Toxicity of arsenic(v) to temperate and tropical marine biota and the derivation of chronic marine water quality guideline values
Lisa A. Golding A * , Maria V. Valdivia B , Joost W. van Dam C , Graeme E. Batley
A * , Maria V. Valdivia B , Joost W. van Dam C , Graeme E. Batley  A and Simon C. Apte A
A and Simon C. Apte A
A CSIRO, Land and Water, Tharawal Country, New Illawarra Road, Lucas Heights, NSW, Australia.
B Grupo Gestiona Consultores, Providencia, Santiago, Chile.
C Australian Institute of Marine Science, Darwin, NT, Australia.
Environmental Chemistry 19(4) 116-131 https://doi.org/10.1071/EN22039
Submitted: 24 April 2022 Accepted: 7 June 2022 Published: 28 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC)
Environmental context. High-quality ecotoxicology data are required to derive reliable water quality guideline values that ensure long-term protection of marine biota from arsenate. Tropical and temperate marine biota have sensitivity to arsenate covering three to four orders of magnitude due to the range of arsenate detoxification mechanisms used to reduce toxicity. The water quality guideline values derived in this study will contribute to robust risk assessments of arsenate in marine environments.
Rationale. There are very few high-quality chronic inorganic arsenate (AsV) toxicity data to assess the risks to marine ecosystems. We aimed to determine the range in chronic toxicity of AsV to marine biota and derive reliable water quality guideline values (GVs) for the long-term protection of marine ecosystems.
Methodology. We generated chronic toxicity data based on measured dissolved (<0.45 µm filtered) AsV concentrations for 13 marine species representing seven taxonomic groups from temperate and tropical environments. Effect concentrations at the 10% level (EC10) were used in a species sensitivity distribution (SSD) to derive water quality GVs.
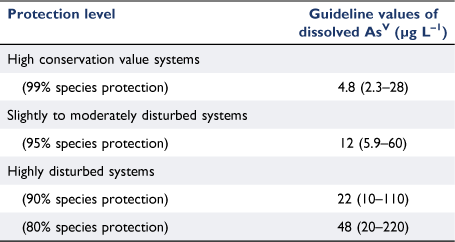
Results. The range of concentrations causing chronic 10, 20 and 50% adverse effects were 13–26 000, 18–34 000 and 32–330 000 µg AsV L–1, respectively. Increased phosphate and nitrate concentrations were found to reduce the toxicity of AsV to certain microalgal, sea urchin and bivalve species. The range in effect concentrations for tropical versus temperate species overlapped at all effect levels. The GVs for the long-term protection of 80, 90, 95 and 99% of marine biota were: 48, 22, 12 and 4.8 µg AsV L–1, respectively.
Discussion. Recommendations on performing toxicity tests with arsenic to prevent artefacts associated with arsenic speciation were made to improve future research on arsenic toxicity. The new data will improve the reliability status of the Australian and New Zealand AsV GVs for marine water quality and fill a data gap for global risk assessments of AsV for marine biota.
Keywords: aquatic ecotoxicology, arsenate, Burrlioz, marine chemistry, metalloid, phosphate, speciation, species sensitivity distribution, water quality criteria.
Introduction
Arsenic remains a priority metalloid of concern globally due to its wide distribution and toxicity to human and ecological health (ATSDR 2019; Chen and Costa 2021). As part of the updates to the Australian and New Zealand Guidelines for Fresh and Marine Water Quality (ANZG 2018) for the long-term protection of the aquatic environment, the marine arsenate (AsV) guideline value (GV) was prioritised for review due to its current status as a low reliability, interim working level that was in need of additional chronic toxicity data (ANZG 2018).
Natural sources of arsenic to marine environments include freshwater inputs and atmospheric deposition of arsenic-containing particulates from pedogenetic weathering, volcanic emissions and forest fires as well as release from hydrothermal vents in deep-sea environments (Bissen and Frimmel 2003; Smith et al. 2003; Morales-Simfors and Bundschuh 2022). Mobilisation of arsenic into seawater from estuarine sediments and coastal acid-sulfate soils occurs as a result of physical disturbance and changing redox conditions (Cullen and Reimer 1989; Johnston et al. 2010). Historical and current anthropogenic activities such as fungicide/herbicide/insecticide manufacture and usage, timber preservation, metal mining and refining and burning of fossil fuels also contribute to elevated arsenic concentrations in coastal seawater (Mandal and Suzuki 2002).
Background dissolved (<0.45 µm filtered) arsenic in oceanic waters ranges from 0.64 to 1.5 µg L–1 with similar concentrations in coastal waters (1.0–1.6 µg L–1) and estuaries (0.11–1.8 µg L–1) (Supplementary Table S1). Coastal ports and harbours can have much higher dissolved arsenic concentrations (0.68–33 µg L–1) depending on the type of anthropogenic activity in the catchment (Supplementary Table S1).
Arsenic exists in four oxidation states: V, III, 0 and −III (Maher and Butler 1988; Sharma and Sohn 2009). Approximately 90% or more of total arsenic in aerobic seawater is present as inorganic arsenate (AsV) with the most abundant oxyanion being HAsO42− (Sadiq 1990; Neff 1997). A minor contribution (< 20% of total arsenic) comes from inorganic arsenite (AsIII) which occurs mostly as the neutral species As(OH)30 (Smedley and Kinniburgh 2002). Elemental arsenic (As0) is insoluble and very rare and As−III occurs only in highly reduced environments making these forms irrelevant to the development of protective GVs due to the low probability of exposure to aquatic life. The methylated forms of arsenic, monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) are much less abundant than the inorganic forms. They are produced by biotransformation of inorganic AsV and are transformed back to inorganic AsV upon release into the marine environment (Duncan et al. 2015). The ability of marine organisms to bioaccumulate arsenic as inorganic and organic forms (predominantly as arsenobetaine (AB)) means that arsenic can be transferred to higher trophic levels of the food chain and conflicting evidence exists for arsenic biomagnification as well as biodilution (Barwick and Maher 2003; Córdoba-Tovar et al. 2022).
Inorganic AsV and AsIII co-exist in seawater in varying ratios due to both abiotic and biotic processes recycling arsenic between the two oxidation states (Maher and Butler 1988). Aerobic conditions favour AsV and anaerobic conditions favour AsIII (Smedley and Kinniburgh 2002). The rates of AsV reduction and AsIII oxidation increase with increasing temperature and are catalysed in the presence of microorganisms (Peterson and Carpenter 1983; Cutter 1992). The biogeochemical cycling of arsenic in seawater is controlled by processes such as oxidation/reduction, adsorption/desorption to particulate organic matter and aluminium, iron and manganese oxyhydroxides and sulfides, temperature/seasonal changes, biotransformation and photodegradation (Cullen and Reimer 1989; WHO 2001; Smedley and Kinniburgh 2002). Where arsenic contamination of marine waters occurs, the dominant form of dissolved inorganic arsenic continues to be AsV (Chang et al. 2011; Hong et al. 2016). Therefore, inorganic AsV is the arsenic form of concern for long-term exposure to marine biota with transient peaks in exposure to relatively lower concentrations of inorganic AsIII also possible.
There are few data to demonstrate differences in chronic toxicity to marine biota based on inorganic arsenic oxidation state. AsIII has been shown to be 1.2–4.6 fold more acutely toxic to marine biota than AsV (Lee et al. 2008; Guţu et al. 2015; Byeon et al. 2020). Comparable data are not available for chronic toxicity with the exception of the marine microalga, Chlorella salina, where AsIII is of similar toxicity to AsV (Karadjova et al. 2008). The majority of toxicity data are based on nominal arsenic concentrations rather than measured concentrations of total or speciated arsenic. This makes it difficult to attribute differences in chronic toxicity to oxidation state especially when AsIII may oxidise to AsV in test solutions (Neff 1997). There are likely to be species-specific differences in the biological response to arsenic oxidation states due to the evolution of detoxification mechanisms of arsenic (De Francisco et al. 2021). Detoxification in many marine organisms involves the biotransformation of AsV to AsIII followed by methylation and metabolism to produce non-toxic organoarsenic forms (for example, AB) that are released into the environment and undergo further transformations (Duncan et al. 2015; Kalia and Khambholja 2015; Wang et al. 2015).
Toxicity of AsV and AsIII is expressed as oxidative stress within cells resulting in effects on growth, larval development, reproduction, respiration and survival at the whole organism level (Neff 1997; Byeon et al. 2021). Arsenic is not an essential element but enters cells by transport proteins designed for essential nutrients and minerals such as phosphate and glycerol (Garbinski et al. 2019). The mechanisms of toxicity differ with oxidation state. AsIII has a high affinity for sulfhydryl groups of biomolecules such as glutathione (GSH) resulting in harm by inhibiting activities of enzymes such as glutathione reductase (Sharma and Sohn 2009). AsV does not bind as readily to sulfhydryl groups but can be reduced to AsIII which will readily bind. Instead, AsV is a chemical analogue of phosphate and can replace inorganic phosphate in enzymes which then uncouples oxidative phosphorylation in mitochondria thereby disrupting cellular energetics (Mandal and Suzuki 2002; McIntyre and Linton 2011). Factors that affect the bioavailability and hence the toxicity of arsenic in seawater include pH, temperature, light, salinity, redox, phosphate, suspended solids, dissolved organic matter and biotransformation (Sharma and Sohn 2009). However, there are insufficient data on the toxicity modifying effects of these parameters to derive bioavailability-based GVs.
Existing and proposed marine GVs for arsenic from a range of jurisdictions are not robust due to sparse chronic toxicity data and the application of assessment factors that result in interim GVs of low reliability. The current ANZG (2018) marine GV is a low reliability, interim working level of 4.5 µg AsV L–1 derived from an insufficient dataset and is to be used for interim guidance. This value was derived by applying an assessment factor of 200 to the lowest chronic toxicity value (no observed effect concentration, NOEC) of 893 µg L–1 obtained for a crustacean. Canada also has a chronic interim marine GV for total arsenic of 12.5 µg L–1 derived by applying an assessment factor of 10 to the lowest toxicity value (lowest observed effect concentration, LOEC) of 125 µg L–1 for a diatom (CCME 2001). A total arsenic marine criterion continuous concentration (CCC) value of 36 µg L–1 was based on AsIII toxicity data by USEPA (1995a). The UK technical advisory group proposed a long-term marine environmental quality standard (EQS) for total arsenic of 0.6 µg L–1 above background arsenic levels based on the lowest EC10 (equivalent to a NOEC) of 6 µg AsV L–1 to sea urchin embryo–larval development with an assessment factor of 10 applied (Lepper et al. 2007). This was a factor of 40 lower than the existing EQS. Karthikeyan et al. (2021) proposed long-term marine arsenic GVs for India based on AsIII ranging from 3.1 to 5.9 µg L–1 depending on the software and endpoints used in the species sensitivity distribution (SSD) to derive the GV. Clearly, there are a range of marine arsenic GVs that vary in their level of reliability, form of arsenic used to derive the GV and method of derivation.
