Selenium cycling in a marine dominated estuary: Lake Macquarie, NSW, Australia a case study
William A. Maher A * , Graeme E. Batley
A * , Graeme E. Batley  B , Frank Krikowa A , Michael J. Ellwood
B , Frank Krikowa A , Michael J. Ellwood  A , Jaimie Potts C , Rebecca Swanson C and Peter Scanes C
A , Jaimie Potts C , Rebecca Swanson C and Peter Scanes C
A Research School of Earth Sciences, Australian National University, Canberra, ACT 2601, Australia.
B CSIRO Land and Water, Lucas Heights, NSW 2232, Australia.
C Science, Economics and Insights Division, NSW Department of Planning and Environment, Sydney, NSW 2141, Australia.

William Maher is the Professor of Environmental Chemistry at the Australian National University. He has a sustained trace record in the understanding biogeochemical cycling of trace metals, metalloids and nutrients in aquatic ecosystems, development of water quality and sampling guidelines and development of analytical procedures for measuring trace contaminants in water, sediment and biota. He has published over 300 papers. He was awarded the RACI Analytical Divisions medal in 2002 and the RACI Environmental Chemistry Divisions medal in 2004. He also co-authored a Handbook of Sediment Quality Assessment that won the Eureka Prize for water research 2006. Recently he was awarded the University of Canberra Institute for Applied Ecology-Senior scientist award 2012. |

Graeme Batley is a Post-retirement Fellow with CSIRO Land and Water in Sydney. He is a leading researcher in the area of trace contaminants in aquatic systems, with a focus on contaminant speciation, bioavailability and toxicity in waters and sediments. He has played an ongoing major role in the development of a water and sediment quality guidelines for Australia and New Zealand. In 2022, he was inducted as a Member of the Order of Australia for significant service to environmental toxicology and chemical science. |

Frank Krikowa I have been employed as the Laboratory Manager of the EcoChemistry Laboratory, at the University of Canberra, since its creation in 1997. I have worked in the fields of Atomic Absorption Spectroscopy, Inductively Coupled Mass Spectrometry, the cycling of trace metals and nutrients in the environment. I have developed new methods, techniques and approaches in analytical and environmental chemistry. This requires in-depth knowledge of Quality Assurance procedures. |

Michael Ellwood is a Professor of Marine Biogeochemistry in the Research School of Earth Sciences at the Australian National University. His research interests include understanding processes influencing trace element speciation and distributions in natural waters, trace metal incorporation into marine organisms, and developing analytical methods for aquatic chemistry. |

Jaimie Potts Experienced Senior Environmental Scientist within the government sector. Skilled in Life Sciences, Ecology, Biogeochemistry, Water Quality Monitoring, Estuary Health Assessments and Impact of Urban and Industrial Pollution on Estuary Condition. Passionate ocean lover and avid surfer with a Doctor of Philosophy (Ph.D.) in Ecochemistry from the University of Canberra. |

Rebecca Swanson has worked as an Environmental Scientist at the NSW Department of Planning and Environment since 2012 in the Estuaries and Catchments science team. Rebecca has worked on a range of projects with local government and state agencies to research and monitor the impacts of industrial, urban and agricultural land use on water quality and ecological health of the receiving waterways. |

Dr Peter Scanes is an Honorary Research Fellow, Waters and Coastal Science Section, Department of Planning Industry and Environment. Peter has a PhD from in marine ecology University of Sydney and has worked as an estuary and coastal river ecologist for over 30 years. |
Environmental Chemistry 19(4) 132-143 https://doi.org/10.1071/EN22032
Submitted: 11 April 2022 Accepted: 7 June 2022 Published: 27 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Environmental context. Knowledge of the fate of selenium in estuaries receiving inputs from coal-fired power stations is essential as these environments are important nursery habitats for marine life and selenium has been shown to cause fish and bird mortality and sublethal effects including oedema, chromosomal aberrations and reproductive success. Understanding selenium cycling allows risk assessment to be undertaken and appropriate action to protect resident organisms.
Abstract. The fate of selenium (Se) inputs from coal-fired power station operations in a marine dominated estuary, Lake Macquarie NSW, is explored, as well as Se toxicity, including sublethal and population effects. Selenium is rapidly adsorbed to sediments, and food webs are based on benthic food sources. Selenium is remobilised from sediments by volatilisation and diffusional processes following bioturbation. It is then transferred into food chains via benthic microalgae, deposit feeders and filter-feeding organisms processing suspended sediments. Historically, Se has been found to accumulate in fish to levels above those considered safe for human consumption. After the remediation of a major ash dam in 1995, Se inputs to Lake Macquarie have declined, and the Se concentrations of sediments have also reduced partially due to the deposition of cleaner sediment but also due to the formation of volatile dimethyl selenide. Bioturbation of oxidised surface sediments also results in the release of inorganic Se. In response to decreases in sediment Se concentrations, molluscs and fish Se concentrations have also reduced below deleterious levels, with most fish now being safe for human consumption. Selenium cycling involves the transformation of inorganic species (Se0, SeII, SeIV, SeVI) in sediments and the water column to dimethylselenide and dimethyl diselenide by bacteria with the accumulation of organic Se species in plant detritus (selenomethionine) and animals (selenomethionine and selenocysteine). Dissolved Se concentrations in Lake Macquarie, except near ash dam inputs, have always been well below those that cause toxicity. There is evidence based on Se sediment-spiking studies, however, that Se is probably causing sublethal effects. When undertaking risk assessments of Se, careful consideration should be given to understanding the fate of Se inputs and remobilisation into food webs as not all systems act in accordance with published studies that generally have high Se concentrations in the water column and phytoplankton-based food webs.
Keywords: bioaccumulation, biogeochemical cycling, biomagnification, inputs, Lake Macquarie, selenium, speciation, toxicity.
Introduction
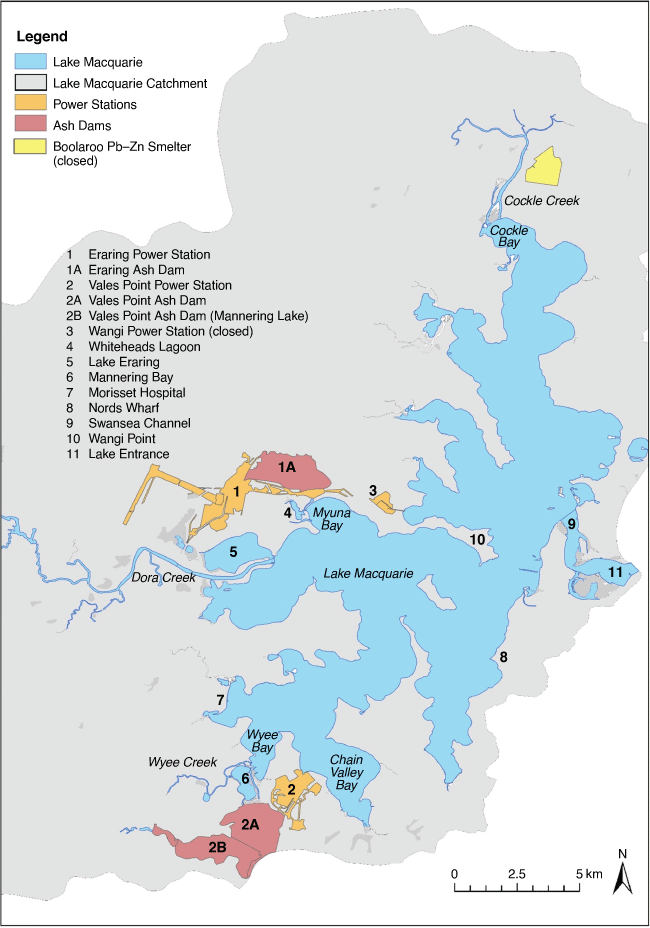
Selenium (Se) is released during coal combustion, and many international reports have documented Se contamination from coal-fired power stations (Besser et al. 1996; Riedel et al. 1996; Lemly 2002, 2004; Chapman et al. 2010). At Lake Macquarie, New South Wales, Australia, coal-fired power stations have been in operation since 1956. These were situated around Lake Macquarie because of the existence of large coal deposits nearby, the availability of water from the estuary for cooling purposes and the proximity to major population centres in Sydney and Newcastle.
