Early history and rationale for outdoor chamber work at the University of North Carolina
Harvey E. Jeffries A B , Richard M. Kamens A and Kenneth Sexton AA Department of Environmental Science and Engineering Gillings School of Global Public Health University of North Carolina at Chapel Hill Chapel Hill, NC 27599, USA.
B Corresponding author. Email: harvey@unc.edu
Environmental Chemistry 10(4) 349-364 https://doi.org/10.1071/EN13901
Published: 28 August 2013
Environmental context. Imagine in 1968 having to tell the largest cities in the US that they would have to spend billions of dollars to reduce human exposure to a gas in their air that no one emitted and that no one knew for sure how it came to be there. This history recalls how scientists and policy makers met this challenge so that by 1985 effective programs were in place.
Abstract. The University of North Carolina (UNC) outdoor chamber facility was established in 1972. The chamber produces reliable and interpretable results using ambient sunlight, temperature and weather, providing an effective physical model system for learning about atmospheric chemistry. This article recounts the 40-year history of the chamber facility, from the early days in understanding ozone–precursor relationship to the latest in studying gas and particulate toxicities on human lung cells.
Preface
Like other models, histories are subject to generalisations, distortions and deletions depending upon who is creating the history. We are sure that this history suffers from these operations. Please forgive us in advance if our recollections and mental constructs are somewhat at odds with your own. Perhaps we can have a ‘conversation’ about this sometime.
Creation of the US Environmental Protection Agency and the Clean Air Act Extension
In 1968, before President Richard Nixon’s creation of the US Environmental Protection Agency (EPA), the National Air Pollution Control Administration (NAPCA) was moved from its association with the Public Health Service program at Cincinnati, Ohio to Raleigh–Durham’s new Research Triangle Park, NC. Many early air pollution researchers, notably Dr Paul Altschuler, Dr Joseph Bufalini and Dr Philip Hanst took up residence in the Raleigh–Durham area and began work at the Administration’s temporary research facility located in the Research Triangle Park (RTP). By 1971, this group became a National Research Laboratory of the EPA and the 1970 Clean Air Act Extension law provided new funding. This group had been conducting indoor chamber air chemistry experiments at the Cincinnati laboratory site but no such facilities existed at the RTP site. In 1972, Hanst had a new idea: why not build an outdoor chamber in North Carolina to help understand how emissions of oxides of nitrogen (NOx = NO + NO2) and non-methane hydrocarbons (NMHC) created ozone and other air pollutants?
As background, NAPCA’s Arthur Stern’s first two volumes of his series Air Pollution, 2nd Edition were published in 1968.[1] This was a period of time in which the problem was well recognised, but the causes of some important health-related effects were poorly understood. These health-effects were thought to be associated with atmospheric chemistry from secondary pollutants like ozone and oxidants in urban atmospheres (‘smog’). At this time, health data were sufficient to establish a National Ambient Air Quality Standard (NAAQS) for photochemical oxidants of 0.08 ppm for 1 h.[2] The state of atmospheric chemical reaction knowledge was essentially contained in Phillip Leighton’s book Photochemistry of Air Pollution.[3] The chapter on ‘Free Radical Reactions’, for example, was 30 pages and the reactions of ‘hydroxyl radicals’ were described in two pages. Leighton stated, ‘With … hydroxyl radicals … existing information is not sufficient to permit an assessment of the relative importance of several possible reactions.’ Most of his discussion of radicals centred on the organic alkoxy free radicals that were created by photolysis of aldehydes. Today we know that hydroxyl radical atmospheric reactions are the most important operators on both the inorganic and organic gases in both the natural and polluted atmospheres. Much of this gain in understanding took place between 1969 and 1990.
As the new EPA was being pushed to control urban air pollution, the basic methods available to them were ‘correlations of pollutants with primary emitted compounds’. This led to the publication in the Federal Register of the Appendix J curve,[4] which plotted afternoon observed ozone (actually ‘photochemical oxidants, including ozone’) at a monitoring site against the morning (0600–0900 hours) observations of the NMHC concentrations (Fig. 1). Only six cities had such monitoring sites. Drawing a smooth line through the highest points produced a curve of highest ozone associated with each level of morning observed NMHC concentration. This implied relationship permitted the estimation of the highest amount of NMHC that (ideally) would not result in photochemical oxidants (including ozone) being higher than the NAAQS of 0.08 ppm for 1 h. Thus, the EPA chose to require NMHC be monitored and reduced to 0.24 ppm C to control ozone and oxidants to the NAAQS for ozone.