The recommended approach for deriving long-term protective water quality GVs for Australia and New Zealand is to use SSDs based on ecologically relevant, chronic biological endpoints and preferably, 10% effect toxicity values (EC10) or no effect concentrations (NECs) (Batley et al. 2018; Warne et al. 2018). The advantages of this approach are that the entire data set contributes to the final GV rather than the most sensitive data point and multiple fractions of the probability distribution can be selected to derive multiple levels of protection for the ecosystem. Also, SSDs avoid adding uncertainty and producing unnecessarily conservative GVs as a consequence of applying arbitrary assessment factors and acute-to-chronic ratios to the most sensitive data point. However, uncertainty is still associated with GVs derived using SSDs particularly when trying to protect 99% of all species and using a distribution that is based on only a few data points. Therefore, minimum recommended guidance provided by Warne et al. (2018) and Batley et al. (2018) on deriving water quality GVs using SSDs has been adopted nationally (ANZG 2018).
In this study, we derived marine water quality GVs for AsV that can be used to provide 80, 90, 95 and 99% levels of long-term protection to temperate and tropical marine biota. The chronic toxicity data consisting of 13 marine species from 7 taxonomic groups, were used to derive the GVs using the SSD approach based on measured dissolved arsenic concentrations. These GVs will be more reliable than the current interim GV and provide certainty to future risk assessments of AsV in marine waters.
Experimental
Preparation of bioassay solutions
Unless otherwise stated, all glass and plasticware used were soaked in dilute (5% or 10% v/v) nitric acid (69% AnalaR NORMAPUR®, BDH PROLABO VWR Chemicals, 70% Ajax Finechem UNIVAR ACS) for at least 24 h and subsequently rinsed at least five times with deionised Milli-Q water (18.2 MΩ cm–1, Millipore) prior to use. All chemical reagents were of analytical grade. Arsenic stock and bioassay solutions were prepared on the same day or 1 day prior to test commencement from disodium arsenate, AsV (Na2HAsO4∙7H2O, May & Baker Ltd./E Merck AG, Darmstadt or Sigma Aldrich, Australia). Bioassays performed by CSIRO Land and Water, Lucas Heights, New South Wales (NSW), Australia and Ecotox Services Australasia (ESA), Lane Cove, NSW, Australia used arsenic stocks prepared in filtered sea water (FSW; 35 PSU, 52.8 mS cm–1, pH 8.1, dissolved organic carbon 1 mg L–1) that were diluted further in FSW. Seawater (FSW) was collected from Cronulla, NSW, Australia (34°04′13.35″S, 151°09′25.69″E), in acid-washed 5 L high density polyethylene containers, filtered (0.45-µm Sartobran P MidiCaps, Sartorius) and stored at 4°C in the dark until time of use. Bioassays performed by the Australian Institute of Marine Science (AIMS), Darwin, Northern Territory (NT), Australia used arsenic stocks prepared in Milli-Q water (Millipore) with small volume aliquots (0.3–4% v/v) that were diluted further with FSW (31–34 PSU) collected from Channel Island, NT (12°33′24″S, 130°51′39″E), UV-sterilised and filtered (0.5 µm ITM spun polypropylene cartridge).
Bioassay overview and quality assurance
A total of 13 marine species (5 tropical and 8 temperate species) representing 7 taxonomic groups (Table 1) were used in bioassays performed by one of three organisations (CSIRO, ESA or AIMS). Test solutions were prepared by respective organisations with the exception of the test solutions used by ESA that were prepared by CSIRO. All bioassays were defined as having chronic exposures, based on life stage, endpoint and bioassay duration (Warne et al. 2018). Bioassays were performed at least twice with at least two replicates for each treatment and control. Physico-chemistry (pH, dissolved oxygen (DO), salinity and conductivity) was measured in the bioassay solutions at the beginning and end of each bioassay with the exception of some algal tests where only pH was measured at the end. Measurements were made using a Hach multiprobe HQ40d or a selection of probes (pH measured with a Thermo Orion Ross (815 600) probe, salinity and conductivity measured with a YSI30 probe, DO measured with a WTW Ox325 probe). Water temperature was recorded continuously with electronic temperature loggers (Hoboware or MultitripTM) or daily with a thermometer positioned in the incubator throughout each test. Dissolved arsenic (<0.45 µm; acid-washed syringe filters; Acrodisc, Pall; Minisart, Sartorius) was measured in test solutions at the beginning of every bioassay as a minimum requirement. Water samples (5 mL) of total arsenic (filtered and unfiltered) were collected from test solutions at the beginning and end of selected bioassays, preserved with 0.13% or 0.2% (v/v) high purity nitric acid (TracePur, Merck or Ajax Finechem) and stored at 4°C in the dark prior to elemental speciation analysis (see below for further details). Copper (as CuSO4∙5H2O, Pronalys Selby Biolab) reference toxicant tests were performed by CSIRO and ESA with every bioassay and periodically performed by AIMS as part of quality assurance. A summary of test conditions and acceptability criteria for the controls and reference toxicant tests as well as the measured physico-chemistry for each bioassay is given in Supplementary Tables S2–S4.
Microalgal growth rate
The effect of arsenic on six microalgal species representing three taxonomic groups from tropical and temperate environments was investigated using diatoms: temperate Ceratoneis closterium (formerly known as Nitzschia closterium), temperate Phaeodactylum tricornutum Bohlin; green algae: temperate Tetraselmis sp., temperate Dunaliella tertiolecta Butcher, temperate Chlorella salina Butcher; and Haptophyte: tropical Tisochrysis lutea (formerly known as Isochrysis galbana). For details on algal taxonomic strain and source please refer to Supplementary Table S2.
All algae were cultured in media, maintained and tested at CSIRO, Lucas Heights with the exception of T. lutea which was cultured and tested at AIMS, Darwin. Chlorella salina was newly imported to CSIRO from the Culture Collection of Algae and Protozoa (CCAP) and cultured in CCAP f/2 media (Guillard and Ryther 1962) at 21°C with 12:12 h light (70–120 µmol photons m–2 s–1 photosynthetically active radiation (PAR))/dark. Bioassays were performed according to standard methods in the OECD (2011) with adjustments described by Stauber et al. (2005), Franklin et al. (2005) and Trenfield et al. (2015).
Bioassays were conducted in borosilicate glass Erlenmeyer flasks (125 or 250 mL) or borosilicate glass scintillation vials (20 mL) (Supplementary Table S2). Both vials and flasks were internally coated with Coatasil (APS Ajax Finechem) silanising solution to reduce metal loss via adsorption to the glass. Controls and treatments were replicated two to three times with 6, 50 or 100 mL replicate volumes. Nutrients were added to all control and AsV test solutions to achieve final concentrations of 1.5 mg NO3− L–1 (as NaNO3) and 0.15 mg PO43− L–1 (as KH2PO4) for all algal species with the exception of T. lutea (2.5 mg NO3− L–1 and 0.025 mg PO43− L–1). Trials were conducted by CSIRO on all algal species with the exception of T. lutea to ensure that nutrient concentrations were sufficient to produce exponential growth throughout the bioassay but were minimised to reduce phosphate competition with arsenic (data not shown). Algae were harvested from cultures during their exponential growth phase by a process of centrifugation and washing with FSW to remove residual culture medium (Supplementary Table S2). An initial algal cell density of 2–4 × 103 cells mL–1 in test solutions was used for all algal species with the exception of T. lutea (1 × 104 cells mL–1). Algal cell density in each replicate was measured daily for 72 h in the case of all algae with the exception of T. lutea (72-h measurements only) and C. salina (daily measurements for 48 h). Flow cytometry (BD-FacsCalibur or BD-Acurri C6, Becton Dickinson) was used to measure algal cell density (Franklin et al. 2005; Stauber et al. 2005). Two algal species (C. closterium and C. salina) required cells to be detached from the glass surface and homogenised gently to reduce clumping of cells prior to cell density measurements. Replicates were manually shaken twice daily or, in the case of C. salina, placed on a shaker table (90 rpm), and spatially randomised twice daily. Bioassays were maintained in temperature-controlled incubators at 21 ± 1 and 28 ± 1°C for respective temperate and tropical species with a light cycle of 12:12 h light (70–120 µmol photons m–2 s–1 PAR)/dark.
Inhibition of the algal population growth rate was calculated as the biological endpoint for each algal species with the exception of T. lutea, as the slope of the linear regression of log10 algal cell density as a function of multiple time measurements where exponential growth was verified. The average specific growth rate endpoint for T. lutea was calculated as the slope of the natural logarithmic increase in average cell density from the initial to the final cell density measurement as a function of exposure time, i.e. 72 h (OECD 2011).
Anemone asexual reproduction
Adult glass anemones (Exaiptasia pallida) used in the test were cultured in the aquaria facility at AIMS; the source population originated from Darwin Harbour, NT, Australia. Culture of the anemones and test methods followed those described by Trenfield et al. (2017) (Supplementary Table S3). Bioassays were conducted in clear, cylindrical polypropylene tubs (500 mL) with lids. Triplicate tubs containing 300 mL of test solution, were prepared for each treatment concentration, stocked with five adult anemones (3–5 mm oral disc) each and incubated for 14 days at 28 ± 1°C in 12:12 h light (60–100 µmol photons m–2 s–1 PAR)/dark conditions. Feeding of anemones and renewal of test solutions were conducted three times weekly as detailed by Trenfield et al. (2017) (Supplementary Table S3). After a 14 day incubation, adult anemones were removed and each tub was examined under a stereomicroscope to determine the number of lacerates (proto-anemones which partitioned from adults) and juvenile anemones produced and the inhibition of lacerate production per adult was calculated as the biological endpoint.