At Lake Macquarie, the power stations built ash dams on the margins of the lake to store the ash from coal combustion (Fig. 1). During wet weather periods, historically, the ash dams have discharged Se into the lake and its contamination of sediments and biota, including bioaccumulation and transfer through the lake’s food web, has been well documented (Furner 1979; Roy and Crawford 1984; Batley et al. 1992, 1993a, 1993b; Kilby and Batley 1993; Peters et al. 1997, 1999a, 1999b; Jung et al. 1998; Kirby et al. 2001a, 2001b; Barwick and Maher 2003; Roach 2005; Roach et al. 2008; Schneider et al. 2014).
Selenium contamination of aquatic ecosystems has been shown to cause fish and bird mortality (Gillespie and Baumann 1986; Hamilton 2004; Ohlendorf et al. 2020) and sublethal effects including oedema, reduced hematocrit and haemoglobin levels, swollen gill lamella with extensive vacuolation, degeneration of ovarian follicles and liver, myocardial and pericardial damage, chromosomal aberrations and reduced reproductive success (Krishnaja and Rege 1982; Sorensen and Bauer 1983; Sorensen et al. 1984; Gillespie and Baumann 1986; Coyle et al. 1993). Concern about the Se contamination of Lake Macquarie became public after the release of a report (Roberts 1994) containing Se concentrations in mullet (Mugil cephalus) and silver biddy (Gerres subfasciatus) from areas close to coal-fired power stations, that at the time were up to 12 times the acceptable limit for human consumption (1 µg g–1 wet mass, NFA (National Food Authority) 1992). Subsequently, other studies of Se concentrations in fish have also shown elevated Se concentrations (Wlodarczkyk and Beath 1997; Roach et al. 2008)
The published literature on Se-contaminated freshwater lakes and estuaries (e.g. Hyco Reservoir North Carolina, Belews Lake North Carolina, Kesterson Reservoir California, Lake Sutton North Carolina, San Francisco Bay, USA), is skewed to studies where Se concentrations in water are typically high ~10 µg L–1 but can be up to 122 µg L–1 (Lemly 1985; Saiki and Lowe 1987; Riedel et al. 1996; Chapman et al. 2010) and transfer through food webs is primarily via phytoplankton (Cloern 1996; Riedel et al. 1996; Doblin et al. 2006). Selenium concentrations in the water column of Lake Macquarie are, by contrast, generally low (<0.05–0.3 µg L–1) as partitioning of Se to sediments occurs. In addition, phytoplankton levels are very low and food webs are sediment based (Thomson 1959). This case study is presented to outline the major processes in a marine benthic food web system where the setting of selenium water quality criteria will not be protective of aquatic ecosystems and human fish consumers.
The question we address is whether Se deposited in sediments is substantially remobilised by biological processes. Specifically, we discuss Se partitioning from water to suspended and benthic sediments, its subsequent remobilisation by the microbial formation of volatile Se species, and bioturbation and Se accumulation by organisms and its transfer through food webs. In addition, we explore Se toxicity, including sublethal and population effects.
Study location
Lake Macquarie estuary is situated approximately 90 km north of Sydney and close to the city of Newcastle. The lake extends approximately 22 km in a north–south direction; it has a maximum width of about 10 km (Fig. 1), a maximum depth of approximately 11 m and an average depth of 8 m (Maunsell and Partners 1974). The lake is separated from the ocean by a narrow entrance channel and sand-bars at Swansea. The tidal range in Lake Macquarie is small, with the spring tidal range being estimated at 0.11 m over the lake basin (MHL 1997). Despite this poor tidal exchange, the lake has a marine character because there is minimal freshwater dilution from the two main fluvial inputs. Shallows between Swansea and Wangi Point effectively prevent deep-water movement within the lake, resulting in a division of the lake into northern and southern components about this latitudinal axis (Fig. 1). As such, water movements in the two portions of the lake are essentially independent (Spencer 1959). Electricity is currently generated from the burning of coal at the Eraring and Vales Point Power Stations and was previously generated at Wangi Power Station (Fig. 1). The Wangi Power Station was decommissioned in 1986 and replaced with the more modern station at Eraring. Vales Point started operations in 1963 and Eraring Power Station in 1981. Both power stations are still operating and have a combined output of 4200 MW, which is a large part of NSW’s generating capacity.
Selenium is derived mainly from fly ash, not bottom ash, with both ending up in ash dams. Ash dam overflows are the primary source of Se to Lake Macquarie (Swaine 1985; Kilby and Batley 1993; Schneider et al. 2014), with Se being soluble in the alkaline ash dam waters. Historically, the Vales Point ash dam (Mannering Lake) discharged into Mannering Bay. This ash dam was semi-enclosed with an outlet via Wyee Creek which feeds Wyee Bay located in the southern part of Lake Macquarie (Fig. 1). Selenium inputs as a result of ash dam discharges led to elevated Se concentrations in surface sediments. In 1995, remediation of the major ash dam at the Vales Point Power Station was undertaken. Since then, water has been removed from the ash dam and recycled back to the power station, where it is mixed with cooling water before being discharged into the bay. This has successfully prevented most of the Se stored in the ash dam from being directly released to the lake, however, it still acts as a conduit for the ash dam water to the lake, albeit diluted.
In the northern part of the lake, the Boolaroo lead–zinc smelter was in operation from 1897 to 2003, resulting in the contamination of Cockle Creek and the northern reaches of Lake Macquarie with primarily Cu, Pb, Zn and Cd (Burt et al. 2007) but there was also some Se contamination.
Selenium partitioning from water to suspended and benthic sediments
Aqueous and suspended sediment Se
Dissolved Se concentrations in waters from around the lake are generally low (<0.05–0.3 µg L–1) and are reflective of the Pacific Ocean (~0.06 µg L–1) and indicate that Se partitioning to sediments and transport by particles is occurring. Exceptions are near the power stations and ash dams where high dissolved Se concentrations have historically been measured in Whiteheads Lagoon (36 µg L–1), Mannering Bay (34 µg L–1), Wyee Creek (11.3 ± 0.4 µg L–1) and other inflows near the two power plants (0.45–1.33 µg L–1) (Batley, unpublished results). Hydrodynamic modelling of water flow patterns indicates that a flow regime for the lake favours suspended particle-associated contaminant deposition near the Morisset Hospital (Fig. 1; Ellwood et al. 2015; Schneider et al. 2016) driven by southerly/southeasterly wind-driven currents. Cooling water discharged continuously from both power stations and the flows from Dora and Wyee Creeks also contribute to the water movement, moving dissolved Se away from the power stations. Using a hydrological model, a density particle model was built based on suspended particles released hypothetically from the ash dams during 1 month to explain Se distribution in the lake’s benthic sediments (Schneider et al. 2016). Particles released from the Vales Point ash dam go north due to the water inflow and west as a result of wind effects. The model also showed that particles started losing kinetic energy near Morisset Hospital (Fig. 1), where most of the particles released by Vales Point would settle creating a Se contamination hot spot. Particles released by Eraring Power Station, via Whiteheads Lagoon, are less influenced by water flow and winds and are expected to settle closer to the point of particle release in Myuna Bay.