|
Atmospheric chemists were not entirely pleased with this approach and set off to design a research program to improve the fundamental understanding. Meanwhile, the EPA moved to require states to begin monitoring and the EPA began to prepare to control NMHC from automobiles as a procedure to attain the NAAQS of 0.08-ppm ozone for 1 h. Shortly after this, the Appendix J curve was criticised for being more reflective of meteorology, that is, issues of simple dilution, than it was of the chemistry that produced ozone. For example, a very similar Appendix J curve could be produced by plotting peak oxidants against carbon monoxide and it was not believed that CO was important in the chemistry that produced high ozone. In recognition of the weakness of this relationship, the EPA eventually dropped the requirement that states violating the NAAQS for ozone had to monitor NMHC.
An alternative way to learn about atmospheric chemistry was to create physical model systems, e.g. chambers with transparent walls and external light sources in which various NMHC and NOx could be introduced and, upon being sufficiently illuminated, would produce ozone and other oxidants.
Dr Basil Dimitriades at the Bureau of Mines Laboratories (BOM) in Bartlesville Oklahoma had built one such system in 1966. His 2.8-m3 BOM chamber could be injected with exhaust from a 1963 automobile and illuminated with constant irradiation for a constant amount of time and the time history of ozone (oxidants) could be recorded. Dimitriades used a matrix of initial conditions of NMHC and NOx to produce conditions covering the likely observed range of precursors in larger cities.[5] Interpreting his results proved challenging. It would require nearly a decade before his chamber data could be used to help solve the precursor–secondary pollutant relationship in a given city. Dimitriades joined the EPA laboratory at RTP in 1973.
In North Carolina, Hanst and Bufalini wanted to be able to use this same type of approach to help understand how to best regulate urban ozone and oxidants. They also had sources of external funding.
Atmospheric chemistry at University of North Carolina Chapel Hill
In the early 1960s, at the University of North Carolina (UNC), Dr Lyman Ripperton became a Professor, having previously worked at the Los Angeles Air Pollution Control District Laboratory investigating the causes of ‘smog’. At UNC, Ripperton had started an atmospheric chemistry research program in the Department of Sanitary Engineering, soon to become the Department of Environmental Science and Engineering (ESE), located in UNC’s School of Public Health. Ripperton’s laboratory had ‘indoor reactor systems’ holding large 50- to 200-L glass flasks surrounded by high intensity mercury and fluorescent lamps. The flasks could be filled with reactant gases, irradiated and then be sampled by an infrared spectrometer with large long-path gas cells that could detect various hydrocarbon reactants and derived products during a photochemical oxidation experiment. Furthermore, Ripperton had access to talented graduate students who worked with support from Public Health Service Fellowship Grants or who had graduate assistantship support.
Ripperton’s interests also included what he called ‘wild ozone’, that is, ozone in the natural troposphere. At that time the common view of ozone was that it was either made-and-stayed in the stratosphere (the earth’s ozone layer) or it was an air pollutant, a product of human activities and was only an issue in a few large cities. Atmospheric scientists, on the other hand, knew that ozone was a natural and vital component of the earth’s atmosphere and was likely present at the surface in nearly all locations. Ripperton was testing hypotheses that ozone was also being produced by atmospheric chemistry near the surface in nearly all locations with an active biosphere. With his colleagues from the Research Triangle Institute (now officially RTI) and the US Forrest Service, Ripperton planned a short ‘Ozone Variability in Mountainous Terrain’ field study in the late summer of 1965 in the Pisgah National Forest of western North Carolina. Harvey Jeffries, a chemist, who had just become a graduate student under Ripperton’s direction, participated in this 1965 field study, before he ever attended a class. The field study results were published in 1967[6] and revealed unusual ozone behaviour among mountaintops and valleys.