Barnacle larval development
Adult barnacles (Amphibalanus amphitrite) used as broodstock had been settled on cement bricks and grown in the aquaria facility at AIMS, Darwin, NT, and originated from a population sourced from Darwin Harbour. Methods followed those described by van Dam et al. (2016) with slight modifications. Bioassays were conducted in bespoke 250 mL borosilicate glass funnels (with lids), which were coated with Coatasil (Ajax Finechem) silanising solution. Triplicate funnels with test solutions (100 mL) were prepared for each treatment concentration, gently aerated from the bottom (~1 bubble s–1 to keep larvae and algal food suspended) and incubated for 96 h at 28 ± 1°C in 13:11 h light (100–120 µmol photons m–2 s–1 PAR)/dark. Larval release was triggered by leaving the broodstock to dry overnight and re-submersion in the morning. Freshly released phototactic stage II nauplii were concentrated by directing a point-source light in a corner of the spawning vessel and collected using a wide-bore pipette. On the morning of test initiation, test vessels were filled with 80 mL of test solution, aeration was commenced and 107 cells of rinsed Chaetoceros muelleri were added from a concentrated stock prepared the day before. Fifty freshly released larvae were randomly selected using a stereomicroscope and added to a 15 mL polypropylene tube containing 10 mL of test solution. As soon as all tubes held 50 larvae, these were transferred to the associated funnels. Tubes were rinsed with 10 mL of test solution to ensure all larvae had been transferred. Aeration was monitored twice daily and algae (107 cells of rinsed C. muelleri) were added to each funnel daily. At 96 h, funnel contents were drained over a 150 μm nitrile mesh and the number of cyprids and settled larvae scored as detailed by van Dam et al. (2016). The percentage of larvae that had transitioned to cyprid stage over 96 h was calculated and used as the biological endpoint.
Copepod larval development
Copepods (Acartia sinjiensis) were cultured and bioassays performed by CSIRO, Lucas Heights using animals originally collected offshore of Townsville, Queensland and cultured long-term in the laboratory at 30 ± 1°C with 18:6 h light (~8 µmol photons m–2 s–1 PAR)/dark conditions, gentle aeration and food consisting of a mixture of algae (Proteomonas sulcata and T. lutea) (Gissi et al. 2013). Standard methods were based on OECD (2005) and adapted to this species of copepod (Binet et al. 2019).
The final test solution volume was 180 mL per chamber after the initial volume of 60 mL on Day 0 was supplemented with 120 mL of newly prepared test solution on Day 2. Four replicate test containers (250 mL polycarbonate with loose lids) per treatment were maintained under the same conditions as those for culturing but without aeration. Food consisting of a mixture of Tetraselmis chuii and T. lutea washed and resuspended in FSW was added on Day 0 and Day 2 (Supplementary Table S3). Physico-chemistry was measured in test solutions on Day 0, Day 2 and test termination.
Adults were isolated for 24 h prior to test initiation and resulting cysts produced by the adults were used to start the bioassay at a density of 40–60 cysts per chamber. After 80 h, the bioassay was terminated by the addition of Rose Bengal (0.002% m/v final concentration) in 10% buffered formalin (5% v/v final concentration) to the test solutions which were then stored at 4°C for 24 h. The number of copepodites and nauplii at different developmental stages were determined by stereomicroscopy (Olympus SZX10, 20× magnification) and the percentage of copepodites as a fraction of the sum of copepodites plus nauplii was calculated in each replicate and averaged for each treatment as the biological endpoint.
Oyster, mussel and sea urchin larval development
Adult oysters (Saccostrea glomerata, formerly Saccostrea commercialis) and mussels (sequenced as Mytilus galloprovincialis Lamarck, 1819, Supplementary Material S5) were harvested from aquaculture farms in NSW and Victoria, Australia while adult sea urchins (Heliocidaris tuberculata) were collected sub-tidally from coastal NSW, Australia. Bioassays were performed by ESA (oyster) and CSIRO (mussel and sea urchin) within 48 h of field collection. Standard methods used in these bioassays were based on APHA (1998) and Krassoi (1995) for the oyster, ASTM (2012) and USEPA (1995b) for the mussel and USEPA (1995b) and Doyle et al. (2003) for the sea urchin.
Test solutions (5 mL) were dispensed into new unwashed disposable borosilicate glass tubes (13 mm × 100 mm, Kimble Chase) with three to four replicates per treatment and covered with thin plastic film. Mussel and sea urchin bioassays were maintained at 20 ± 1°C with 16:8 h light (10–20 µmol photons PAR m–2 s–1)/dark conditions while oysters were maintained at 25 ± 1°C with 12:12 h light (80 µmol photons m–2 s–1 PAR)/dark conditions (Supplementary Tables S3, S4).
Gametes were obtained by induced spawning of mussels and sea urchins using temperature shock and injection of 2 M KCl, respectively, while oysters were strip-spawned. Gametes were inspected for quality by microscopy and then combined in a sperm-to-egg ratio of 100:1 for each species to achieve > 90% fertilisation. An aliquot of embryo solution was added to each glass tube to achieve final densities of 300, 500 and 500 embryos chamber–1 for mussels, oysters and sea urchins, respectively. Bioassays were terminated at 48 h in the case of the mussel and oyster or 72 h in the case of the sea urchin by the addition of buffered formalin (10%). The first 100 larvae of each species was assessed by microscope (100× magnification) as having normal development (D-shape veliger for the bivalves and symmetrical pluteus larva for the sea urchin) or abnormal development (delayed, asymmetrical or deformed development). The percentage of normal larval development was used as the biological endpoint to derive effect concentrations.
Snail larval growth
Adult snails (Nassarius dorsatus) used as broodstock were field collected from an uncontaminated site near Darwin, NT, and maintained in the aquaria facility at AIMS, Darwin, NT. Test methods were based on those described by Trenfield et al. (2016), with the following modifications: the test duration was extended from 4 to 7 days so that the test obtained chronic classification following criteria set by Warne et al. (2018): snail larvae were fed rinsed C. muelleri only (2 × 104 cells mL–1), bioassays were conducted in polystyrene six-well plates (the combined wells within a single plate (6 wells × 10 mL) were considered one replicate) with lids; larval density was increased to 0.5 larva mL–1 (90 larvae per treatment) and all surviving larvae were measured at the end of the test. Triplicate plates were prepared for each treatment concentration to provide true replication and incubated for 168 h (7 days) at 28 ± 1°C in 12:12 h light (60–100 µmol photons m–2 s–1 PAR)/dark.
Larvae were obtained from adult snails that deposited egg cases on cut polycarbonate pipes that were transferred to tanks containing flow-through 0.5 µm filtered FSW. After 2 days, the tanks were left static and transferred to incubators. Once the eggs had hatched into trochophore larvae, they were fed with unwashed C. muelleri at approximately 2 × 104 cells mL–1. Veliger larvae (48 h old) were randomly selected to start the test and a sub-sample (20–35 individuals) was photographed at 250x magnification (Leica DFC320 camera on a Leica DM4000B microscope) to determine mean start-of-test shell size using image software (GIMP, V2.8.18). Larvae were transferred daily to clean exposure solutions and dead individuals removed. At test finalisation, shell size was measured for all surviving larvae to determine daily growth rates. The daily growth rates of the larvae surviving for 7 days were used as the biological endpoint for deriving effect concentrations.
Influence of nutrients on arsenic toxicity
Bioassays using C. closterium, P. tricornutum, Tetraselmis sp., H. tuberculata and M. galloprovincialis were performed according to the methods described above with a range of phosphate (0, 0.015, 0.15 mg L–1 added as KH2PO4) and nitrate (0, 0.15, 1.5 mg L–1 added as NaNO3) added at a 16:1 ratio of N/P in FSW. These concentrations were 0× (i.e. background phosphate and nitrate in FSW), 0.1× and 1× the normal concentration of phosphate and nitrate that was added to an algal bioassay, respectively. Nutrients are not normally added to the sea urchin and mussel bioassays, however for the purposes of this experiment, the same nutrient concentrations as the algal bioassays were applied for consistency. Arsenic(v) concentrations (1–100 000 µg L–1) for each nutrient treatment were extended over a broad range to determine the extent of toxicity mitigation by the addition of the nutrients. Because this was a range-finder experiment, treatments were not replicated in the microalgal bioassays but were in triplicate for the sea urchin and mussel bioassays. Matrix-matched controls were incorporated into the standard suite of quality control procedures and dissolved (<0.45 µm) phosphorus at the beginning and end of each bioassay was measured as described below.
Arsenic and metal analyses
Filtered (<0.45 µm) and unfiltered water samples collected from bioassay test solutions were preserved and stored as mentioned above. They were analysed for multiple metals, together with total arsenic and phosphorus, using either inductively coupled plasma atomic emission spectrometry (ICP-AES, Agilent 720 performed by CSIRO, NATA-accredited, limit of detection (LOD) = 6.4 µg As L–1) or inductively coupled plasma mass spectrometry after high performance liquid chromatography (HPLC-ICPMS Agilent LC1260 and Agilent 7700×, performed by Envirolab Services Pty Ltd, NATA accredited laboratory, practical quantitation limit (PQL) = 1 µg As L–1). The ICP-AES and HPLC-ICPMS operating conditions and calculation of the LOD and PQL are given in the Supplementary Material (see section that includes Supplementary Table S6). Matrix-matched standards were used and the requirement of acceptability for the drift standards, duplicates and metal recovery of standard waters that were incorporated into each analytical batch for quality assurance and control are given in the Supplementary Material (see section that includes Supplementary Table S7). Measured values that were less than the LOD, i.e. in the controls, were substituted as half the LOD or PQL for the derivation of effect concentrations.
Arsenic speciation was measured in selected bioassay solutions at initiation and termination. Filtered water samples were taken from a N. dorsatus (snail) test, an E. pallida (anemone) test and a T. lutea (microalga) test, preserved in hydrochloric acid (3 mL of 6 M HCl L–1) and kept frozen until analysis. AB, DMA, MMA, inorganic AsIII and AsV were all analysed using HPLC-ICPMS by Envirolab Services Pty Ltd.
Statistical analyses
Concentrations of dissolved arsenic that resulted in 10, 20 and 50% biological effects (i.e. IC/ECx where x = 10, 20 or 50) relative to the control were calculated using prescribed statistical procedures from OECD (2006), and the R package drc (Ritz and Streibig 2005; R Development Core Team 2016). Response data (as defined in the methods above) from multiple bioassays for each test organism were pooled and effect concentrations were based on initial measured dissolved arsenic assuming all arsenic was present as AsV which was verified on a subset of test solutions (see results on arsenic speciation in bioassays below). Non-linear regression models evaluated included logistic, log-logistic and Weibull models of different levels of parameterisation. Models were compared using the Akaike Information Criterion (Pinheiro and Bates 2000) and a visual assessment of fit and those that best described the data were applied to derive estimates of toxicity. The associated 95% confidence limits were estimated using the delta method (Ritz and Streibig 2005).