Selenium in benthic sediments
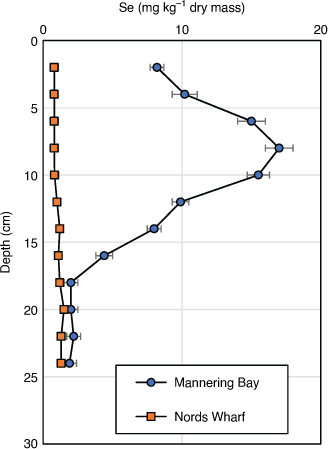
Pre 2000, the Se concentration in surface sediments in Mannering Bay (9 ± 2 mg kg–1 dry mass), Chain Valley Bay (6 ± 3 mg kg–1 dry mass) and Wyee Bay (3 ± 1 mg kg–1 dry mass) were significantly higher than other lake sites (0.4–2.5 mg kg–1 dry mass) (Peters et al. 1999a) and those found in other Australian uncontaminated locations (Maher and Batley 1990). Sediments sampled close to Vales Point Power Station had Se concentrations that exceed 17 mg kg–1 dry mass at depth (Fig. 2, Peters et al. 1999a, 1999b). High Se concentrations have also been measured in sediments close to the Swansea Channel (5 ± 4 mg kg–1 dry mass). Dredging in the Swansea Channel occurred from 1975 to 1981 to allow barges carrying heavy equipment for the power stations to enter the lake (AWACS 1995), and intermittently since then to allow commercial fishing boats to enter the lake. It is possible that during dredging, sediments containing higher sub-surface concentrations of Se were dumped near Swansea and mixed with less contaminated surficial sediment resulting in higher Se concentrations at this site. In contrast, Se-contaminated freshwater lakes and estuaries in the USA can contain up to 100 mg kg–1 dry mass of Se in sediments (Saiki and Lowe 1987).
|
More recent sediment measurements in 2011 (Schneider et al. 2015) showed that Se concentrations in southern lake sediments ranged from 0.4 to 5.6 mg kg–1 dry mass with the highest Se concentrations near Morisset Hospital. Surface concentrations in Mannering Bay, Chain Valley Bay and Wyee Bay sediments are now 5 ± 3, 2.1 ± 0.6 and 1 ± 1 mg kg–1 dry mass, respectively. These Se concentrations are significantly lower than those found in ash dams (~25 mg kg–1 dry mass).
The high concentration of Se in benthic sediments at the southern end of the lake is as predicted by hydrodynamic modelling (Ellwood et al. 2016; Schneider et al. 2016) with Se adsorbed to particles being dispersed by wind-driven currents. Selenium and total organic carbon (TOC) concentrations in sediments are significantly correlated (R2 = 0.34) (Schneider et al. 2016) and Se in reduced sediments is associated with operationally defined organic and sulfide phases (Peters et al. 1977). Roach et al. (2005) investigated the associations of Se with grain size, TOC, Fe and Al in Lake Macquarie sediments and showed that Se was mainly associated with TOC. Elemental Se and selenide minerals, e.g. FeSe, and selenosulfides are stable over a wide intermediate range of pH and redox potentials and usually coexist with organic matter (Masscheleyn et al. 1990, 1991). Sediment depth profiles using Pb210 dating clearly show the increase (over 30 years) and later a decrease of Se over time (Fig. 2). The reductions in Se concentrations in recently deposited sediments that have been observed at several locations are associated with a decline in Se discharge from the ash ponds at Vales Point following dam remediation. A comparison of sediment layers before and after ash handling procedures were implemented, shows that Se concentrations have substantially decreased (Schneider et al. 2014). With knowledge of long-term sedimentation rates for Lake Macquarie (7 mm year–1 near creeks or other inputs but close to 2–3 mm year–1 elsewhere (Kilby and Batley 1993), the decrease in Se concentrations is larger than expected. This may be partially due to high sedimentation rates from urban development that commenced in 1979, to Se volatilisation or to bioturbation (see below).
Porewater Se
There are few studies of Se concentrations in pore waters, with Se concentrations ranging from 0.1 to 5 µg L–1 being reported for Whiteheads Lagoon and Mannering Bay (Batley et al. 1992, 1994; Jung et al. 1998). Peters et al. (1997, 1999b) clearly showed that Se is associated with bulk sediments having a low redox potential, as is typically measured in reduced sediments, and is not present in the dissolved phase, i.e. pore water.
Care must be taken in interpreting porewater data as Se can be remobilised from sediments if reducing conditions are not maintained during collection of pore waters from bulk sediments (Peters et al. 1999b). In addition, experiments performed by Jung and Batley (2004) have suggested that if sediment cores are frozen, an increase in Se concentrations in pore waters can result from rupturing the cells of selenium-accumulating bacteria and algae present in the samples.
Se remobilisation from sediments
Volatile Se fluxes
Studies in our laboratory (Peters et al. 1999b) have isolated seven types of bacteria, all capable of transforming selenite (but not selenate) quantitatively to elemental Se. Mass balances showed that for three bacterial strains, total Se was conserved, selenate decreased while Se (0, II−) increased, indicating the production of non-volatile organoselenium compounds. For two bacterial strains, both total Se and selenate decreased with no increase in selenium (0, II−), indicating a net loss of Se from the media.
Methylation of sedimentary Se to volatile dimethylselenide (DMSe) and dimethyldiselenide (DMDSe) is known to be a natural remediation process with volatile Se species being produced by the microbial biomethylation of inorganic species (+VI, +IV and 0 oxidation states), and organoselenium species (Chau et al. 1976; Doran 1982). Sediments from near the Morisset Hospital have been the subject of field sampling and monitoring to determine the extent to which Se was being lost to the atmosphere as DMSe and DMDSe (Ellwood et al. 2015). Flux estimates were obtained by trapping volatile Se species using benthic domes. Measurements in both summer and winter showed distinct seasonal differences, with a higher summer DMSe flux of 53 ± 25 ng Se m–2 h–1 compared to 8 ± 5 ng Se ng Se m–2 h–1 in winter. No DMDSe was detected. Scaling of the DMSe sediment efflux results to the greater Mannering Bay area (5 km2) produced an annual selenium loss of 1330 ± 660 g Se year–1. Surface sediments within the area have a Se concentration between 3 and 6 mg kg–1 dry mass, which represents 600 kg of selenium within the upper 2 cm of the sediment profile, assuming a sediment density of 2 g cm–3. Thus, the current annual DMSe loss to the atmosphere represents 0.2% of the total selenium from Mannering Bay sediments. These fluxes are similar to those measured in Europe and North America and represent an annual loss of ~1.3 kg of selenium per year from this area alone. Lake-wide, this would represent a significant loss to the atmosphere.
This represents the current status, but Peters et al. (1999a) reported historical sediment Se concentrations at depth of 17 mg kg–1 dry mass compared to 3–6 mg kg–1 dry mass now, so fluxes at that time could have been at least 3–5 times greater. As a percentage, that is around 1% year–1 or 10% in a decade, so is significant. Over a 30-year period, even without the deposition of cleaner sediment, this would reduce Se in highly contaminated sediments to below 10 mg kg–1.
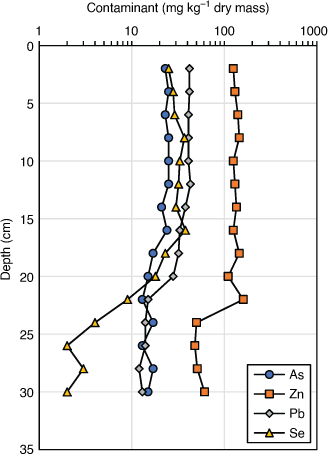
Volatilisation of Se in ash dam ponds can also be inferred from sediment Se and metal depth profiles (Fig. 3). While elements such as Pb, As and Zn have been conserved in the sediment profile, Se has become depleted in the surface layers. Either Se volatilisation is occurring or Se is being remobilised into the water column under aerobic conditions (see below).
|
Bioturbation
Selenium present in sediments is mobilised and released under oxygenated conditions with bound Se in sediments moving from organic/sulfide phases to exchangeable/Fe/Mn oxyhydroxide phases (Peters et al. 1999b). Bioturbation by sediment-dwelling organisms, especially bivalves and polychaetes (Bird 1994), can transport oxygen deep into sediments (~25–30 cm) through porewater irrigation (Meadows and Tait 1989). Sediment oxidation by irrigation results in the release of Se into pore waters and the overlying water column. As the density of bivalves and polychaetes in shallow sediments is substantial (MacIntyre 1959), oxygenation of sediments and the release of Se is also high. Laboratory experiments have confirmed that a substantial release of Se occurs when sediments are oxidised (Peters et al. 1999b). Sediment corer reactor experiments have also shown that fluxes of Se from sediments could be as high as 90 µg m–2 day–1 at some locations, e.g. Whiteheads Lagoon (Jung et al. 1998). The release of Se, however, may not be evident in field-collected pore waters or overlying waters as benthic microalgae can take up released Se and overlying waters dilute released pore water.