Shortly after coming to the new RTP NAPCA headquarters in 1968, Professor Stern joined the Department of ESE and later became a member of Jeffries’ Ph.D. Committee. Jeffries, with National Science Foundation funding, went on to conduct more complex and definitive studies of ‘wild ozone’ in North Carolina as part of his 1971 Ph.D. thesis An experimental method for measuring the rate of synthesis, destruction, and transport of ozone in the lower atmosphere.[7] This study simultaneously sampled ambient air from four levels on an instrumented 36-m (120-foot) research tower located in RTP near the EPA. Interestingly, Jeffries’ method was recently re-invented by Cazorla and Brune and applied in Houston, TX.[8] Dr Donald Fox had come to UNC in this same period to study oxides of nitrogen as a post-doctoral researcher under the direction of Ripperton. Richard Kamens, after completing 2 years as a Peace Corps volunteer in Thailand, had come to the Department of ESE as a Master’s Student under Ripperton’s direction. After graduating in 1971, Kamens worked with Professor Stern on a National Academy of Science panel to evaluate the 1975 light duty emission standards, as mandated by the new Clean Air Amendments. Kamens focussed on the ‘linear rollback’ approach that was used to link emission standards for oxides of nitrogen, non-methane hydrocarbons and carbon monoxide to the ambient air quality standards for these species.[9] Simply, the amount of reductions in emissions could be estimated from the needed ‘rollback’ in ambient concentrations that would be necessary to achieve air quality standards or the hydrocarbon guideline. A large uncertainty was the estimated growth factor in future ambient concentrations that would result if no controls were implemented. From this early experience with standards and policy issues it became clear to the young UNC atmospheric chemistry group that an endless array of questions related to air pollution control strategies needed to be explored.
Hanst and colleagues at the nearby EPA laboratories approached Ripperton in 1972 with the idea of funding UNC to build and operate an ‘outdoor smog chamber’ to be used to explore urban-like atmospheric chemistry issues, especially from the viewpoint of effectiveness of controlling air pollution problems. After several exploratory meetings, a proposal was submitted to the EPA to build and operate in a rural area south of Chapel Hill a large (312 000-L), two-sided, outdoor A-frame chamber using fluorinated ethylene propylene (FEP) Teflon film for walls. Fox developed the design of the A-frame and the Teflon film-panels. A unique aspect of the chamber design was to have two large side-by-side chambers in which the initial and physical conditions would be identical except for one chemical condition that was the subject of the test. Typically, all conditions were the same except for the total concentration of the test volatile organic compound (VOC) or VOC mixture. Thus, regardless of the external meteorological conditions, the effect of a 50 % reduction in (for example) initial NMHC on the formation of NO2 would be demonstrated in a single run. Of course it was important to show that, when identical initial conditions were injected, the two sides give identical results – and they did.
The School of Public Health Shop personnel, Fox, Kamens, Jeffries, students and staff built the chamber at a rented site on a farm ~32 km south of Chapel Hill in a semi-wooded rural area near Pittsboro, NC. Fig. 2 shows a photograph from 2002 of the 312-m3 UNC dual outdoor gas chamber in the foreground, the two 25-m3 wood-smoke chambers, built by Kamens in 1983 and the larger 270-m3 dual outdoor aerosol chamber built by Kamens in 2002.

|
The somewhat complex operation of a dual-sided chamber, which often began at 0400 hours and did not end until 1700 hours on a run day, was achieved in part by sharing a single set of measurement instruments between the two sides; values on the instrument inlets were automatically switched between the two sets of sample lines at intervals appropriate for the measurement system. A Digital Equipment Corporation PDP-11 Minicomputer was wired to a large data acquisition system and chamber control systems were installed to automate the operation of the chamber, the injections of gases and the sampling of the chamber by the instruments. Jeffries and several students did the hardware and software design and installation of this large computer system.
Shortly after construction began, Ripperton took a Senior Scientist position at the nearby RTI and started another chamber program there. Dr Jeffries accepted an Assistant Professor position at UNC’s Department of ESE. Soon after, Fox and Kamens join the ESE faculty too.
Initial study in the UNC dual outdoor chamber
The first atmospheric problem to be studied was, ‘Given that EPA would require reductions of NMHC, how will the urban ambient concentrations of NO2 change?’ There was debate among atmospheric chemists as to whether the NAAQS-regulated pollutant NO2 would increase or would decrease if NMHC were reduced. Recall this is a period before there were any photochemical reaction mechanisms or smog models or computers to run them on. Even with a reasonably complete understanding of the reactions involved, the prediction of the outcome of a large network of reactions with feedback paths and termination processes exceeded the reasoning capacity of even Dr Paul Altschuler, a most respected urban atmospheric chemist of that time. The UNC researchers also wanted to investigate how well the proposed EPA NMHC regulations would work to reduce ozone, but they were told that the EPA had decided this issue and did not need such data. The researchers collected it anyway, and, of course, later the EPA did care.