EC/IC10 values representing chronic biological responses to AsV were used to derive the water quality GVs. Toxicity data for marine biota were collated from the literature cited in the ANZECC/ARMCANZ (2000) document as well as peer-reviewed publications since 2000. A quality assessment protocol was applied to the literature which scored data according to measures of quality control and assurance (Warne et al. 2018). Only high-quality publications that scored ≥80% (which is more stringent than the recommended score of ≥50% (Warne et al. 2018)), used measured arsenic concentrations and were chronic endpoints, were considered for inclusion in the GV derivation process.
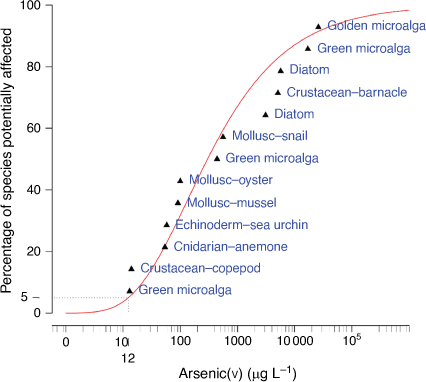
An SSD was used to derive the GVs as it best represents the diversity in biological sensitivity to arsenic rather than focusing on the most sensitive response. The Burrlioz software (Version 2.0) automatically fits a three-parameter Burr Type III distribution to the data when there are greater than eight data points. The 80th, 90th, 95th and 99th percentiles of the distribution were derived with 95% confidence limits to provide four levels of ecosystem protection. Protection of 99 and 95% of species (PC99 and PC95) was applied to areas of high conservation value and areas which had slightly to moderately disturbed ecosystems, respectively. Protection of 90 and 80% of species (PC90 and PC80) was applied to areas that were highly disturbed as agreed by stakeholders.
Results and discussion
Arsenic speciation in bioassays
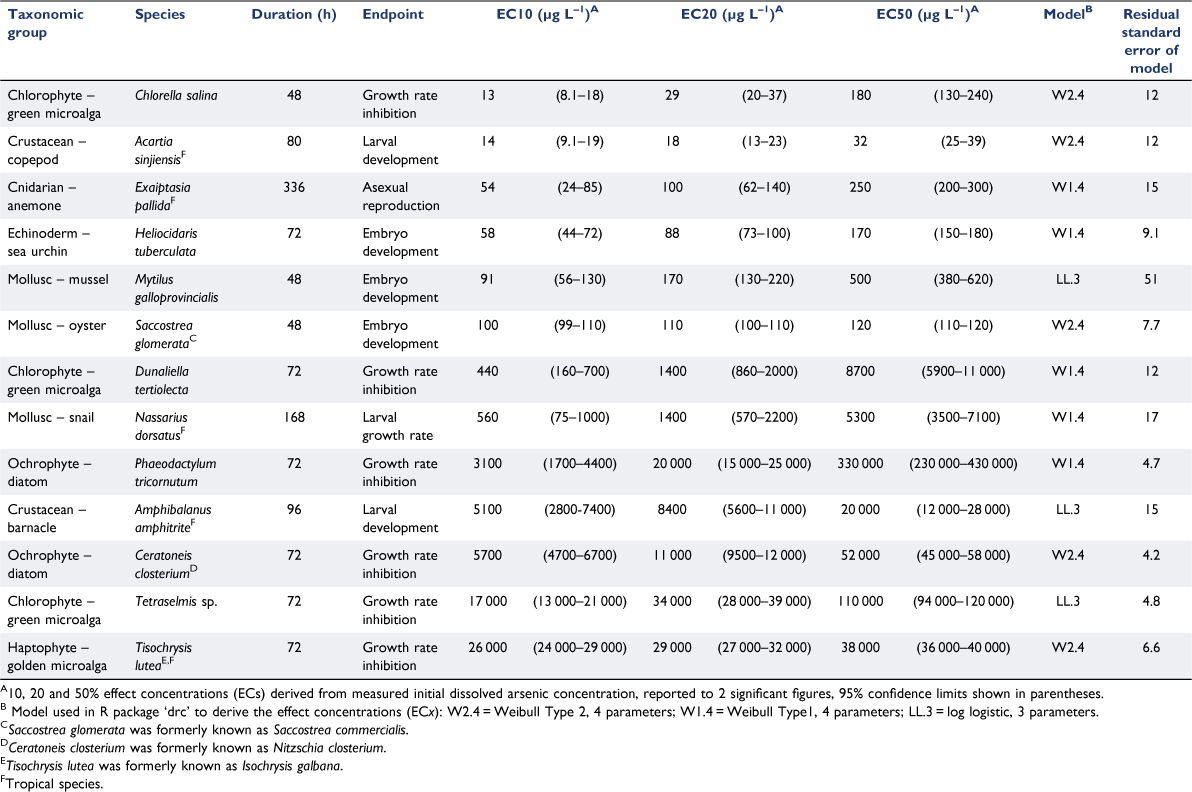
Arsenic was highly soluble in all bioassay solutions, with 98 ± 7.5% (mean ± s.d., n = 266) of total arsenic in a dissolved (<0.45 µm filtered) form. The close 1:1 relationship between measured dissolved and total arsenic concentrations was consistent across the range of arsenic concentrations analysed at the beginning and end of selected bioassays (Fig. 1). Measured dissolved arsenic concentrations did not change (factor of 1.0 ± 0.22 (mean ± s.d., n = 83)) over bioassay durations of 48–80 h at temperatures of 20–30°C. These data verified that arsenic in bioassay solutions did not precipitate in seawater up to 100 000 µg L–1 total arsenic, the dissolved arsenic concentration remained unchanged over the bioassay duration covering a range of temperatures and, by inference, that loss from solution by adsorption to surfaces was insignificant. Therefore, effect concentrations were calculated based on the initial dissolved arsenic concentrations.
|
Analysis of inorganic and organic arsenic forms in the test solutions of select bioassays showed that 95 ± 15% (mean ± s.d., n = 28) of the dissolved arsenic was present as inorganic AsV and all other forms of arsenic were below the detection limit (1 µg L–1 AsIII, AB, DMA; 5 µg L–1 MMA) over the duration of the bioassay (Supplementary Table S8). The exception was the anemone bioassay, where up to 3.3 and 1.9% of total dissolved arsenic was present as AB and DMA, respectively, by the end of the 14 day bioassay.
Overall, these data confirm that the derived biological effect concentrations were a function of exposure to dissolved inorganic AsV rather than dissolved inorganic AsIII, methylated or precipitated arsenic forms. While these data demonstrate the stability of AsV in chronic bioassays, future chronic studies using inorganic AsIII should carefully consider changes in speciation over time and how best to preserve and analyse the samples.
Quality assurance of toxicity bioassays
All bioassays met the requirements of acceptability in the controls and reference toxicant tests as described in Supplementary Tables S2–S4. The chronic toxicity data for all bioassays are available in Supplementary Table S9. Dissolved arsenic measured in the control treatments that used FSW sourced from Cronulla and Channel Island was less than the LOD of 6.4 and 3.0–4.5 µg L–1, respectively.
Chronic toxicity of AsV to microalgae and the influence of nutrients
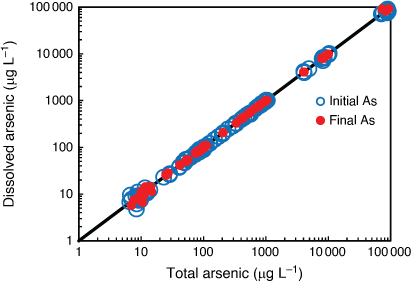
AsV toxicity to the six microalgal species was species-specific with EC10 values ranging from 13 to 26 000 µg L–1 representing the most and least sensitive of the 13 marine organisms tested (Table 1). There was no taxon-specific sensitivity to arsenic with the order of the most to the least sensitive microalga being C. salina (green alga) > D. tertiolecta (green alga) > P. tricornutum (diatom) > C. closterium (diatom) > Tetraselmis sp. (green flagellate) > T. lutea (golden microalga). The only tropical microalga (T. lutea) was also the most tolerant species of all 13 species tested. The shape of the concentration–response curves was also varied between species with the steepest portion of the curves for all microalgae encompassing 1–4 orders of magnitude in AsV concentration (Fig. 2) suggesting that different mechanisms of toxicity and detoxification occurred with each microalga. The strategies that different microalgae use to detoxify AsV include cell surface binding, biotransformation to less toxic organoarsenic forms and complexation with GSH and phytochelatins followed by sequestration in vacuoles and excretion (Levy et al. 2005; Morelli et al. 2005; Karadjova et al. 2008; Wang et al. 2015). However, above a threshold concentration of arsenic, detoxification strategies become overwhelmed and toxicity occurs.
|
AsV toxicity values (EC50) obtained from the literature for four of the tested microalgal species ranged from 2.3 to 340 000 µg L–1 demonstrating a 250 times wider range in the EC50 values from the current study (Supplementary Table S10). Comparing the microalgal toxicity values from this study with those from the literature was complicated by the fact that differences existed in the quantification of algal growth (i.e. chlorophyll a, optical density, cellular counts), initial algal cell densities used, test media (growth medium, synthetic seawater, natural seawater) and the concentrations of phosphate added (Supplementary Table S10). These factors (particularly the phosphate concentration) require standardisation in order to make valid comparisons of toxicity results between different microalgae. For example, the most sensitive microalga in the current study (C. salina) had a 48 h EC50 of 180 µg L–1 (130–240 µg L–1, 95% confidence limits) in the presence of 0.15 mg L–1 phosphate compared to the same species tested by Karadjova et al. (2008) that had 72 h EC50 values ranging from 2.3 to 1200 µg L–1 depending on the concentration of phosphate (0.031–4.0 mg L–1) in the test solution (Supplementary Table S10). There was no similar phosphate concentration or initial algal cell density to enable a valid comparison between the studies. Similar incompatibilities occurred where literature values could be found for D. tertiolecta, Tetraselmis sp., and T. lutea (Supplementary Table S10). It is well known that experimental differences such as test medium, pH, temperature, redox potential, exposure duration, light intensity and photoperiod influence the toxicity of arsenic to microalgae (Karadjova et al. 2008; Wang et al. 2015). For future arsenic toxicity tests using microalgae, we recommend that: (i) a low environmentally relevant initial algal cell density i.e. 1 × 103 cells mL–1 be used, (ii) phosphate and nitrate concentrations be reported and that concentrations are low enough to maintain exponential growth but not high enough to artificially reduce arsenic toxicity and (iii) algal population growth rate (as opposed to algal cell yield) based on cell density be used as the endpoint so that tests of duration ≥ 48 h can be compared.