Se accumulation by organisms and transfer through food webs
The trophic structure of the dominant seagrass ecosystem in Lake Macquarie has been determined using carbon and nitrogen isotopes (Schneider et al. 2015). The data revealed that invertebrates had three dietary sources, benthic microalgae, and suspended organic matter such as plankton and seagrass detritus. Grazers were feeding on benthic microalgae, while filter-feeding molluscs were filtering water containing plankton, sediment and detrital material. All fish had diets reflective of some ingestion of detritus and associated epiphytes, with herbivores grazing on benthic algae as well. Carnivore isotope signatures reflected feeding on animals. Although organisms will ingest sediment, especially filter feeders and bottom-feeding fish such as mullet, benthic microalgae and bacteria are the primary food sources and play an important role in Se cycling as a link between sediments and higher organisms. Phytoplankton biomass in the lake is low and is a relatively unimportant vector for the accumulation of Se in food webs.
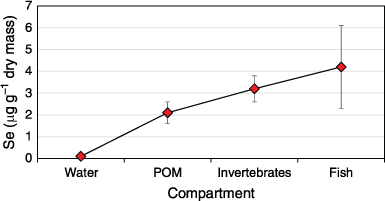
Selenium concentrations in organisms from Lake Macquarie (Fig. 3) are higher than in organisms from other uncontaminated Australian estuaries (Maher et al. 1992; Maher and Batley 1990). Still, they are considerably lower than Se concentrations reported in organisms from freshwater lakes receiving coal-fired power station inputs in the United States, Canada, and elsewhere that can exceed 300–400 mg kg–1 dry mass in algae, submerged rooted plants, chironomids and fish and detritus (Saiki and Lowe 1987; Chapman et al. 2010; Lemly 2014). This can be attributed to the relatively low Se concentrations in Australian coals and the lower Se concentration of water and sediments.
Sediments as the primary source of Se is evident from results of field-collected specimens and laboratory experiments that show that benthic animals accumulate more Se from contaminated sediments (Peters et al. 1999a; Taylor and Maher 2012, 2014). Selenium concentrations in muscle tissues of three benthic-feeding fish species (Mugil cephalus, Platycephalus fuscus and Acanthopagrus australis) were significantly correlated (r2 = 0.398, P < 0:05; r2 = 0.562, P < 0:05 and r2 = 0.740, P < 0:01 respectively) with surficial sediment Se concentrations (Peters et al. 1999a). The reduction in fish Se concentrations has clearly occurred when Se sources were remediated and Se concentration in sediments reduced (Kirby et al. 2001a). When sediment Se concentrations in the southern basin were decreased from 1 to 20 mg kg–1 dry mass to 0.8–1 mg kg–1 dry mass from 1995, fish Se concentrations in Mugil cephalus reduced from 2 ± 0.4 mg kg–1 wet mass in 1993 to 1.2 ± 0.1 mg kg–1 wet mass in 1997 to 0.6 ± 0.2 mg kg–1 wet mass in 2000, the latter well below the current human consumption guideline (2 mg kg–1 wet mass, FSANZ 2001).
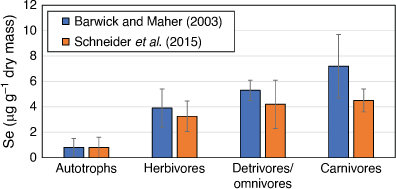
When Se concentrations were measured in seagrass ecosystems in Lake Macquarie (Barwick and Maher 2003; Schneider et al. 2015), the highest mean Se concentrations were observed in carnivores, followed by planktivores, omnivores, detritivores, herbivores and autotrophs (Fig. 4). Selenium was found to biomagnify, with a mean magnification factor of 1.39 per trophic level. Some fish species had Se concentrations up to 2.5 µg g–1 wet mass, with individuals of five fish species exceeding the maximum permitted concentrations for human consumption (2 mg kg–1 wet mass). Selenium concentrations measured in some fish were also above those shown to elicit sublethal effects in fish (see toxicity section below). The ecosystem-scale model of Presser and Luoma (2010) was applied to determine how Se in the seagrass food web was processed from sediments through diet to predators (Fig. 5, Schneider et al. 2015). Trophic position, habitat and feeding zone were examined as possible factors influencing Se bioaccumulation. Habitat and feeding zone influenced Se concentrations in invertebrates, while trophic level and feeding zone were significant factors influencing Se concentrations in fish. The sediment/water partition coefficient (Kd) was 4180, showing that partitioning of Se to sediments was occurring, and thus, benthic sediment food sources were an important Se source to benthic feeders. Measured trophic transfer factors for Lake Macquarie were similar to those reported for other water bodies, showing that input source was not the main determinant of the magnitude of Se bioaccumulation in the food web. Rather, the initial partitioning of Se into bioavailable particulate organic matter (POM) is the main factor in Se bioaccumulation. Presser and Luoma (2010) modelled the differences in the Se magnitude of biomagnification in various aquatic ecosystems and found that the Se concentration in POM depends on the partitioning of Se from the water column (in lake Macquarie initially via suspended particles), whether the POM is pelagic or benthic phytoplankton, vascular plants (such as seagrass) or detritus and the trophic transfer of Se from POM through the food web to consumers. The trophic transfer of Se from POM to initial consumers can be highly variable (0.5–4.2), whereas transfers between higher trophic levels are relatively constant (1.1–1.8).
|
|
Other factors that are generally understudied and may significantly influence the intake of Se include the habitat (sediment or water column) and the feeding zone (benthos or water column) used by organisms. These factors are important in environments with long water-residence times where Se concentrations are higher in the sediments than in the overlying water column. Organisms capable of obtaining Se directly from sediments via ingestion of associated microalgae and microorganisms are more likely to accumulate Se to high concentrations than pelagic species (Peters et al. 1999a; Schlenk et al. 2007). Thus organisms living in sediments and feeding on benthic organisms are expected to have higher Se concentrations than pelagic biota. In Se contaminated freshwater systems, Muscatello et al. (2008) and Muscatello and Janz (2009) also reported low Se concentrations in water (0.43–5 µg L–1) and sediments (0.54 mg kg–1 dry mass). Biomagnification of Se, however, resulted in an approximately 1.5–6 fold increase in the Se content between plankton, invertebrates and fish but not between forage fish and predatory fish. Selenium concentrations in organisms from exposure areas exceeded a proposed 3–11 μg g–1 dry mass dietary toxicity threshold for freshwater fish (DeForest et al. 1999). The authors concluded that the Se released into these aquatic systems had the potential to reach levels that could impair fish reproduction.
Selenium species and ecosystem cycling
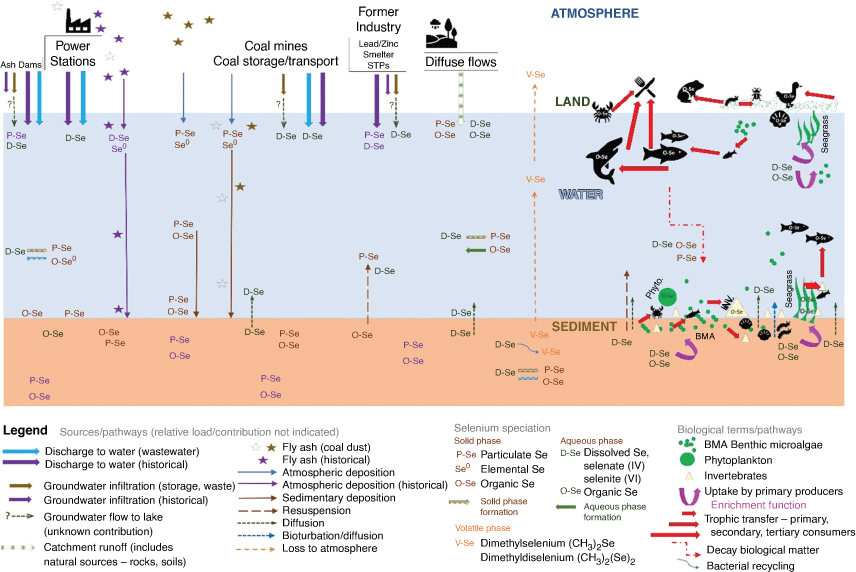
The major Se species identified in environmental compartments (Maher et al. 1997; Peters et al. 1999b; Maher and Krikowa 2007; Ellwood et al. 2016; Jagtap et al. 2016) and their cycling are shown in Fig. 6. Selenium in sediments is present primarily as inorganic species (Se0, SeII, SeIV, SeVI) with organic Se present in detritus (selenomethionine (SeMet) and selenocysteine (SeCyst)). Selenium(iv) is the major species in water. As previously mentioned, bacteria convert Se into (CH3)2Se and (CH3)2Se2. Once Se is taken up by primary producers, it is converted into SeMet and SeCyst and consumers also have SeMet and SeCyst. The pathway of SeCyst formation in consumers is unknown as SeCyst can be formed from SeII and/or SeMet (Craig and Maher 2003; Maher et al. 2010).