Shortly after the UNC project began, Dr Basil Dimitriades joined the atmospheric chemistry group at the EPA and he became the UNC group’s ‘Project Monitor’ and with his experience in smog chambers, became a mentor and strong supporter of the UNC Outdoor Chamber Program.
In addition to building the large chamber and equipping the adjacent laboratory and its data acquisition and control system, the project had to plan experiments that clearly demonstrated that the chamber produced reliable and interpretable results using ambient sunlight, temperature and weather. For the actual tests, the researchers had to create a nationally representative NMHC mixture for the initial VOCs. This was greatly aided by the help and data of Dr William Lonneman and Robert Seila of EPA, who had collected VOC canister samples during the morning rush hour from more than 40 cities and had analysed more than 2000 of them for 200 individual HC compounds on their gas chromatographic system. Their data was used to create a stable, reliably injectable mixture of gases in cylinders and a liquid mixture injected with a syringe to create a highly representable average urban morning 54 VOC species mixture in the chambers. This was subsequently named SynUrban54 Mix,[10] and has been used for more than 30 years of test experiments in the UNC and other chambers around the world. The chamber design involved some trial and error testing and rebuilding, but the final design did function as expected and was able to tolerate rain, high winds and hail storms.
A more difficult issue was to convince the EPA scientists and other ‘indoor chamber’ researchers that the results in this ‘ambient-condition-driven chamber’ were meaningful and useful. That is, no one had ever seen time series of NO, NO2, various HC and O3 from a variability illuminated chamber over a summer season; all indoor chambers (including the BOM chamber) went from dark to illuminated fully at a constant level for a maximum duration of 6 h. In the outdoor chamber, all of the initial conditions were established in the dark before sunrise, and then the natural progression of the solar day began at near zero actinic flux intensity just before sunrise and increased gradually to an intensity often three to ten times that in the artificially illuminated indoor chambers. Furthermore, the UNC experiments lasted until late afternoon, resulting in 12-h duration experiments instead of the 4–5-h indoor experiments. Thus, the time series graphs of species concentrations from the UNC chambers appeared very different than what the ‘indoor chamber’ community were used to seeing.
In June 1975, Jeffries, Fox and Kamens completed a technical report to the EPA entitled ‘Outdoor Smog Chamber Studies: Effect of Hydrocarbon Reduction on Nitrogen Dioxide’,[11] in which the design, construction and performance of the new UNC outdoor chamber were described. In 1976, the team published a paper comparing the photochemical transformations in the outdoor chamber with those in indoor chambers.[12] For this, Jeffries modified a new FORTRAN program for mathematical modelling of photochemical reaction systems that was being developed by Dr Marcia Dodge and John Overton at the EPA-RTP. Jeffries added the ability to have diurnal varying conditions including varying photolysis rates. For a reaction mechanism for propylene and NOx, Jeffries used one developed by Kenneth Demerjian[13] in his Ph.D. thesis work under Dr Jack Calvert at Ohio State. Jeffries was then able to simulate both indoor and outdoor chamber experiments and to show the effects of just changing the photolysis time profiles. Of course, the outdoor, naturally illuminated chamber, is the much more realistic condition. These were the first simulations of outdoor chamber data to be published, and Jeffries began to develop an extensive expertise with modelling codes and photochemical reaction mechanisms as a means of interpreting the UNC outdoor chamber data.
Over the course of years, the UNC outdoor chamber has been shown to be highly reproducible, both from the perspective of side-to-side performance and year-to-year reproducibility of the same test condition at nearly the same time of year. That is, a ‘standard condition’, e.g. a simple VOC experiment, is repeated throughout the run season (typically mid-May to end of October) and the results compared side-to-side and year-to-year. The side-to-side matches are usually within 5–10 % duplication. For clear sky experiments that fall within a few days of each other on the calendar, but one or more years apart, the experimental graphs also match closely in time and magnitude, and are sometimes virtually identical. So although conditions are variable in outdoor experiments, they are repeatable, sometimes over a 5-year span.