The addition of phosphate and nitrate have the potential to reduce arsenic toxicity to marine microalgae although there is uncertainty regarding the mechanism of amelioration (Wang et al. 2015). Karadjova et al. (2008) showed that increasing the phosphate concentration 12 fold, decreased the toxicity of AsV to C. salina 520 fold. This corresponded with a decrease in the internalisation flux of AsV and a decrease in the intracellular arsenic concentration but did not significantly change the conditional binding constant for AsV at the membrane transport sites, therefore, the interaction between phosphate and AsV could not be explained by competitive inhibition at the transport site. The pilot trial conducted in the present study also found a trend of decreasing AsV toxicity with increasing nutrients (phosphate and nitrate at a constant ratio of 16:1) in two (diatoms C. closterium and P. tricornutum) of the three microalgae used (Supplementary Fig. S11). A very different response occurred with the green microalga (Tetraselmis sp.) whereby increasing AsV without additional nutrients stimulated microalgal growth and had little to no effect on microalgal growth when nutrients were added (Supplementary Fig. S11). Therefore, the interaction between phosphate and AsV was specific to the microalgal species. This was also demonstrated by the different patterns of 72 h phosphorus depletion by the microalgae in the corresponding test solutions (Supplementary Fig. S11). The AsV-sensitive diatom C. closterium and the AsV-tolerant green microalga, Tetraselmis sp., both markedly depleted the phosphorus from the test solutions with increasing AsV within 72 h, while P. tricornutum did not. While these are pilot trial findings, they warrant a deeper investigation to determine the role of phosphate and nitrate as AsV toxicity modifying factors for marine microalgae.
Chronic toxicity of AsV to crustacea
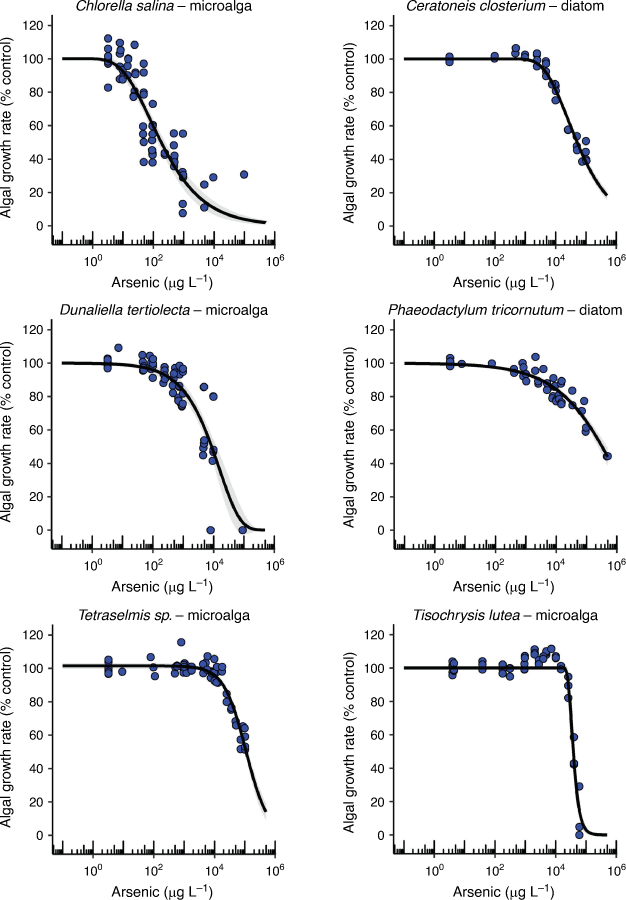
The two tropical crustacea varied 364-fold in their chronic EC10 values (14 and 5100 µg L–1) with larval development of the copepod, A. sinjiensis, being the second most sensitive endpoint to AsV (Table 1, Fig. 3). There are no comparable chronic AsV toxicity data available in the literature that are based on measured arsenic concentrations for marine copepods. However, if acute LC50 data were converted into estimated chronic EC10 data using a default acute-to-chronic ratio of 10, values for Paracyclopina nana and Tigriopus japonicus would be 1080 and 1720 µg L–1, respectively (Lee et al. 2008; Byeon et al. 2020). This would suggest that larval development of A. sinjiensis was 77–123 times more sensitive than either P. nana or T. japonicus to AsV.
|
Adult barnacles have traditionally been used as biomonitors for arsenic contamination in marine environments (Rainbow and Blackmore 2001). These are the first toxicity data for the chronic effects of AsV on barnacle larval development (5100 µg L–1 EC10) and indicate that A. amphitrite is tolerant to AsV which makes it ideal as a biomonitor (Table 1).
Chronic toxicity of AsV to an anemone
Asexual reproduction in the tropical E. pallida was the third most sensitive endpoint to AsV toxicity with an EC10 value of 54 µg L–1 (Table 1, Fig. 3). While bioaccumulation and the unusual speciation of arsenic in anemones has been studied (Ninh et al. 2008), there are no other known arsenic toxicity data for comparison. The unusual speciation of organoarsenicals in anemones is related to the presence of microalgal symbionts and may have contributed to the detection of organoarsenicals in the test solutions (Supplementary Table S8). The role of Symbiodinium sp. in the toxicity of AsV to anemones is unknown but worthy of further investigation given the role of phosphate in modifying toxicity to some microalgae.
Chronic toxicity of AsV to an echinoderm and the influence of nutrients
H. tuberculata 72 h larval development had an EC10 value of 58 µg L–1 that was very similar to the EC10 for effects on asexual reproduction in E. pallida (Table 1, Fig. 4). There are no comparable chronic EC10 values in the literature, however, Garman et al. (1997) found a significant reduction in normal sea urchin (Strongylocentrotus purpuratus) larval development at the lowest nominal AsV concentration of 11 µg L–1 (48 h LOEC) which suggests that the different species may have similar sensitivities. These developmental abnormalities were found to correlate with DNA protein cross-linkage induction which indicates that AsV was causing DNA damage that could translate into development abnormalities (Garman et al. 1997).
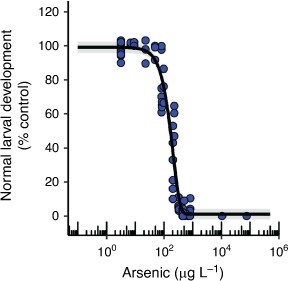
|
Additional phosphate (0.015 and 0.15 mg L–1 as KH2PO4) and nitrate (0.15 and 1.5 mg L–1 as NaNO3) in the seawater significantly reduced the toxicity of AsV to sea urchin larval development by as much as fifty times at AsV concentrations of 95 µg L–1 and above (Supplementary Fig. S12). This relationship was not accompanied by a marked reduction in dissolved phosphorus in the test solutions over time as was observed for two of the microalgal species (Supplementary Fig. S12). It has been found that contaminants can be more toxic at certain sea urchin larval developmental stages with the 8-cell stage being sensitive to AsV toxicity (Garman et al. 1997; Gaion et al. 2013). Certain stages of sea urchin embryo development are also reliant on accelerated phosphate uptake which could make that developmental stage vulnerable to toxicity from AsV if the same uptake channel is shared. However, AsV toxicity may be reduced in the presence of additional phosphate due to competitive inhibition of AsV uptake at the phosphate uptake channel (Chambers and Whiteley 1966). Therefore, the role of phosphate as a modifying factor for AsV toxicity to sea urchin larval development requires further investigation.
Chronic toxicity of AsV to molluscs and the influence of nutrients
The mussel (M. galloprovincialis) and oyster (S. glomerata) embryo–larval development were similarly sensitive to AsV with chronic EC10 values of 91 and 100 µg L–1, respectively (Table 1, Fig. 3). The growth rate of the tropical marine snail (N. dorsatus) was less sensitive and more variable with an EC10 value of 560 µg L–1 (Table 1, Fig. 3). The concentration response curves for all three species differed with a very steep partial response section of the curve for the oyster between 100 and 102 µg L–1 and much larger slopes for the mussel and snail that covered two orders of magnitude suggesting different mechanisms of toxicity occurred between the species for these endpoints (Fig. 3).
While there were no chronic (≥ 48 h) EC10 values for AsV toxicity to oyster larval development based on measured AsV, there were 48 h EC50 values of 660 and 19 µg L–1 based on AsV measured in the stock solutions for larval development of Crassotrea gigas and Crassotrea angulata, respectively (Moreira et al. 2018). The small (3 mL) test solution volume in a microplate prevented measurements of AsV in the actual test solutions making the exposure concentrations estimated rather than measured concentrations (Moreira et al. 2018). The 48 h EC50 of 120 µg L–1 for S. glomerata larval development (Table 1) was in between the values for C. gigas and C. angulata and demonstrated the broad range in sensitivity to AsV for oyster species. Moreira et al. (2018) also found salinity (20–33 psu) and temperature (20–28°C) to have species-specific effects on AsV toxicity. Increasing salinity caused a marked decrease in AsV toxicity to C. gigas larval development in contrast to C. angulata. Effects of temperature on AsV toxicity were inconsistent for both species.
There were no AsV chronic larval development toxicity data reported in the literature for other mussel or gastropod species to compare with M. galloprovincialis and the tropical N. dorsatus. However, there were studies investigating biomarker and omics responses of AsV exposure in M. galloprovincialis which demonstrated oxidative stress and effects on energy metabolism in both the larval D shape and juvenile stages (Wu et al. 2016; Yu et al. 2016; Coppola et al. 2018; Moreira et al. 2018). The ability of bivalves and gastropods to bioaccumulate arsenic and their high EC10 values (91–560 µg L–1) relative to natural seawater concentrations suggest that these species were tolerant of AsV which justifies their use as sessile biomonitors of contaminants in the case of the bivalves (Neff 1997).
The addition of phosphate and nitrate to the seawater reduced the toxicity of AsV to embryo–larval development of M. galloprovincialis by up to 1.5 times at AsV concentrations of 1000 µg L–1 and above, with no change to the dissolved phosphorus over time in the test solutions (Supplementary Fig. S12). While the mechanism by which phosphate reduces AsV toxicity is unknown, there is likely to be competitive inhibition of AsV uptake in the presence of excess phosphate that should be investigated in the first instance.