Selenium ecotoxicology
Marine organisms have a limited capacity to detoxify Se. Selenium is an essential element and primary producers synthesise SeMet and SeCyst that is accumulated by consumers. SeCyst is incorporated into proteins and these are ubiquitous in the animal kingdom (Lee et al. 1990). Selenomethione is not required by animals and excess SeMet is toxic to fish and birds (Spallholz and Hoffman 2002; Kupsco and Schlenk 2016).
Selenium ecotoxicology includes traditional acute or chronic toxicity testing with ecologically relevant endpoints such as lethality, immobilisation, growth, development, population growth or reproduction, together with biomarker measures of organism fitness and population-scale measurements.
Sublethal effects
Laboratory studies exposing two indigenous Lake Macquarie bivalve species A. trapezia and Tellina deltoidalis to sediment dosed with 5 and 20 mg Se kg–1 dry mass (Taylor and Maher 2012, 2014) found that Se-exposed organisms had decreased antioxidant capacity, increased lipid peroxidation and increased lysosomal destabilisation and that they were able to detoxify only a small percentage of accumulated Se, which suggested a limited detoxification and storage capacity for this element.
Population effects
Roach et al. (2008) noted larval abnormalities in fish near power station outflows. ‘Based on the fish Se concentrations at the time, it would have been expected that some increase in rates of fish larvae deformities would be found lake-wide (i.e. ambient and Whiteheads Lagoon and Wyee Bay)’. As this was not the case, it is now thought that the larval deformities observed were due to the discharge of hot cooling water.
Toxicity data
Hyne et al. (2002) tested the toxicity of SeMet to amphipod species occurring in Lake Macquarie using water-only tests, with only Paracalliope australis being sensitive to Se concentrations that are only found near power stations outlets and ash dams (96 h LC50 of 2.58 µg Se L–1)
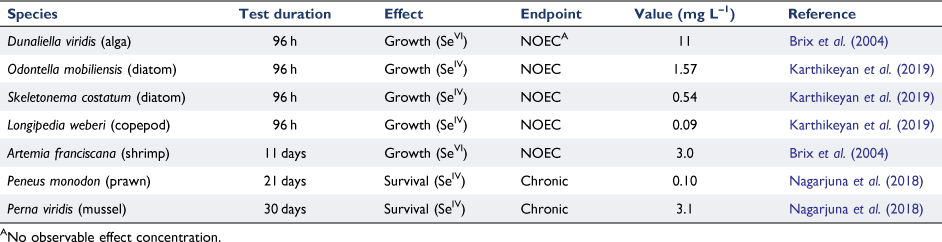
Internationally, there have been many reports of Se toxicity in marine waters, as shown in Table 1. Boisson et al. (1995) showed that SeIV was more toxic than SeVI to the marine alga Cricosphaera elonga. Karthikeyan et al. (2019) used a species sensitivity distribution of toxicity data to derive a low reliability marine guideline value (GV) of 22 µg L–1 for 95% species protection; however, as Se bioaccumulates, 99% species protection would be recommended in Australia and New Zealand (ANZG 2018). Again, except occasionally in close proximity to power stations and in ash dams, Se concentrations approaching these values are not found.
|
Luoma and Presser (2009) stated that because dietary exposure was more important than water exposure, GVs based on the latter are unlikely to be protective, especially of fish. Tissue residue-based GVs, as applied by the USEPA (2004) for Se in freshwaters, are more appropriate. A value near 1–5 µg Se L–1 is likely a more protective marine GV (Janz 2012). Again, these values are not found in most of the lake.
Summary and concluding remarks
In most of the documented cases of gross Se contamination in freshwater and estuarine systems outside Australia, high Se concentrations are measurable in the water column, and food webs are based on phytoplankton. Lake Macquarie is different in that Se is rapidly adsorbed to sedimentparticles, and food webs are based on benthic food sources. Selenium is remobilised from sediments by volatilisation and bioturbation, and transferred into food chains via benthic microalgae, deposit feeders and filter-feeding organisms processing suspended sediments. Since 1995, after the remediation of a major ash dam, Se inputs to the lake have been reduced and Se concentrations in sediments have reduced partially due to the deposition of cleaner sediment but mainly due to Se volatilisation as dimethylselenide and possibly bioturbation release of SeIV. In response to decreases in sediment Se concentrations, Se concentrations in molluscs and fish have also drastically reduced.
Except near ash dam outlets, Se water concentrations have been below those expected to cause toxicity; however, Se historically, has been found to accumulate in fish to levels above those considered safe for human consumption but now most fish are safe for human consumption. Based on laboratory studies using Se spiked sediments it is likely that sublethal effects to benthic organisms are occurring. An assessment of population effects still needs to be undertaken. When undertaking risk assessments of Se, careful consideration should be given to understanding the fate of Se and remobilisation into food webs as not all systems act in accordance with published studies that generally have high Se concentrations in the water column and phytoplankton based food webs. In these cases, the setting of selenium water quality criteria may not be protective of aquatic ecosystems, as has been found for freshwater systems, with guidelines based on tissue residues likely to be more appropriate (DeForest et al. 1999; Hamilton 2004).
Data availability
All data cited has been obtained from papers given in the reference section.
Conflicts of interest
Graeme Batley is an Editor for Environmental Chemistry and was blinded from the peer review process for this manuscript.
Declaration of funding
This research did not receive any specific funding.
References
ANZG (2018) ‘Australian and New Zealand Guidelines for Fresh and Marine Water Quality.’ (Australian and New Zealand Governments and Australian state and territory governments: Canberra ACT, Australia) Available at www.waterquality.gov.au/anz-guidelinesAWACS (1995) ‘Lake Macquarie Process Study.’ (Australian Water and Coastal Studies with JH & ES Laxton Pty. Ltd.: Sydney, Australia)
Barwick M, Maher W (2003). Biotransference and biomagnification of selenium, copper, cadmium, zinc, arsenic and lead in a temperate seagrass community from lake Macquarie, NSW, Australia. Marine Environmental Research 56, 471–502.
| Biotransference and biomagnification of selenium, copper, cadmium, zinc, arsenic and lead in a temperate seagrass community from lake Macquarie, NSW, Australia.Crossref | GoogleScholarGoogle Scholar |
Batley GE, Brockbank CI, Jones DR, Lincoln-Smith M (1992) Trace elements in waters, sediments and fish near Vales Point Power Station. CSIRO Division of Coal and Energy Technology Investigation Report CET/IR065. p. 15. (Lucas Heights, NSW, Australia)
Batley GE, Brockbank CI, Jones DR, Lincoln-Smith M (1993a) Trace elements in waters and fish near Vales Point Power Station. CSIRO Division of Coal and Energy Technology Investigation Report No. CET/IR158. p. 9. (Lucas Heights, NSW, Australia)
Batley GE, Brockbank CI, Lincoln-Smith M (1993b) Trace elements in sediments and fish near Eraring Power Station. CSIRO Division of Coal and Energy Technology Investigation Report No. CET/IR159. p. 18 (Lucas Heights, NSW, Australia)
Batley GE, Brockbank CI, Lincoln-Smith M (1994) Trace elements in water, sediments and fish near Eraring Power Station, December 1993 Survey. CSIRO Division of Coal and Energy Technology Investigation Report No. CET/IR235. p. 20. (Lucas Heights, NSW, Australia)
Besser JM, Giesy JP, Brown RW, Buell JM, Dawson GA (1996). Selenium bioaccumulation and hazards in a fish community affected by coal fly ash effluent. Ecotoxicology and Environmental Safety 35, 7–15.