One hundred and thirty 12-h duration, dual-outdoor-chamber runs were conducted in the first EPA study. In addition to having plotted time series data, a non-linear multiple regression equation for NO2 maximum concentration accounted for 92 % of the total variance. The conclusions were ‘…for initial NMHC and NOx levels similar to those typically present in current urban atmospheres, reduction of the NMHC reactant by 60–90 % caused a 20–35 % reduction in both NO2 dosage and maximum NO2 concentration. … The results under these real conditions differed from those that had been previously obtained in indoor chambers with constant conditions of light, temperature, and humidity.’
Emergence of photochemical reaction mechanisms and computer modelling
By the mid-1970s, the discovery of the importance of the hydroxyl radical (•OH) in oxidising organic compounds in the atmosphere by Greiner,[14] and by Stedman et al.,[15] led to a paradigmatic shift in the science of atmospheric chemistry. The groundbreaking modelling work of H. Niki at Ford Laboratories and the effect of Kenneth Demerjian’s Ph.D. thesis under Dr Jack Calvert at Ohio State[13] became the paradigms for the ‘promise of modelling’ to have a central role in the implementation of the EPA’s Air Quality Management Plans as solutions to the regulation of ozone by effective regulation of its precursors.
After the first UNC chamber project had finished, the team was approached by Dr Marcia Dodge (at EPA-RTP), who had the task of creating projects to formulate and improve the photochemical reaction mathematical models and to find methods for applying these computer models to make future predictions of urban air quality under various scenarios of precursor reductions or changes. The modelling team, led by Dr Gary Whitten at System Applications Inc. (later officially SAI), was already funded by the EPA to develop new formulations of the important chemistry.
Whitten already had experience with the fact that the fundamental kinetics data were limited, that the details of many important reaction pathways were unknown, and that formulating a complex, positive and negative feedback controlled system without constraints was unlikely to produce a reliable model. The 1974 Hecht, Seinfeld and Dodge[16] mechanism was one example of formulating a generalised mechanism directly. The mechanism was formulated based on generalised principles of VOC reactions and was tested and fitted with 13 different indoor smog chamber runs. The conclusions stated, ‘Based on our results and the principles of formulation, the kinetics mechanism developed here appears to hold substantial promise for incorporation into urban simulations models.’. And indeed, when this mechanism was used to simulate complex behaviour of a NOx and HC system, it was the first to be able to produce such behaviour as shown by Fig. 3. This was the first published model-produced ozone isopleth diagram that could reproduce features of several older laboratory results, including those of Dimitriades. The paper described several potential limitations for the mechanism and its formulation and cautioned about reaching qualitative conclusions from its predictions. When the mechanism was later tested with UNC outdoor chamber data for the same types of HCs, however, it failed rather badly, consistent with the paper’s statement, ‘Clearly, however, a considerably more accurate and complete data base is required if the adequacy of the lumped mechanism is to be critically evaluated.’

|
Whitten, in his work,[17] had developed a different strategy: the concept of ‘hierarchy of species approach’, which was similar to the approach initially used by Demerjian in his modelling study, but more formal. In this approach, one uses all the kinetics and laboratory isolated systems data available, the general principles of organic reactions and thermodynamics principles to formulate an ‘explicit’ photochemical reaction mechanism, i.e. one in which all the model entities are assumed to represent ‘real’ molecules with ‘real’ properties (as best known). This was in contrast to the generalised, reduced form mechanism in the Hecht, Seinfeld and Dodge approach in which the model’s reacting entities were not ‘real’ molecules, but were ‘tuned’ model species.