Arsenic GV derivation
Overall, the broad range in chronic EC10 values (13–26 000 µg AsV L–1), EC20 values (18–34 000 µg AsV L–1) and EC50 values (32–330 000 µg AsV L–1) covered three to four orders of magnitude and demonstrates that there are species-specific mechanisms of detoxification that have evolved for exposure to AsV (De Francisco et al. 2021). There was no one taxon that was more sensitive than all others and the range in EC10 values for temperate (13–17 000 µg AsV L–1) and tropical (14–26 000 µg AsV L–1) species overlapped, as was the case for the EC20 and EC50 values so that there were no apparent biases in the data set related to taxonomic group or tropical versus temperate species.
Chronic EC10 values for 13 marine species representing 7 taxonomic groups were based on measured dissolved AsV concentrations and were used in an SSD to derive chronic GVs for long-term protection of 80, 90, 95 and 99% of marine species (Tables 1, 2, Figs 2–5). The EC10 values from both tropical (25–30°C) and temperate (20 and 21°C) species were among the lowest and highest values and were combined for deriving the GVs using the SSD.
|
|
A literature search was conducted for AsV toxicity values to augment those produced from the current study. Unfortunately, none of the studies met our quality assessment criteria of scoring greater than 80% using scoring criteria from Warne et al. (2018) and being based on measured AsV from test solutions. The use of nominal rather than measured arsenic concentrations and no verification of arsenic speciation was a common problem. Microalgal toxicity data were also problematic due to the variability of the test conditions used as described earlier and did not score above 80%. Moreira et al. (2018) came close to meeting the requirements with scoring greater than 80% but used measured arsenic from stock solutions rather than test solutions because of the small test volume (3 mL) utilised, and derived chronic EC50 values which would have required an assessment factor to be applied to convert them into chronic EC10 values. To improve the reliability of the final GV, we prioritised EC10 values from fewer data over using arbitrary assessment factors to adjust the toxicity values and increase the number of data points, i.e. prioritising data quality over data quantity. A literature search for AsV toxicity data for marine fish revealed no chronic toxicity data and identified a data gap for future research.
The GVs for the 80–99% species protection levels ranged by a factor of 10 from 4.8 to 48 µg L–1 and the 95% confidence limits were similarly broad (Table 2). The PC95 of 12 µg L–1 dissolved AsV will be the most commonly applied GV as it aims to protect 95% of marine species in slightly to moderately disturbed systems (Table 2). The PC95 is 2.7 times higher than the current low reliability interim GV (4.5 µg L–1, (ANZG 2018)), is very similar to the chronic interim GV derived for total arsenic in Canada of 12.5 µg L–1 (CCME 2001) and is not at the extreme ends of the range that encompasses the other arsenic GVs previously mentioned. The PC95 is protective of the most sensitive endpoint in the database for the population growth rate inhibition of the microalga C. salina (EC10 of 13 µg L–1) and is not overly conservative when compared to measured background concentrations of AsV in coastal waters (Supplementary Table S1). It should be noted that the PC95 was above the LOD for ICP-AES (6.4 µg L–1) and PQL for ICP-MS (1 µg L–1), however, the PC99 was below the ICP-AES LOD but still above the ICP-MS PQL which should be the preferred analytical instrument of the two available options in this case, if trying to measure arsenic concentrations at the PC99 level of protection. In the case of site-specific marine AsV GVs, background AsV concentrations may need to be accounted for.
The classification of the reliability of the GVs was based on the number of datapoints used in the SSD, whether assessment factors were applied and the adequacy of the visual fit of the data in the SSD (Warne et al. 2018). In this case, there was a good sample size of 13 chronic EC10 values with no assessment factors used, however, there was a poor fit of the inverse Weibull model automatically selected by Burrlioz for the SSD (Fig. 5). This resulted in the GVs being classified as being of moderate reliability which was an improvement on the low reliability classification of the interim GV.
An alternative SSD software package to the recommended default Burrlioz software, known as shinyssdtools version 0.0.3.9001 (Dalgarno 2018), has been built from the ssdtools R package version 0.3.3 (Thorley and Schwarz 2018) and has potential advantages over the Burrlioz software. The main advantage is that shinyssdtools has a wider range of model distributions that can be applied to the data and has a model-averaging function to derive the final GVs. This is very relevant to improving the fit of the SSD when there are limited data. The PCx (where x = 80, 90, 95, and 99%) values and 95% confidence limits of the GVs using shinyssdtools with model-averaging were: 53 (10–390), 15 (2.3–150), 5.2 (0.68–70), and 0.78 (0.056–18) µg L–1, respectively (Supplementary Table S13, Supplementary Fig. S14). Shinyssdtools gave GVs that were 1.5, 2.3 and 6.2 times lower than Burrlioz GVs at the 90, 95 and 99% protection levels, respectively. The 95% confidence limits were similarly broad and overlapped at each species protection level suggesting no significant differences between GVs from the two software packages. The fit of the model to the data continued to be poor regardless of the software used. Therefore, while the SSD from shinyssdtools gave more protective GVs, there was no improvement in the fit of the model to the data or the reliability of the GVs. This was a consequence of the ongoing issue of working with limited toxicity data. The current guidance defaults to the use of Burrlioz software, however, this may be modified as more appropriate methods for handling small data are developed in the future.
Conclusion
Chronic toxicity data for AsV were generated for 13 temperate and tropical marine species representing 7 taxonomic groups that enabled the derivation of far more reliable marine AsV GVs. There was no one taxa that was more or less sensitive to AsV and no bias to the data with the combined use of tropical and temperate species. The chronic toxicity of AsV to marine species had a broad range of EC10 values from 13 to 26 000 µg L–1 which reflects the broad range of species-specific arsenic detoxification mechanisms. Speciation analysis of the test solutions confirmed that exposure was to dissolved AsV alone with some organoarsenicals produced in the chronic anemone bioassay likely as a result of biotransformation. There was insufficient evidence from the literature of inorganic AsIII being more or less chronically toxic than AsV to marine organisms which supports the need to repeat this investigation with the same marine biota using inorganic AsIII. Initial investigations of the effect of additional phosphate and nitrate on the toxicity of AsV to microalgae growth rate inhibition, and embryo–larval development of sea urchins and bivalves showed that these nutrients reduced AsV toxicity to marine biota but the reduction was species-specific and occurred at AsV concentrations that would be present in highly contaminated marine waters (>80 µg As L–1). Further investigations with varying molar ratios of As, P and N would assist in defining the importance of the ameliorative effect at environmentally relevant concentrations.
This work highlighted important requirements for future research on the toxicity of arsenic to marine organisms: (i) the form of arsenic must be measured in the test solutions to verify the exposure form and concentration with priority given to the speciation of inorganic AsV and AsIII, especially when studying chronic AsIII toxicity due to the possibility of changes to the oxidation state of arsenic by redox or biotransformation, (ii) the modifying effects of phosphate, nitrate, salinity, temperature and dissolved organic carbon require further investigation to assist in predicting arsenic bioavailability under certain water quality conditions, (iii) because the effects of arsenic on microalgae may be confounded by the phosphate and nitrate requirements for their growth, the addition of nutrients to the test medium should be reported and minimised to ensure that exponential growth over the exposure period is achieved without artificially reducing the toxicity of AsV, additionally, microalgal growth rate should be prioritised over yield as an endpoint, to enable the comparison of toxicity data from different exposure periods and (iv) there is a data gap for the chronic toxicity of AsV to marine fish that would enable vertebrates to be represented in the SSD.
The PC80, PC90, PC95 and PC99 values of 48, 22, 12, and 4.8 µg L–1 dissolved AsV respectively, will provide protection with moderate reliability and enable evidence-based water quality management and risk assessment of AsV to marine ecosystems globally.
Data availability
Please contact the authors for additional data requests.
Conflicts of interest
Graeme Batley is an Editor for Environmental Chemistry and was blinded from the peer review process for this manuscript.
Declaration of funding
Funding for research conducted by J. van Dam (AIMS) was supplied by Rio Tinto. A travel scholarship was awarded to M.V. Valdivia from Becas Chile.
Supplementary material
Genetic sequencing data for the mussel; measured dissolved arsenic in marine waters; tables of test conditions for all species; speciation of arsenic measured in test solutions; chronic toxicity data used in concentration–response curves; literature AsV toxicity values and test conditions for microalgae; figures of toxicity modifying effects of additional nutrients to marine biota and total phosphorus levels in test solutions and SSD output from shinyssdtools are provided. Supplementary material is available online.
Acknowledgements
The authors thank Culture Collection of Algae and Protozoa (CCAP), SAMs Ltd, Scottish Marine Institute, Scotland, United Kingdom for supply of the Chlorella salinas algal strain used in toxicity testing; Australian National Algae Culture Collection (ANACC), Tasmania, Australia for supply of microalgal species used in toxicity testing. Myti Blue, Victoria; Eden Farms, NSW for supply of blue mussels, Sarah Stephenson CSIRO, Lucas Heights, NSW for DNA extraction and processing of mussels for taxonomic identification, Monique Binet and Francesca Gissi CSIRO, Lucas Heights, NSW for assistance with the tropical chronic copepod bioassay and Josh King and Chad Jarolimek CSIRO, Lucas Heights, NSW for assistance with arsenic analyses.