| Selenium bioaccumulation and hazards in a fish community affected by coal fly ash effluent.Crossref | GoogleScholarGoogle Scholar |
Bird FL (1994) The effects of bioturbation in Port Phillip Bay. Port Phillip Bay Environmental Study, Technical Report 14. (CSIRO Institute of Natural Resources and Environment: Melbourne, Vic, Australia)
Boisson F, Gnassia-Barelli M, Romeo M (1995). Toxicity and accumulation of selenite and selenate in the unicellular marine alga Cricosphaera elongatae. Archives of Environmental Toxicology and Chemistry 28, 487–493.
| Toxicity and accumulation of selenite and selenate in the unicellular marine alga Cricosphaera elongatae.Crossref | GoogleScholarGoogle Scholar |
Brix KV, Deforest DK, Cardwell RD, Adams WJ (2004). Derivation of a chronic site-specific water quality standard for selenium in the Great Salt Lake, Utah, USA. Environmental Toxicology and Chemistry 23, 606–612.
| Derivation of a chronic site-specific water quality standard for selenium in the Great Salt Lake, Utah, USA.Crossref | GoogleScholarGoogle Scholar |
Burt A, Maher W, Roach A, Krikowa F, Honkoop P, Bayne B (2007). The accumulation of Zn, Se, Cd, and Pb and physiological condition of Anadara trapezia transplanted to a contamination gradient in Lake Macquarie, New South Wales, Australia. Marine Environmental Research 64, 54–78.
| The accumulation of Zn, Se, Cd, and Pb and physiological condition of Anadara trapezia transplanted to a contamination gradient in Lake Macquarie, New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Chapman PM, Adams WJ, Brooks M, Delos CG, Luoma SN, Maher WA, Ohlendorf HM, Presser TS, Shaw P (2010) ‘Ecological Assessment of Selenium in the Aquatic Environment.’ (CRC Press: Boca Raton, FL, USA)
Chau YK, Wong PTS, Silverberg BA, Luxon PL, Bengert GA (1976), Methylation of selenium in the aquatic environment. Science, 192, 1130–1131,
| Crossref |.
Cloern JE (1996). Phytoplankton bloom dynamics in coastal ecosystems: A review with some general lessons from sustained investigation of San Francisco Bay, California. Reviews of Geophysics 34, 127–168.
| Phytoplankton bloom dynamics in coastal ecosystems: A review with some general lessons from sustained investigation of San Francisco Bay, California.Crossref | GoogleScholarGoogle Scholar |
Coyle JJ, Buckler DR, Ingersoll CG, Fairchild JF, May TW (1993). Effect of dietary selenium on the reproductive success of bluegills (Lepomis macrochirus). Environmental Toxicology and Chemistry 12, 551–565.
| Effect of dietary selenium on the reproductive success of bluegills (Lepomis macrochirus).Crossref | GoogleScholarGoogle Scholar |
Craig P, Maher WA (2003) Organoselenium compounds in the environment. Chapter 10. In ‘Organometallic Compounds in the Environment’. (Ed. P Craig) pp. 391–399. (Longman: UK)
DeForest DK, Brix KV, Adams WJ (1999). Critical review of proposed residue-based selenium toxicity thresholds for freshwater fish. Human and Ecological Risk Assessment 5, 1187–1228.
| Critical review of proposed residue-based selenium toxicity thresholds for freshwater fish.Crossref | GoogleScholarGoogle Scholar |
Doblin MA, Baines SB, Cutter LS, Cutter GA (2006). Sources and biogeochemical cycling of particulate selenium in the San Francisco Bay estuary. Estuarine, Coastal and Shelf Science 67, 681–694.
| Sources and biogeochemical cycling of particulate selenium in the San Francisco Bay estuary.Crossref | GoogleScholarGoogle Scholar |
Doran JW (1982) Microorganisms and the biological cycling of selenium. In ‘Advances in Microbial Ecology’, Vol. 6. (Ed. KC Marshall) Ecology. pp. 1–32. (Springer: Boston, MA, USA)
Ellwood MJ, Schneider L, Potts J, Batley GE, Floyd J, Maher WA (2015). Volatile selenium fluxes from selenium-contaminated sediments in an Australian coastal lake. Environmental Chemistry 13, 68–75.
| Volatile selenium fluxes from selenium-contaminated sediments in an Australian coastal lake.Crossref | GoogleScholarGoogle Scholar |
FSANZ (2001) Generally expected levels (GELs) for metal contaminants. Food Standards Australia New Zealand, Majura, ACT, Australia. Available at https://www.foodstandards.gov.au/code/userguide/documents/GELs_0801.pdf
Furner I (1979) Lead, cadmium, zinc and copper in organisms of Lake Macquarie. BSc honours thesis (unpublished), University of Newcastle, NSW, Australia.
Gillespie RB, Baumann PC (1986). Effects of high tissue concentrations of selenium on reproduction by bluegills. Transactions of the American Fisheries Society 115, 208–213.
| Effects of high tissue concentrations of selenium on reproduction by bluegills.Crossref | GoogleScholarGoogle Scholar |
Hamilton SJ (2004). Review of selenium toxicity in the aquatic food chain. Science of The Total Environment 326, 1–31.
| Review of selenium toxicity in the aquatic food chain.Crossref | GoogleScholarGoogle Scholar |
Hyne RV, Hogan AC, Pablo F, Roach AC (2002). Toxicity of selenomethionine- and seleno- contaminated sediment to the amphipod Corophium sp. Ecotoxicology and Environmental Safety 52, 30–37.
| Toxicity of selenomethionine- and seleno- contaminated sediment to the amphipod Corophium sp.Crossref | GoogleScholarGoogle Scholar |
Jagtap R, Maher W, Krikowa F, Ellwood MJ, Foster S (2016). Measurement of selenomethionine and selenocysteine in fish tissues using HPLC-ICP-MS. Microchemical Journal 128, 248–257.
| Measurement of selenomethionine and selenocysteine in fish tissues using HPLC-ICP-MS.Crossref | GoogleScholarGoogle Scholar |
Janz DM (2012) Selenium. In ‘Homeostasis and Toxicology of Essential Metals, Fish Physiology Vol 31A’. (Eds CM Wood, AP Farrell, CJ Brauner) pp. 329–375. (Academic Press: London, UK)
Jung RF, Batley GE (2004). Freezing of sediments inappropriate for pore water selenium analysis. Marine Pollution Bulletin 49, 295–298.
| Freezing of sediments inappropriate for pore water selenium analysis.Crossref | GoogleScholarGoogle Scholar |
Jung RF, Brockbank CI, Batley GE, Ford PW (1998) Selenium in waters and sediments from Lake Macquarie. CSIRO Energy Technology Investigation Report ET/IR142. p. 42. (Lucas Heights, NSW, Australia)
Karthikeyan P, Marigoudar SR, Nagarjuna A, Sharma KV (2019). Toxicity assessment of cobalt and selenium on marine diatoms and copepods. Environmental Chemistry and Ecotoxicology 1, 36–42.
| Toxicity assessment of cobalt and selenium on marine diatoms and copepods.Crossref | GoogleScholarGoogle Scholar |
Kilby GW, Batley GE (1993). Chemical indicators of sediment chronology. Australian Journal of Marine and Freshwater Research 44, 635–647.
| Chemical indicators of sediment chronology.Crossref | GoogleScholarGoogle Scholar |
Kirby J, Maher W, Harasti D (2001a). Changes in selenium, copper, cadmium and zinc concentrations in mullet (Mugil cephalus) from the southern basis of Lake Macquarie, Australia in response to alteration of coal fire power station fly ash handling procedures. Archives of Environmental Contamination and Toxicology 41, 171–181.
| Changes in selenium, copper, cadmium and zinc concentrations in mullet (Mugil cephalus) from the southern basis of Lake Macquarie, Australia in response to alteration of coal fire power station fly ash handling procedures.Crossref | GoogleScholarGoogle Scholar |
Kirby J, Maher W, Krikowa F (2001b). Selenium, cadmium, copper and zinc concentrations in sediments and mullet (Mugil cephalus) from the southern basin of Lake Macquarie, New South Wales, Australia. Archives of Environmental Contamination and Toxicology 40, 246–256.