Because the inorganic reactions cannot be ‘generalised’, the model must include the minimum set of inorganic species and reactions that provide all the important transformations and losses for these. This reaction set can be tested against various organic-free (to the extent possible) data to assure that the inorganic processes in the model have behavioural reliability, i.e. that the model makes predictions that ‘fit’ the observations in a reasonable manner without undue compensating errors. After this has been shown, carbon monoxide is then added to the model and chamber experiments with only oxides of nitrogen and CO are performed. The model is again evaluated with all prior experiments and with experiments having CO and NOx. Only adjustments to the CO reactions are done as needed within the uncertainty of the model formulation data. Next, experiments are done with NOx and formaldehyde (HCHO), which makes CO when it reacts. The model is tested to see if it ‘fits’ that system. The parts that represent the CO cannot be changed at this stage. Next experiments are done with NOx and methane, which makes HCHO when it reacts, and the model is again tested without varying any of the previous test components. This experimenting and testing process then continues to ethylene (the first olefin and is produced in automobile exhaust), which gives HCHO when it reacts but includes important •OH + ethylene and O3 + ethylene reactions. Testing then continues to acetaldehyde (CH3CHO), the next higher aldehyde, which makes peroxy acetyl nitrate (PAN), an organic nitrate that is a human lachrymator and potent plant phytotoxin. Next, propylene (the second olefin, present in automobile exhaust) is tested. Propylene produces both formaldehyde and acetaldehyde when reacted with hydroxyl radicals and with ozone. Propylene is the most studied hydrocarbon in chambers because it was thought to exhibit all the essential characteristics of urban smog. In general, this process proposes to test individually all the products of a parent organic compound before testing the mechanism for the parent compound. This procedure helps avoid compensating errors among the mechanism parts. It also requires a specialised set of chamber experiments to test the model’s behaviour.
Another Whitten idea was that after a mechanism had been shown to reproduce the behaviours of all these various systems, it could become the source of a ‘model of a model’. That is, the modelling processes of ‘generalisation, distortion and deletion’ can be systematically applied to the full explicit mechanism to produce a simpler, less computationally expensive, yet reasonably reliable (accurate prediction) representation for use in many urban like simulations to test policies. The model of the model can be tested against the fully explicit model over a wide range to understand the effects of the simplifications and adjust them as needed. Thus, the essential elements of the internal workings of the overall mechanism are preserved with fewer reactions and removal of non-essential products and intermediates. Later, Jim Killus, Whitten’s modelling partner and electrical engineer, would introduce some model techniques that greatly reduced the numbers of model species (and therefore storage) yet preserved the effective overall ozone productivity of the model.
Dodge saw great advantage in coupling the model formulation work of Whitten at SAI with the outdoor dual-chamber work at UNC to provide a solid source of observational data to test and evaluate the evolving mechanisms. She funded the UNC chamber over several multiple year projects to work closely with her and with Whitten and other SAI modellers, to create a highly accurate, yet very efficient photochemical model urban-scale mechanism for use in air quality models. This was the sequence of ‘Carbon Bond’ mechanisms, named for their simplification principles of creating ‘model species’ that represented the type of carbon-to-carbon bonding and to build up the model representation of real organic molecules from the additive properties of the limited number of carbon bonding model entities. But first there had to be ‘fully explicit’ or nearly explicit mechanisms created so that the entire complexity of the reacting system could be captured.
By January 1979, an SAI report to the EPA entitled ‘Modeling of Simulated Photochemical Smog with Kinetics Mechanisms’[15] described the results of SAI’s computer modelling for detailed chemical mechanisms for formaldehyde, acetaldehyde, ethylene, propylene, butane, 1-butene, trans-2-butene and 2,3-dimethylbutane. These then became the basis of a generalised mechanism containing a condensed version of the explicit schemes and a semi-empirical scheme for aromatics. The latter was the evaluated first version of the Carbon Bond Mechanism. Dual experiments from the UNC outdoor chamber were used extensively. A smaller number of experiments from other chambers were also simulated.
Based on this successful collaboration and the success of the model photochemical reaction mechanisms, Dodge decided to extend the UNC outdoor chamber program to produce a large comprehensive test set. The effort extended over more than a decade. The work will be discussed briefly in a section below.
A successful chamber and mechanistic-based model for ozone–precursor relationships
While the UNC Outdoor Chamber Database was being developed and used to test more comprehensive reaction mechanism models that would eventually be useful in full three-dimensional air quality models, there was a need to produce simpler models for immediate application by states to calculate their NMHC or NOx reductions to achieve the NAAQS for O3. An informal collaborative effort among the chamber group at UNC, Whitten and his modelling team at SAI and Dimitriades and Dodge at the EPA were able to make significant progress.