References
ANZECC/ARMCANZ (2000) ‘Australian and New Zealand Guidelines for Fresh and Marine Water Quality.’ (Australia and New Zealand Environment and Conservation Council/Agricultural and Resource Management Council of Australia and New Zealand: Canberra, Australia)ANZG (2018) ‘Australian and New Zealand Guidelines for Fresh and Marine Water Quality.’ (Australian and New Zealand Governments and Australian state and territory governments: Canberra ACT, Australia) Available at https://www.waterquality.gov.au/anz-guidelines
APHA (1998) ‘Standard Methods for the Examination of Water and Wastewater’, 20th edn. (Ed. MAH Franson) (American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC)
ASTM (2012). ‘Conducting Static Acute Toxicity Tests Starting with Embryos of Four Species of Saltwater Bivalve Molluscs. E724-98(2012).’ (ASTM International)
ATSDR (2019) Substance Priority List. Agency for Toxic Substances and Disease Registry. Available at https://www.atsdr.cdc.gov/spl/index.html [Verified 9 April 2022]
Barwick M, Maher W (2003). Biotransference and biomagnification of selenium copper, cadmium, zinc, arsenic and lead in a temperate seagrass ecosystem from Lake Macquarie Estuary, NSW, Australia. Marine Environmental Research 56, 471–502.
| Biotransference and biomagnification of selenium copper, cadmium, zinc, arsenic and lead in a temperate seagrass ecosystem from Lake Macquarie Estuary, NSW, Australia.Crossref | GoogleScholarGoogle Scholar |
Batley GE, van Dam RA, Warne MSJ, Chapman JC, Fox DR, Hickey CW, Stauber JL (2018) ‘Technical Rationale for Changes to the Method for Deriving Australian and New Zealand Water Quality Guideline Values for Toxicants. Prepared for the revision of the Australian and New Zealand Guidelines for Fresh and Marine Water Quality’. p. 49. (Australian and New Zealand Governments and Australian state and territory governments: Canberra, ACT) Available at https://www.waterquality.gov.au/sites/default/files/documents/batley-technical-rationale-2018.pdf
Binet MT, Gissi F, Stone S, Trinh C, McKnight KS (2019). Use of scanning and image recognition technology to semi-automate larval development assessment in toxicity tests with a tropical copepod. Ecotoxicology and Environmental Safety 180, 1–11.
| Use of scanning and image recognition technology to semi-automate larval development assessment in toxicity tests with a tropical copepod.Crossref | GoogleScholarGoogle Scholar |
Bissen M, Frimmel FH (2003). Arsenic―A review. Part I: Occurrence, toxicity, speciation, mobility. Acta Hydrochimica et Hydrobiologica 31, 9–18.
| Arsenic―A review. Part I: Occurrence, toxicity, speciation, mobility.Crossref | GoogleScholarGoogle Scholar |
Byeon E, Yoon C, Lee JS, Lee YH, Jeong CB, Lee JS, Kang HM (2020). Interspecific biotransformation and detoxification of arsenic compounds in marine rotifer and copepod. Journal of Hazardous Materials 391, 122196
| Interspecific biotransformation and detoxification of arsenic compounds in marine rotifer and copepod.Crossref | GoogleScholarGoogle Scholar |
Byeon E, Kang HM, Yoon C, Lee JS (2021). Toxicity mechanisms of arsenic compounds in aquatic organisms. Aquatic Toxicology 237, 105901
| Toxicity mechanisms of arsenic compounds in aquatic organisms.Crossref | GoogleScholarGoogle Scholar |
CCME (2001) Canadian Water Quality Guidelines for the Protection of Aquatic Life. Arsenic. Prepared by the Canadian Council of Ministers of the Environment, 1999, updated 2001.
Chambers EL, Whiteley AH (1966). Phosphate transport in fertilized sea urchin eggs. I. Kinetic aspects. Journal of Cellular Physiology 68, 289–308.
| Phosphate transport in fertilized sea urchin eggs. I. Kinetic aspects.Crossref | GoogleScholarGoogle Scholar |
Chang JS, Lee JH, Kim IS (2011). Bacterial aox genotype from arsenic contaminated mine to adjacent coastal sediment: Evidences for potential biogeochemical arsenic oxidation. Journal of Hazardous Materials 193, 233–242.
| Bacterial aox genotype from arsenic contaminated mine to adjacent coastal sediment: Evidences for potential biogeochemical arsenic oxidation.Crossref | GoogleScholarGoogle Scholar |
Chen QY, Costa M (2021). Arsenic: a global environmental challenge. Annual Review of Pharmacology and Toxicology 61, 47–63.
| Arsenic: a global environmental challenge.Crossref | GoogleScholarGoogle Scholar |
Coppola F, Almeida Â, Henriques B, Soares AMVM, Figueira E, Pereira E, Freitas R (2018). Biochemical responses and accumulation patterns of Mytilus galloprovincialis exposed to thermal stress and arsenic contamination. Ecotoxicology and Environmental Safety 147, 954–962.
| Biochemical responses and accumulation patterns of Mytilus galloprovincialis exposed to thermal stress and arsenic contamination.Crossref | GoogleScholarGoogle Scholar |
Córdoba-Tovar L, Marrugo-Negrete J, Barón PR, Díez S (2022). Drivers of biomagnification of Hg, As and Se in aquatic food webs: A review. Environmental Research 204, 112226
| Drivers of biomagnification of Hg, As and Se in aquatic food webs: A review.Crossref | GoogleScholarGoogle Scholar |
Cullen WR, Reimer KJ (1989). Arsenic speciation in the environment. Chemical Reviews 89, 713–764.
| Arsenic speciation in the environment.Crossref | GoogleScholarGoogle Scholar |
Cutter GA (1992). Kinetic controls on metalloid speciation in seawater. Marine Chemistry 40, 65–80.
| Kinetic controls on metalloid speciation in seawater.Crossref | GoogleScholarGoogle Scholar |
Dalgarno S (2018) ssdtools: A shiny web app to analyse species sensitivity distributions. Prepared by Poisson Consulting for the Ministry of the Environment, British Columbia. Available at https://bcgov-env.shinyapps.io/ssdtools/
De Francisco P, Martín-González A, Rodriguez-Martín D, Díaz S (2021). Interactions with arsenic: Mechanisms of toxicity and cellular resistance in eukaryotic microorganisms. International Journal of Environmental Research and Public Health 18, 12226
| Interactions with arsenic: Mechanisms of toxicity and cellular resistance in eukaryotic microorganisms.Crossref | GoogleScholarGoogle Scholar |
Doyle CJ, Pablo F, Lim RP, Hyne RV (2003). Assessment of metal toxicity in sediment pore water from Lake Macquarie, Australia. Archives of Environmental Contamination and Toxicology 44, 343–350.
| Assessment of metal toxicity in sediment pore water from Lake Macquarie, Australia.Crossref | GoogleScholarGoogle Scholar |
Duncan EG, Maher WA, Foster SD (2015). The formation and fate of organoarsenic species in marine ecosystems: Do existing experimental approaches appropriately simulate ecosystem complexity?. Environmental Chemistry 12, 149–162.
| The formation and fate of organoarsenic species in marine ecosystems: Do existing experimental approaches appropriately simulate ecosystem complexity?.Crossref | GoogleScholarGoogle Scholar |
Franklin NM, Stauber J, Adams M (2005) Improved methods of conducting microalgal bioassays using flow cytometry. In ‘Techniques in Aquatic Toxicology’. (Ed. GK Ostrander) Vol. 2, pp. 735–756. (CRC Press: FL, USA)
Gaion A, Scuderi A, Pellegrini D, Sartori D (2013). Arsenic exposure affects embryo development of sea urchin, Paracentrotus lividus (Lamarck, 1816). Bulletin of Environmental Contamination and Toxicology 91, 565–570.
| Arsenic exposure affects embryo development of sea urchin, Paracentrotus lividus (Lamarck, 1816).Crossref | GoogleScholarGoogle Scholar |
Garbinski LD, Rosen BP, Chen J (2019). Pathways of arsenic uptake and efflux. Environment International 126, 585–597.
| Pathways of arsenic uptake and efflux.Crossref | GoogleScholarGoogle Scholar |
Garman GD, Anderson SL, Cherr GN (1997). Developmental abnormalities and DNA-protein crosslinks in sea urchin embryos exposed to three metals. Aquatic Toxicology 39, 247–265.
| Developmental abnormalities and DNA-protein crosslinks in sea urchin embryos exposed to three metals.Crossref | GoogleScholarGoogle Scholar |
Gissi F, Binet MT, Adams MS (2013). Acute toxicity testing with the tropical marine copepod Acartia sinjiensis: Optimisation and application. Ecotoxicology and Environmental Safety 97, 86–93.
| Acute toxicity testing with the tropical marine copepod Acartia sinjiensis: Optimisation and application.Crossref | GoogleScholarGoogle Scholar |
Guillard RR, Ryther JH (1962). Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Canadian Journal of Microbiology 8, 229–239.
| Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran.Crossref | GoogleScholarGoogle Scholar |
Guţu CM, Olaru OT, Purdel NC, Ilie M, Neamţu MC, Miulescu RD, Avramescu ET, Margină DM (2015). Comparative evaluation of short-term toxicity of inorganic arsenic compounds on Artemia salina. Romanian Journal of Morphology and Embryology 56, 1091–1096.
Hong S, Kwon H-O, Choi S-D, Lee J-S, Khim JS (2016). Arsenic speciation in water, suspended particles, and coastal organisms from the Taehwa River Estuary of South Korea. Marine Pollution Bulletin 108, 155–162.
| Arsenic speciation in water, suspended particles, and coastal organisms from the Taehwa River Estuary of South Korea.Crossref | GoogleScholarGoogle Scholar |
Johnston SG, Keene AF, Burton ED, Bush RT, Sullivan LA, McElnea AE, Ahern CR, Smith CD, Powell B, Hocking RK (2010). Arsenic mobilization in a seawater inundated acid sulfate soil. Environmental Science and Technology 44, 1968–1973.
| Arsenic mobilization in a seawater inundated acid sulfate soil.Crossref | GoogleScholarGoogle Scholar |
Kalia K, Khambholja DB (2015) Arsenic contents and its biotransformation in the marine environment. In ‘Handbook of Arsenic Toxicology’. (Ed. SJS Flora) pp. 675–700. (Academic Press, Elsevier: London, UK)
| Crossref |
Karadjova IB, Slaveykova VI, Tsalev DL (2008). The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater. Aquatic Toxicology 87, 264–271.
| The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater.Crossref | GoogleScholarGoogle Scholar |
Karthikeyan P, Marigoudar SR, Mohan D, Sharma KV, Ramana Murthy MV (2021). Prescribing sea water quality criteria for arsenic, cadmium and lead through species sensitivity distribution. Ecotoxicology and Environmental Safety 208, 111612
| Prescribing sea water quality criteria for arsenic, cadmium and lead through species sensitivity distribution.Crossref | GoogleScholarGoogle Scholar |
Krassoi R (1995). Salinity adjustment of effluents for use with marine bioassays: effects on the larvae of the doughboy scallop Chlamys asperrimus and the Sydney rock oyster Saccostrea commercialis. Australasian Journal of Ecotoxicology 1, 143–148.