| Selenium, cadmium, copper and zinc concentrations in sediments and mullet (Mugil cephalus) from the southern basin of Lake Macquarie, New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Krishnaja AP, Rege MS (1982). Induction of chromosomal aberrations in fish Boleophthalmus dussumerieri after exposure in vivo to mitomycin C and heavy metals mercury, selenium and chromium. Mutation Research 102, 71–82.
| Induction of chromosomal aberrations in fish Boleophthalmus dussumerieri after exposure in vivo to mitomycin C and heavy metals mercury, selenium and chromium.Crossref | GoogleScholarGoogle Scholar |
Kupsco A, Schlenk D (2016). Molecular mechanisms of selenium-Induced spinal deformities in fish. Aquatic Toxicology 179, 143–150.
| Molecular mechanisms of selenium-Induced spinal deformities in fish.Crossref | GoogleScholarGoogle Scholar |
Lee BJ, Rajagopalan M, Kim YS, You KH, Jacobson KB, Hatfield D (1990). Selenocysteine tRNA[Ser]sec gene is ubiquitous within the animal kingdom. Molecular and Cellular Biology 10, 1940–1949.
| Selenocysteine tRNA[Ser]sec gene is ubiquitous within the animal kingdom.Crossref | GoogleScholarGoogle Scholar |
Lemly AD (1985). Toxicology of selenium in a freshwater reservoir: Implications for environmental hazard evaluation and safety. Ecotoxicology and Environmental Safety 10, 314–338.
| Toxicology of selenium in a freshwater reservoir: Implications for environmental hazard evaluation and safety.Crossref | GoogleScholarGoogle Scholar |
Lemly AD (2002). Symptoms and implications of Se toxicity in fish: the Belews Lake case example. Aquatic Toxicology 57, 39–49.
| Symptoms and implications of Se toxicity in fish: the Belews Lake case example.Crossref | GoogleScholarGoogle Scholar |
Lemly AD (2004). Aquatic selenium pollution is a global environmental safety issue. Ecotoxicology and Environmental Safety 59, 44–56.
| Aquatic selenium pollution is a global environmental safety issue.Crossref | GoogleScholarGoogle Scholar |
Lemly AD (2014). Teratogenic effects and monetary cost of selenium poisoning of fish in Lake Sutton, North Carolina. Ecotoxicology and Environmental Safety 104, 160–167.
| Teratogenic effects and monetary cost of selenium poisoning of fish in Lake Sutton, North Carolina.Crossref | GoogleScholarGoogle Scholar |
Luoma SN, Presser TS (2009). Emerging opportunities in management of selenium contamination. Environmental Science and Technology 43, 8483–8487.
| Emerging opportunities in management of selenium contamination.Crossref | GoogleScholarGoogle Scholar |
MacIntyre RJ (1959). Some aspects of the ecology of Lake Macquarie, N.S.W., with regard to an alleged depletion of fish. VII. The benthic macrofauna. Australian Journal of Marine and Freshwater Research 10, 341–353.
| Some aspects of the ecology of Lake Macquarie, N.S.W., with regard to an alleged depletion of fish. VII. The benthic macrofauna.Crossref | GoogleScholarGoogle Scholar |
Maher W, Batley G (1990). Organometallics in the near shore marine environment of Australia. Applied Organometallic Chemistry 4, 419–439.
| Organometallics in the near shore marine environment of Australia.Crossref | GoogleScholarGoogle Scholar |
Maher WA, Krikowa F (2007) Measurement of total Se and Se species in seafood by quadrupole inductively coupled plasma mass spectrometry, electrothermal atomization atomic absorption spectrometry and high-performance liquid chromatography inductively coupled plasma mass spectrometry. In ‘The Analysis of Chemical Elements in Food: Applications for atomic and mass spectrometry’, Chapter 20. (Ed. S Caroli). pp. 643–669. (Wiley Interscience)
Maher W, Baldwin S, Deaker M, Irving M (1992). Characteristics of selenium in Australian marine biota. Applied Organometallic Chemistry 6, 103–112.
| Characteristics of selenium in Australian marine biota.Crossref | GoogleScholarGoogle Scholar |
Maher W, Deaker M, Jolley D, Krikowa F, Roberts B (1997). Selenium occurrence, distribution and speciation in the cockle Anadara trapezia and the mullet Mugil cephalus. Applied Organometallic Chemistry 11, 313–326.
| Selenium occurrence, distribution and speciation in the cockle Anadara trapezia and the mullet Mugil cephalus.Crossref | GoogleScholarGoogle Scholar |
Maher B, Roach T, Doblin M, Fan T, Foster S, Garrett R, Moller G, Oram L, Wallschlager D (2010) Environmental sources, speciation and partitioning of selenium. In ‘Ecological Assessment of Selenium in the Aquatic Environment’, 1 edn. (Eds PM Chapman, WJ Adams, M Brooks, CG Delos, SN Luoma, WA Maher, HM Ohlendorf, TS Presser, P Shaw) pp. 47–92. (CRC Press: Boca Raton, FL, USA)
Masscheleyn PH, Delaune RD, Patrick WH (1990). Transformations of selenium as affected by sediment oxidation-reduction potential and pH. Environmental Science and Technology 24, 91–96.
| Transformations of selenium as affected by sediment oxidation-reduction potential and pH.Crossref | GoogleScholarGoogle Scholar |
Masscheleyn PH, Delaune RD, Patrick WH (1991). Arsenic and selenium chemistry as affected by sediment redox potential and pH. Journal of Environmental Quality 20, 522–527.
| Arsenic and selenium chemistry as affected by sediment redox potential and pH.Crossref | GoogleScholarGoogle Scholar |
Maunsell and Partners (1974) ‘A review of selenium and heavy metal contamination of Lake Macquarie, NSW due to power generation and lead/zinc smelting’. B. Carroll (1996). (Environmental Research Group, Department of Chemical Engineering, University of Sydney: NSW, Australia)
Meadows PS, Tait J (1989). Modification of sediment permeability and shear strength by two burrowing invertebrates. Marine Biology 101, 75–82.
| Modification of sediment permeability and shear strength by two burrowing invertebrates.Crossref | GoogleScholarGoogle Scholar |
MHL (1997) Swansea Channel and Lake Macquarie Data Collection March-June 1996, Manly Hydraulics Laboratory Report No. 770. (Manly, NSW, Australia)
Muscatello JR, Janz DM (2009). Selenium accumulation in aquatic biota downstream of a uranium mining and milling operation. Science of the Total Environment 407, 1318–1325.
| Selenium accumulation in aquatic biota downstream of a uranium mining and milling operation.Crossref | GoogleScholarGoogle Scholar |
Muscatello JR, Belknap AM, Janz DM (2008). Accumulation of selenium in aquatic systems downstream of a uranium mining operation in northern Saskatchewan, Canada. Environmental Pollution 156, 387–393.
| Accumulation of selenium in aquatic systems downstream of a uranium mining operation in northern Saskatchewan, Canada.Crossref | GoogleScholarGoogle Scholar |
Nagarjuna A, Karthikeyan P, Mohan D, Marigoudar SR (2018). Effect of selenium on Penaeus monodon and Perna viridis: enzyme activities and histopathological responses. Chemosphere 199, 340–350.
| Effect of selenium on Penaeus monodon and Perna viridis: enzyme activities and histopathological responses.Crossref | GoogleScholarGoogle Scholar |
NFA (National Food Authority) (1992) ‘Australian food standards code.’ (Australian Government Publication Service: Canberra)
Ohlendorf HM, Santolo GM, Byron ER, Eisert MA (2020). Kesterson Reservoir: 30 Years of selenium risk assessment and management. Integrated Environmental Assessment and Management 16, 257–268.
| Kesterson Reservoir: 30 Years of selenium risk assessment and management.Crossref | GoogleScholarGoogle Scholar |
Peters GM, Maher WA, Barford JP, Gomes VG (1997). Selenium associations in estuarine sediments: redox effects. Water Air and Soil Pollution 99, 275–282.
