoogle Scholar |
Ittekkot V (1982). Variations of dissolved organic matter during a plankton bloom: Qualitative aspects, based on sugar and amino acid analyses. Marine Chemistry 11, 143–158.
| Variations of dissolved organic matter during a plankton bloom: Qualitative aspects, based on sugar and amino acid analyses.Crossref | GoogleScholarGoogle Scholar |
Ittekkot V, Degens ET, Brockmann U (1982). Monosaccharide composition of acid‐hydrolyzable carbohydrates in particulate matter during a plankton bloom1. Limnology and Oceanography 27, 770–776.
| Monosaccharide composition of acid‐hydrolyzable carbohydrates in particulate matter during a plankton bloom1.Crossref | GoogleScholarGoogle Scholar |
Jayarathne T (2017) Chemical characterization of biomass burning and sea spray aerosol. PhD thesis, University of Iowa, Iowa City, IA, USA.
| Crossref |
Jayarathne T, Stockwell CE, Yokelson RJ, Nakao S, Stone EA (2014). Emissions of fine particle fluoride from biomass burning. Environmental Science & Technology 48, 12636–12644.
| Emissions of fine particle fluoride from biomass burning.Crossref | GoogleScholarGoogle Scholar |
Jayarathne T, Sultana CM, Lee C, Malfatti F, Cox JL, Pendergraft MA, Moore KA, Azam F, Tivanski AV, Cappa CD, Bertram TH, Grassian VH, Prather KA, Stone EA (2016). Enrichment of saccharides and divalent cations in sea spray aerosol during two phytoplankton blooms. Environmental Science & Technology 50, 11511–11520.
| Enrichment of saccharides and divalent cations in sea spray aerosol during two phytoplankton blooms.Crossref | GoogleScholarGoogle Scholar |
Jayarathne T, Gamage DK, Prather KA, Stone EA (2022) Data from: Enrichment of saccharides at air-water interface: A quantitative comparison of sea surface microlayer and foam [Dataset]. UC San Diego Library Digital Collections.
| Crossref |
Jiao N, Herndl GJ, Hansell DA, Benner R, Kattner G, Wilhelm SW, Kirchman DL, Weinbauer MG, Luo T, Chen F, Azam F (2010). Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nature Reviews Microbiology 8, 593–599.
| Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean.Crossref | GoogleScholarGoogle Scholar |
Kundu S, Langevin D, Lee LT (2008). Neutron reflectivity study of the complexation of DNA with lipids and surfactants at the surface of water. Langmuir 24, 12347–12353.
| Neutron reflectivity study of the complexation of DNA with lipids and surfactants at the surface of water.Crossref | GoogleScholarGoogle Scholar |
Kuznetsova M, Lee C, Aller J (2005). Characterization of the proteinaceous matter in marine aerosols. Marine Chemistry 96, 359–377.
| Characterization of the proteinaceous matter in marine aerosols.Crossref | GoogleScholarGoogle Scholar |
Larsson K, Odham G, Södergren A (1974). On lipid surface films on the sea. I. A simple method for sampling and studies of composition. Marine Chemistry 2, 49–57.
| On lipid surface films on the sea. I. A simple method for sampling and studies of composition.Crossref | GoogleScholarGoogle Scholar |
Lee C, Sultana CM, Collins DB, Santander MV, Axson JL, Malfatti F, Cornwell GC, Grandquist JR, Deane GB, Stokes MD, Azam F, Grassian VH, Prather KA (2015). Advancing model systems for fundamental laboratory studies of sea spray aerosol using the microbial loop. The Journal of Physical Chemistry A 119, 8860–8870.
| Advancing model systems for fundamental laboratory studies of sea spray aerosol using the microbial loop.Crossref | GoogleScholarGoogle Scholar |
Levinson AA (1968). Oceans (foundations of earth science series). Geochimica et Cosmochimica Acta 32, 1367–1368.
| Oceans (foundations of earth science series).Crossref | GoogleScholarGoogle Scholar |
Li Y, McClements DJ (2014). Modulating lipid droplet intestinal lipolysis by electrostatic complexation with anionic polysaccharides: Influence of cosurfactants. Food Hydrocolloids 35, 367–374.
| Modulating lipid droplet intestinal lipolysis by electrostatic complexation with anionic polysaccharides: Influence of cosurfactants.Crossref | GoogleScholarGoogle Scholar |
Liebezeit G, Bolter M, Brown I, Dawson R (1980). Dissolved free amino-acids and carbohydrates at pycnocline boundaries in the sargasso sea and related microbial activity. Oceanologica Acta 3, 357–362.