Lee KW, Raisuddin S, Hwang DS, Park HG, Dahms HU, Ahn IY, Lee JS (2008). Two-generation toxicity study on the copepod model species Tigriopus japonicus. Chemosphere 72, 1359–1365.
| Two-generation toxicity study on the copepod model species Tigriopus japonicus.Crossref | GoogleScholarGoogle Scholar |
Lepper P, Sorokin N, Maycock D, Crane M, Atkinson C, Hope S-J, Comber S (2007) Preconsultation report: Proposed EQS for water framework directive annex VIII substances: arsenic (total dissolved). Science report: SC040038/SR3. SNIFFER Report: WFD52(iii). (Environment Agency: Bristol, UK) p. 91. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/291226/scho0407blvu-e-e.pdf
Levy JL, Stauber JL, Adams MS, Maher WA, Kirby JK, Jolley DF (2005). Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum). Environmental Toxicology and Chemistry 24, 2630–2639.
| Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum).Crossref | GoogleScholarGoogle Scholar |
Maher W, Butler E (1988). Arsenic in the marine environment. Applied Organometallic Chemistry 2, 191–214.
| Arsenic in the marine environment.Crossref | GoogleScholarGoogle Scholar |
Mandal BK, Suzuki KT (2002). Arsenic round the world: A review. Talanta 58, 201–235.
| Arsenic round the world: A review.Crossref | GoogleScholarGoogle Scholar |
McIntyre DO, Linton TK (2011) Chapter 6 Arsenic. In ‘Homeostasis and toxicology of non-essential metals’. (Eds CM Wood, AP Farrell, CJ Brauner) Vol. 31A, pp. 297–349. (Academic Press, Elselvier: London, UK)
| Crossref |
Morales-Simfors N, Bundschuh J (2022). Arsenic-rich geothermal fluids as environmentally hazardous materials – A global assessment. Science of the Total Environment 817, 152669
| Arsenic-rich geothermal fluids as environmentally hazardous materials – A global assessment.Crossref | GoogleScholarGoogle Scholar |
Moreira A, Figueira E, Libralato G, Soares AMVM, Guida M, Freitas R (2018). Comparative sensitivity of Crassostrea angulata and Crassostrea gigas embryo-larval development to As under varying salinity and temperature. Marine Environmental Research 140, 135–144.
| Comparative sensitivity of Crassostrea angulata and Crassostrea gigas embryo-larval development to As under varying salinity and temperature.Crossref | GoogleScholarGoogle Scholar |
Morelli E, Mascherpa MC, Scarano G (2005). Biosynthesis of phytochelatins and arsenic accumulation in the marine microalga Phaeodactylum tricornutum in response to arsenate exposure. BioMetals 18, 587–593.
| Biosynthesis of phytochelatins and arsenic accumulation in the marine microalga Phaeodactylum tricornutum in response to arsenate exposure.Crossref | GoogleScholarGoogle Scholar |
Neff JM (1997). Ecotoxicology of arsenic in the marine environment. Environmental Toxicology and Chemistry 16, 917–927.
| Ecotoxicology of arsenic in the marine environment.Crossref | GoogleScholarGoogle Scholar |
Ninh TD, Nagashima Y, Shiomi K (2008). Unusual arsenic speciation in sea anemones. Chemosphere 70, 1168–1174.
| Unusual arsenic speciation in sea anemones.Crossref | GoogleScholarGoogle Scholar |
OECD (2005) ‘OECD draft guidelines for testing of chemicals. Proposal for a new guideline, calanoid copepod development and reproduction test with Acartia tonsa. Final draft document.’ (Organisation for Economic Cooperation and Development: Paris, France)
OECD (2006) ‘Current approaches in the statistical analysis of ecotoxicity data.’ (Organisation for Economic Cooperation and Development: Paris, France)
OECD (2011) ‘Guidelines for testing of chemicals No. 201: freshwater algae and cyanobacteria, growth Inhibition test’, Vol. 1, pp. 1–25. (Organisation for Economic Cooperation and Development: Paris, France)
Peterson ML, Carpenter R (1983). Biogeochemical processes affecting total arsenic and arsenic species distributions in an intermittently anoxic fjord. Marine Chemistry 12, 295–321.
| Biogeochemical processes affecting total arsenic and arsenic species distributions in an intermittently anoxic fjord.Crossref | GoogleScholarGoogle Scholar |
Pinheiro JP, Bates D (2000). ‘Mixed-effects models in S and S-PLUS.’ (Springer-Verlag: New York)
R Development Core Team (2016) ‘R: A language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/
Rainbow PS, Blackmore G (2001). Barnacles as biomonitors of trace metal availabilities in Hong Kong coastal waters: Changes in space and time. Marine Environmental Research 51, 441–463.
| Barnacles as biomonitors of trace metal availabilities in Hong Kong coastal waters: Changes in space and time.Crossref | GoogleScholarGoogle Scholar |
Ritz C, Streibig JC (2005). Bioassay analysis using R. Journal of Statistical Software 12, 1–22.
| Bioassay analysis using R.Crossref | GoogleScholarGoogle Scholar |
Sadiq M (1990). Arsenic chemistry in marine environments: a comparison between theoretical and field observations. Marine Chemistry 31, 285–297.
| Arsenic chemistry in marine environments: a comparison between theoretical and field observations.Crossref | GoogleScholarGoogle Scholar |
Sharma VK, Sohn M (2009). Aquatic arsenic: toxicity, speciation, transformations, and remediation. Environment International 35, 743–759.
| Aquatic arsenic: toxicity, speciation, transformations, and remediation.Crossref | GoogleScholarGoogle Scholar |
Smedley PL, Kinniburgh DG (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry 17, 517–568.
| A review of the source, behaviour and distribution of arsenic in natural waters.Crossref | GoogleScholarGoogle Scholar |
Smith E, Smith J, Smith L, Biswas T, Correll R, Naidu R (2003). Arsenic in Australian environment: an overview. Journal of Environmental Science and Health, Part A Toxic/Hazardous Substances and Environmental Engineering A38, 223–239.
| Arsenic in Australian environment: an overview.Crossref | GoogleScholarGoogle Scholar |
Stauber J, Franklin NM, Adams M (2005) Microalgal toxicity tests using flow cytometry. In ‘Small-scale Freshwater Toxicity Investigations Vol 1 - Toxicity Test Methods’. (Eds C Blaise, JF Férard) pp. 203–241. (Springer: Dordrecht, The Netherlands)
Thorley J, Schwarz CJ (2018). ssdtools: An R package to fit species sensitivity distributions. Journal of Open Source Software 3, 1082
| ssdtools: An R package to fit species sensitivity distributions.Crossref | GoogleScholarGoogle Scholar |
Trenfield MA, van Dam JW, Harford AJ, Parry D, Streten C, Gibb K, van Dam RA (2015). Aluminium, gallium, and molybdenum toxicity to the tropical marine microalga Isochrysis galbana. Environmental Toxicology and Chemistry 34, 1833–1840.
| Aluminium, gallium, and molybdenum toxicity to the tropical marine microalga Isochrysis galbana.Crossref | GoogleScholarGoogle Scholar |
Trenfield MA, van Dam JW, Harford AJ, Parry D, Streten C, Gibb K, van Dam RA (2016). A chronic toxicity test for the tropical marine snail Nassarius dorsatus to assess the toxicity of copper, aluminium, gallium, and molybdenum. Environmental Toxicology and Chemistry 35, 1788–1795.
| A chronic toxicity test for the tropical marine snail Nassarius dorsatus to assess the toxicity of copper, aluminium, gallium, and molybdenum.Crossref | GoogleScholarGoogle Scholar |
Trenfield MA, van Dam JW, Harford AJ, Parry D, Streten C, Gibb K, van Dam RA (2017). Assessing the chronic toxicity of copper and aluminium to the tropical sea anemone Exaiptasia pallida. Ecotoxicology and Environmental Safety 139, 408–415.
| Assessing the chronic toxicity of copper and aluminium to the tropical sea anemone Exaiptasia pallida.Crossref | GoogleScholarGoogle Scholar |
USEPA (1995a) Water quality criteria. United States Environmental Protection Agency. Office of Science and Technology, Office of Water, Washington DC. September 1995.
USEPA (1995b) Short-term methods for estimating the chronic toxicity of effluent and receiving waters to west coast marine and estuarine organisms. United States Environmental Protection Agency. Office of Research and Development, Washington DC, August 1995. EPA/600/R-95/136.
van Dam JW, Trenfield MA, Harries SJ, Streten C, Harford AJ, Parry D, van Dam RA (2016). A novel bioassay using the barnacle Amphibalanus amphitrite to evaluate chronic effects of aluminium, gallium and molybdenum in tropical marine receiving environments. Marine Pollution Bulletin 112, 427–435.
| A novel bioassay using the barnacle Amphibalanus amphitrite to evaluate chronic effects of aluminium, gallium and molybdenum in tropical marine receiving environments.Crossref | GoogleScholarGoogle Scholar |
Wang Y, Wang S, Xu P, Liu C, Liu M, Wang Y, Wang C, Zhang C, Ge Y (2015). Review of arsenic speciation, toxicity and metabolism in microalgae. Reviews in Environmental Science and Biotechnology 14, 427–451.
| Review of arsenic speciation, toxicity and metabolism in microalgae.Crossref | GoogleScholarGoogle Scholar |
Warne M, Batley G, van Dam R, Chapman J, Fox D, Hickey C, Stauber J (2018) Revised Method for Deriving Australian and New Zealand Water Quality Guideline Values for Toxicants – update of 2015 version. Prepared for the revision of the Australian and New Zealand Guidelines for Fresh and Marine Water Quality. Australian and New Zealand Governments and Australian state and territory governments, Canberra, p. 48. Available at https://www.waterquality.gov.au/sites/default/files/documents/warne-wqg-derivation2018.pdf
WHO (2001) ‘Arsenic and arsenic compounds. Environmental Health Criteria 224’, 2nd edn. (World Health Organisation: Geneva, Switzerland)
Wu H, Xu L, Ji C, Yu D (2016). Proteomic and metabolomic responses in D-shape larval mussels Mytilus galloprovincialis exposed to cadmium and arsenic. Fish and Shellfish Immunology 58, 514–520.
| Proteomic and metabolomic responses in D-shape larval mussels Mytilus galloprovincialis exposed to cadmium and arsenic.Crossref | GoogleScholarGoogle Scholar |
Yu D, Ji C, Zhao J, Wu H (2016). Proteomic and metabolomic analysis on the toxicological effects of As (III) and As (V) in juvenile mussel Mytilus galloprovincialis. Chemosphere 150, 194–201.
| Proteomic and metabolomic analysis on the toxicological effects of As (III) and As (V) in juvenile mussel Mytilus galloprovincialis.Crossref | GoogleScholarGoogle Scholar |