| Selenium associations in estuarine sediments: redox effects.Crossref | GoogleScholarGoogle Scholar |
Peters GM, Maher WA, Krikowa F, Barford JP, Gomes VG, Jeswani HK, Reible DD, Scholz D (1999a). Selenium in sediments, pore water and benthic infauna of Lake Macquarie, NSW, Australia. Marine Environmental Research 47, 491–508.
| Selenium in sediments, pore water and benthic infauna of Lake Macquarie, NSW, Australia.Crossref | GoogleScholarGoogle Scholar |
Peters GM, Maher WA, Jolley D, Carroll BI, Gomes VG, Jenkinson AV, McOrist GD (1999b). Selenium contamination, redistribution and remobilisation in sediments of Lake Macquarie, New South Wales. Organic Geochemistry 30, 1287–1300.
| Selenium contamination, redistribution and remobilisation in sediments of Lake Macquarie, New South Wales.Crossref | GoogleScholarGoogle Scholar |
Presser TS, Luoma SN (2010). A methodology for ecosystem-scale modelling of selenium. Integrated Environmental Assessment and Management 6, 685–710.
| A methodology for ecosystem-scale modelling of selenium.Crossref | GoogleScholarGoogle Scholar |
Riedel GF, Sanders JG, Gilmour CC (1996). Uptake, transformation, and impact of selenium in freshwater phytoplankton and bacterioplankton communities. Aquatic Microbial Ecology 11, 43–51.
| Uptake, transformation, and impact of selenium in freshwater phytoplankton and bacterioplankton communities.Crossref | GoogleScholarGoogle Scholar |
Roach AC (2005). Assessment of metals in sediments from Lake Macquarie, New South Wales, Australia, using normalisation models and sediment quality guidelines. Marine Environmental Research 59, 453–472.
| Assessment of metals in sediments from Lake Macquarie, New South Wales, Australia, using normalisation models and sediment quality guidelines.Crossref | GoogleScholarGoogle Scholar |
Roach AC, Maher W, Krikowa F (2008). Assessment of metals in fish from Lake Macquarie, New South Wales, Australia. Archives of Environmental Contamination and Toxicology 54, 292–308.
| Assessment of metals in fish from Lake Macquarie, New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Roberts B (1994) The accumulation and distribution of selenium in fish from Lake Macquarie, NSW. Honours thesis, University of Canberra, Belconnen, ACT, Australia.
Roy PS, Crawford EA (1984). Trace metals in a contaminated Australian estuary-dispersion and accumulation trend. Estuarine, Coastal and Shelf Science 19, 341–358.
| Trace metals in a contaminated Australian estuary-dispersion and accumulation trend.Crossref | GoogleScholarGoogle Scholar |
Saiki MK, Lowe PT (1987). Selenium in aquatic organisms from subsurface agricultural drainage water, San Joaquin Valley, California. Archives for Environmental Contamination and Toxicology 16, 657–670.
| Selenium in aquatic organisms from subsurface agricultural drainage water, San Joaquin Valley, California.Crossref | GoogleScholarGoogle Scholar |
Schlenk D, Batley G, King C, Stauber J, Adams M, Simpson S, Maher W, Oris JT (2007). Effects of light on microalgae concentrations and selenium uptake in bivalves exposed to selenium-amended sediments. Archives of Environmental Contamination and Toxicology 53, 365–370.
| Effects of light on microalgae concentrations and selenium uptake in bivalves exposed to selenium-amended sediments.Crossref | GoogleScholarGoogle Scholar |
Schneider L, Maher W, Potts J, Gruber B, Batley G, Taylor A, Chariton A, Krikowa F, Zawadzki A, Heijnis H (2014). Recent history of sediment metal contamination in Lake Macquarie, Australia, and an assessment of ash handling procedure effectiveness in mitigating metal contamination from coal-fired power stations. Science of the Total Environment 490, 659–670.
| Recent history of sediment metal contamination in Lake Macquarie, Australia, and an assessment of ash handling procedure effectiveness in mitigating metal contamination from coal-fired power stations.Crossref | GoogleScholarGoogle Scholar |
Schneider L, Maher WA, Potts J, Taylor AM, Batley G, Krikowa F, Chariton AA, Gruber B (2015). Modelling food web structure and selenium biomagnification in Lake MacquariNew South Wales, Australia, using stable carbon and nitrogen isotopes. Environmental Toxicology and Chemistry 34, 608–617.
| Modelling food web structure and selenium biomagnification in Lake MacquariNew South Wales, Australia, using stable carbon and nitrogen isotopes.Crossref | GoogleScholarGoogle Scholar |
Schneider L, Maher WA, Floyd J, Potts J, Gruber B (2016). Transport and fate of metal contamination in estuaries: Using a model network to predict the contributions of physical and chemical factors. Chemosphere 153, 227–236.
| Transport and fate of metal contamination in estuaries: Using a model network to predict the contributions of physical and chemical factors.Crossref | GoogleScholarGoogle Scholar |
Sorensen EMB, Bauer TL (1983). Haematological dyscrasia in teleost chronically exposed to selenium-laden effluent. Archives of Environmental Contamination and Toxicology 12, 135–141.
| Haematological dyscrasia in teleost chronically exposed to selenium-laden effluent.Crossref | GoogleScholarGoogle Scholar |
Sorensen EMB, Cumbie PM, Bauer TL, Bell JS, Harlan CW (1984). Histopathological, haematological, condition factor and organ weight changes associated with selenium contamination in fish from Belews Lake, North Carolina. Archives of Environmental Contamination and Toxicology 13, 153–162.
| Histopathological, haematological, condition factor and organ weight changes associated with selenium contamination in fish from Belews Lake, North Carolina.Crossref | GoogleScholarGoogle Scholar |
Spallholz E, Hoffman DJ (2002). Selenium toxicity: cause and effects in aquatic birds. Aquatic Toxicology 57, 27–37.
| Selenium toxicity: cause and effects in aquatic birds.Crossref | GoogleScholarGoogle Scholar |
Spencer R (1959). Some aspects of the ecology of Lake Macquarie, NSW, with regard to an alleged depletion of fish. II. Hydrology. Australian Journal of Marine and Freshwater Research 10, 279–296.
| Some aspects of the ecology of Lake Macquarie, NSW, with regard to an alleged depletion of fish. II. Hydrology.Crossref | GoogleScholarGoogle Scholar |
Swaine D (1985) Trace inorganic constituents in fly ash. In ‘Symposium on Coal Combustion’. (University of Newcastle: New South Wales)
Taylor AM, Maher WA (2012). Exposure-dose-response of Anadara trapezia to metal contaminated sediments. 3. Selenium spiked sediments. Aquatic Toxicology 124/125, 152–162.
| Exposure-dose-response of Anadara trapezia to metal contaminated sediments. 3. Selenium spiked sediments.Crossref | GoogleScholarGoogle Scholar |
Taylor AM, Maher WA (2014). Exposure-dose-response of Tellina deltoidalis to contaminated estuarine sediments 3. Selenium spiked sediments. Comparative Biochemistry and Physiology, Part C 159, 34–43.
| Exposure-dose-response of Tellina deltoidalis to contaminated estuarine sediments 3. Selenium spiked sediments.Crossref | GoogleScholarGoogle Scholar |
Thomson JM (1959). Some aspects of the ecology of Lake Macquarie, N.S.W. with regard to an alleged depletion of fish. XII. Summary review. Australian Journal of Marine and Freshwater Research 10, 399–408.
| Some aspects of the ecology of Lake Macquarie, N.S.W. with regard to an alleged depletion of fish. XII. Summary review.Crossref | GoogleScholarGoogle Scholar |
USEPA (2004) Draft Aquatic Life Water Quality Criterion for Selenium. United States Environmental Protection Agency Report No. EPA-82-D-04-001. (Washington, DC, USA)
Wlodarczkyk J, Beath K (1997) Heavy metals in seafood in Lake Macquarie: a cross-sectional survey. Report to the Hunter Public Health Unit. (University of Newcastle Research Association: Callaghan NSW and John Wlodarczyk Consulting Services: New Lambton, NSW, Australia)