In late 1976, Dimitriades organised at RTP, NC, ‘The International Conference on Photochemical Oxidant Pollution and its Control’, which attracted an impressive number of European and US researchers for its 23 sessions.[18] Papers were presented on a wide range of topics including mathematical models of ozone air quality, oxidant precursor relations and health effects. Most of the conference was to provide evidence, opinions and support for an idea that Dimitriades put forth in his talk near the end of the conference ‘An Alternative to the Appendix-J Method For Calculating Oxidant- and NO2-related Control Requirements’,[19] which was an analysis beyond his 1972 paper.[20] Subsequently, an extended version of this talk was published.[21] Jeffries, Kamens, Fox and Dimitriades presented a paper entitled ‘Outdoor Smog Chamber Studies: Effect of Diurnal Light, Dilution and Continuous Emission on Oxidant Precursor Relationships’[22] immediately following Dimitriades’ paper. Dodge then presented the key paper, ‘Combined Use of Modeling Techniques and Smog Chamber Data To Derive Ozone-Precursor Relationships’, which for all practical purposes was the highlight of the conference.[23]
The essence of Dimitriades and Dodge’s idea was that smog chambers can be used as physical models of precursor–ozone relations, with the advantage of being ‘real’, but having disadvantages of being opaque in operation and not easily manipulated. There were two main sets of smog chamber data under consideration: the real automobile exhaust BOM indoor-conditions dataset covering ~17 points of initial precursor and subsequent ozone, and the UNC outdoor chamber data following Whitten’s ‘hierarchy of species approach’. The latter allowed Whitten to develop reliable mechanisms for ethylene, propylene and n-butane that performed well under ‘real ambient sunshine and temperatures’. Furthermore, as part of this collaboration, several other outdoor chamber test conditions were produced at UNC and evaluated by Whitten. These were the ones described at the conference by Jeffries et al.[22]
The important steps in Dodge’s idea were: (a) to use current state-of-the-art outdoor chamber evaluated chemical mechanisms to ‘model’ the BOM indoor chamber results; (b) to use this evaluated mechanism to simulate ‘typical ambient solar and dilution conditions’ to predict location specific precursor–ozone relationships and (c) to use the predicted relationships to estimate the likely effects of various potential controls on the precursor emissions. That is, should the city use NMHC reductions or NOx reductions to reach 0.08-ppm ozone?
As Dodge undertook the task of simulating the BOM chamber, she had to solve the problem of simulating the automobile exhaust used in the chamber. Because the VOC composition of real automobile exhaust is very complex and was beyond the ability to simulate explicitly with any existing photochemical mechanism, a ‘model’ approach was needed. The two most important classes of VOCs in automobile exhaust are olefins and paraffins; the third class is aromatics. Little was known about aromatic VOC reactions in 1976. The ‘model’ approach was to test if a single representative olefin (propylene) and a single representative paraffin (n-butane) were sufficient to represent the ozone forming potential of the BOM automobile exhaust under all the test conditions. This is called a ‘surrogate’ approach in modelling. Dodge was able to successfully simulate all seventeen 6-h duration, constant light runs in the BOM chamber with a model VOC mixture of 25 % propylene and 75 % n-butane. Apparently, the reactivity of the aromatic fraction was similar to that of the combined olefin + paraffin fractions. Dodge’s next steps were to remove any chamber dependent chemistry (deposition, initial radical sources) and to change to a diurnal light profile typical of Los Angeles summer solstice and to run the model between 0700 and 1600 hours during which there was a 100-m increase in mixing height but no horizontal dispersion. A matrix of initial NOx and NMHC conditions were simulated and an ozone isopleths diagram was produced (Fig. 4).

|
This work became the basis of the EPA’s Empirical Kinetic Modelling Approach (EKMA) for computing the relationship among ozone’s precursors (NOx and NMHC) and ozone’s maximum concentration in a Lagrangian box-model framework for a given city with its unique emissions.[24] This method was used by the EPA’s policy office for nearly two decades. This model could eventually be run on IBM PCs; it has spread worldwide. The subsequent development of three-dimensional, Eulerian-formulated models that combined meteorological, emissions and chemistry sub-models took much longer than anticipated. Furthermore, the states needing to apply the large models had to await the availability of affordable computer hardware needed to run them.