Marty JC, Ẑutić V, Precali R, Saliot A, Ćosović B, Smodlaka N, Cauwet G (1988). Organic matter characterization in the northern adriatic sea with special reference to the sea surface microlayer. Marine Chemistry 25, 243–263.
| Organic matter characterization in the northern adriatic sea with special reference to the sea surface microlayer.Crossref | GoogleScholarGoogle Scholar |
McCarthy M, Hedges J, Benner R (1996). Major biochemical composition of dissolved high molecular weight organic matter in seawater. Marine Chemistry 55, 281–297.
| Major biochemical composition of dissolved high molecular weight organic matter in seawater.Crossref | GoogleScholarGoogle Scholar |
Modini RL, Russell LM, Deane GB, Stokes MD (2013). Effect of soluble surfactant on bubble persistence and bubble-produced aerosol particles. Journal of Geophysical Research: Atmospheres 118, 1388–1400.
| Effect of soluble surfactant on bubble persistence and bubble-produced aerosol particles.Crossref | GoogleScholarGoogle Scholar |
Mopper K, Dawson R, Liebezeit G, Ittekkot V (1980). The monosaccharide spectra of natural waters. Marine Chemistry 10, 55–66.
| The monosaccharide spectra of natural waters.Crossref | GoogleScholarGoogle Scholar |
Mopper K, Zhou J, Sri Ramana K, Passow U, Dam HG, Drapeau DT (1995). The role of surface-active carbohydrates in the flocculation of a diatom bloom in a mesocosm. Deep Sea Research Part II: Topical Studies in Oceanography 42, 47–73.
| The role of surface-active carbohydrates in the flocculation of a diatom bloom in a mesocosm.Crossref | GoogleScholarGoogle Scholar |
Mühlenbruch M, Grossart H-P, Eigemann F, Voss M (2018). Mini-review: Phytoplankton-derived polysaccharides in the marine environment and their interactions with heterotrophic bacteria. Environmental Microbiology 20, 2671–2685.
| Mini-review: Phytoplankton-derived polysaccharides in the marine environment and their interactions with heterotrophic bacteria.Crossref | GoogleScholarGoogle Scholar |
Norrman B, Zwelfel UL, Hopkinson CS, Brian F (1995). Production and utilization of dissolved organic carbon during an experimental diatom bloom. Limnology and Oceanography 40, 898–907.
| Production and utilization of dissolved organic carbon during an experimental diatom bloom.Crossref | GoogleScholarGoogle Scholar |
Ogura N (1974). Molecular weight fractionation of dissolved organic matter in coastal seawater by ultrafiltration. Marine Biology 24, 305–312.
| Molecular weight fractionation of dissolved organic matter in coastal seawater by ultrafiltration.Crossref | GoogleScholarGoogle Scholar |
Ogura N (1977). High molecular weight organic matter in seawater. Marine Chemistry 5, 535–549.
| High molecular weight organic matter in seawater.Crossref | GoogleScholarGoogle Scholar |
Pérez-Bibbins B, Torrado-Agrasar A, Salgado JM, Mussatto SI, Domínguez JM (2016). Xylitol production in immobilized cultures: A recent review. Critical Reviews in Biotechnology 36, 691–704.
| Xylitol production in immobilized cultures: A recent review.Crossref | GoogleScholarGoogle Scholar |
Pramanik A, Sundararaman M, Das S, Ghosh U, Mukherjee J (2011). Isolation and characterization of cyanobacteria possessing antimicrobial activity from the sundarbans, the world’s largest tidal mangrove forest. Journal of Phycology 47, 731–743.
| Isolation and characterization of cyanobacteria possessing antimicrobial activity from the sundarbans, the world’s largest tidal mangrove forest.Crossref | GoogleScholarGoogle Scholar |
Quinn PK, Bates TS, Schulz KS, Coffman DJ, Frossard AA, Russell LM, Keene WC, Kieber DJ (2014). Contribution of sea surface carbon pool to organic matter enrichment in sea spray aerosol. Nature Geoscience 7, 228–232.
| Contribution of sea surface carbon pool to organic matter enrichment in sea spray aerosol.Crossref | GoogleScholarGoogle Scholar |
Rastelli E, Corinaldesi C, Dell’anno A, Lo Martire M, Greco S, Cristina Facchini M, Rinaldi M, O’dowd C, Ceburnis D, Danovaro R (2017). Transfer of labile organic matter and microbes from the ocean surface to the marine aerosol: An experimental approach. Scientific Reports 7, 11475
| Transfer of labile organic matter and microbes from the ocean surface to the marine aerosol: An experimental approach.Crossref | GoogleScholarGoogle Scholar |
Russell LM, Hawkins LN, Frossard AA, Quinn PK, Bates TS (2010). Carbohydrate-like composition of submicron atmospheric particles and their production from ocean bubble bursting. Proceedings of the National Academy of Sciences 107, 6652–6657.
| Carbohydrate-like composition of submicron atmospheric particles and their production from ocean bubble bursting.Crossref | GoogleScholarGoogle Scholar |
Schill S, Burrows S, Hasenecz E, Stone E, Bertram T (2018). The impact of divalent cations on the enrichment of soluble saccharides in primary sea spray aerosol. Atmosphere 9, 476
| The impact of divalent cations on the enrichment of soluble saccharides in primary sea spray aerosol.Crossref | GoogleScholarGoogle Scholar |
Skoog A, Benner R (1997). Aldoses in various size fractions of marine organic matter: Implications for carbon cycling. Limnology and Oceanography 42, 1803–1813.
| Aldoses in various size fractions of marine organic matter: Implications for carbon cycling.Crossref | GoogleScholarGoogle Scholar |
Stokes MD, Deane GB, Prather K, Bertram TH, Ruppel MJ, Ryder OS, Brady JM, Zhao D (2013). A marine aerosol reference tank system as a breaking wave analogue for the production of foam and sea-spray aerosols. Atmospheric Measurement Techniques 6, 1085–1094.
| A marine aerosol reference tank system as a breaking wave analogue for the production of foam and sea-spray aerosols.Crossref | GoogleScholarGoogle Scholar |
Thornton DCO (2014). Dissolved organic matter (dom) release by phytoplankton in the contemporary and future ocean. European Journal of Phycology 49, 20–46.
| Dissolved organic matter (dom) release by phytoplankton in the contemporary and future ocean.Crossref | GoogleScholarGoogle Scholar |
van Pinxteren M, Müller C, Iinuma Y, Stolle C, Herrmann H (2012). Chemical characterization of dissolved organic compounds from coastal sea surface microlayers (baltic sea, germany. Environmental Science & Technology 46, 10455–10462.
| Chemical characterization of dissolved organic compounds from coastal sea surface microlayers (baltic sea, germany.Crossref | GoogleScholarGoogle Scholar |
Vazquez de Vasquez MG, Rogers MM, Carter-Fenk KA, Allen HC (2022). Discerning poly- and monosaccharide enrichment mechanisms: Alginate and glucuronate adsorption to a stearic acid sea surface microlayer. ACS Earth and Space Chemistry 6, 1581–1595.
| Discerning poly- and monosaccharide enrichment mechanisms: Alginate and glucuronate adsorption to a stearic acid sea surface microlayer.Crossref | GoogleScholarGoogle Scholar |
Verdugo P, Alldredge AL, Azam F, Kirchman DL, Passow U, Santschi PH (2004). The oceanic gel phase: A bridge in the dom–pom continuum. Marine Chemistry 92, 67–85.
| The oceanic gel phase: A bridge in the dom–pom continuum.Crossref | GoogleScholarGoogle Scholar |
Wang X, Sultana CM, Trueblood J, Hill TCJ, Malfatti F, Lee C, Laskina O, Moore KA, Beall CM, Mccluskey CS, Cornwell GC, Zhou Y, Cox JL, Pendergraft MA, Santander MV, Bertram TH, Cappa CD, Azam F, DeMott PJ, Grassian VH, Prather KA (2015). Microbial control of sea spray aerosol composition: A tale of two blooms. ACS Central Science 1, 124–131.
| Microbial control of sea spray aerosol composition: A tale of two blooms.Crossref | GoogleScholarGoogle Scholar |
Whatley FR, Ordin L, Arnon DI (1951). Distribution of micronutrient metals in leaves and chloroplast fragments. Plant Physiology, 26, 414–418.
| Distribution of micronutrient metals in leaves and chloroplast fragments.Crossref | GoogleScholarGoogle Scholar |
Wurl O, Holmes M (2008). The gelatinous nature of the sea-surface microlayer. Marine Chemistry 110, 89–97.
| The gelatinous nature of the sea-surface microlayer.Crossref | GoogleScholarGoogle Scholar |
Xu H, Lv H, Liu X, Wang P, Jiang H (2016). Electrolyte cations binding with extracellular polymeric substances enhanced Microcystis aggregation: Implication for Microcystis bloom formation in eutrophic freshwater lakes. Environmental Science & Technology 50, 9034–9043.
| Electrolyte cations binding with extracellular polymeric substances enhanced Microcystis aggregation: Implication for Microcystis bloom formation in eutrophic freshwater lakes.Crossref | GoogleScholarGoogle Scholar |
Zhou J, Mopper K, Passow U (1998). The role of surface‐active carbohydrates in the formation of transparent exopolymer particles by bubble adsorption of seawater. Limnology and Oceanography 43, 1860–1871.
| The role of surface‐active carbohydrates in the formation of transparent exopolymer particles by bubble adsorption of seawater.Crossref | GoogleScholarGoogle Scholar |