In the early 1980s, a student, Michael Gery, joined the UNC Fox–Jeffries group and undertook a Ph.D. thesis on gas-phase reactions of hydroxyl radical and toluene using a unique continuous stirred tank reactor at the UNC Chamber; he published his results in 1985.[25] Subsequently, Dr Gery joined Whitten’s team at SAI. Given Gery’s knowledge and experience at UNC chambers, including working with Dodge, and his experience in investigating aromatic product reaction mechanisms, he was ideally suited to help with the further development of the Carbon Bond Mechanisms that were under development at SAI. In 1988, SAI submitted to the EPA a large final report[26] and in 1989 published a paper[27] on the creation and testing of a new Carbon Bond IV model mechanism, suitable for use in both Ozone Isopleth Plotting Program (OZIPP) and EKMA applications and in Eulerian air quality models. It described the hierarchy of species approach used, the creation and testing of a ‘detailed’ or ‘semi-explicit’ mechanism and the further condensation of this to produce a compact, yet accurate mechanism. This mechanism, sometimes called CB4 and sometimes CB-IV, became the most widely used mechanism in the world. It was not only used extensively in the US, but it also appeared in models applied to England, Europe, China, Korea, Brazil, Chile, Australia and Japan. The Gery et al. 1989 paper[27] is an excellent paradigm for how to develop and test a photochemical reaction mechanism. It would benefit atmospheric chemistry students to read it even today.
The work of creating the UNC outdoor chamber database
Dynamic experiments
Charles Feigley was Jeffries’ first Ph.D. student. As part of the effort to evaluate smog chamber data as a means of determining the relationship between oxidant concentrations and its precursors, in 1977, Jeffries received funding from the EPA to attempt smog chamber validation using Lagrangian atmospheric data.[28] The new EKMA method described above operated a Lagrangian box-model through an urban area, with emissions entering the bottom and the top of the well mixed box (representing the mixed layer) increasing as the mixing layer depth increased, all resulting in a ‘dynamic system’ of injection and dilution. Almost all the smog chamber data used to test the chemistry was from batch-mode, i.e. initial injections of reactants and virtually no dilution for the duration of experiment. In 1973, the Coordinating Research Council and the EPA funded a special field study called the Los Angeles Reactive Pollutant Program (LARPP), in which constant altitude tetroons were released to follow the wind and EPA instrumented helicopters flew multi-altitude square patterns around the tetroons as they drifted in the wind. The positions of both were detected by precision radar each minute. The purpose of the project was to assemble a data package for studying the processes of transport, diffusion and chemical reaction associated with photochemically reactive air pollutants. The analysis of the most successful of these, Operation 33, to obtain chamber operating conditions and atmospheric comparison data[29] and the UNC chamber experiments to simulate these data[30] were the subjects of Feigley’s Ph.D. thesis. In general, within the limits of measurement error and analysis error the UNC chamber was able to simulate the ozone concentrations for several trials. The use of acetaldehyde to represent all of the ambient aldehydes was judged to reduce the ozone production in the chamber.
In 1976, a new M.Sc. student with excellent prior experience, Kenneth Sexton, joined the Jeffries–Kamens group. He had a natural talent for working at the chamber site and soon contributed to measuring more nitrogen compounds as part of his Master’s thesis.[31] As the EPA became more interested in testing ‘dynamic systems’ and testing alternative mechanisms, Sexton undertook a Ph.D. thesis[32] that created a quality matched test set of conditions.
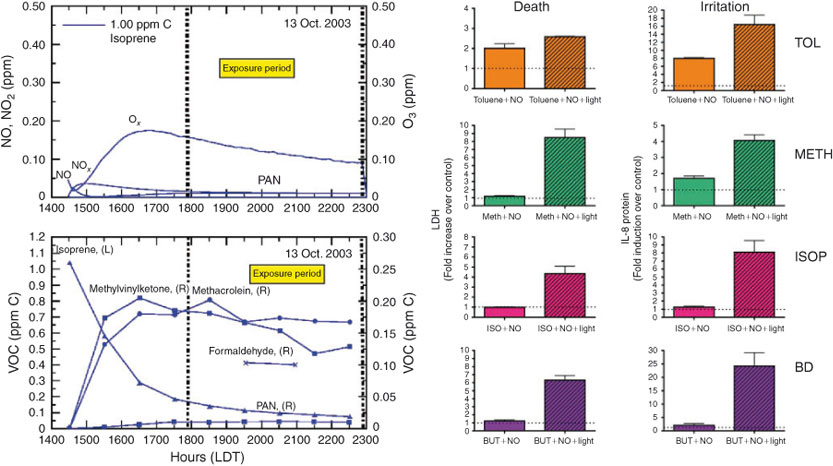
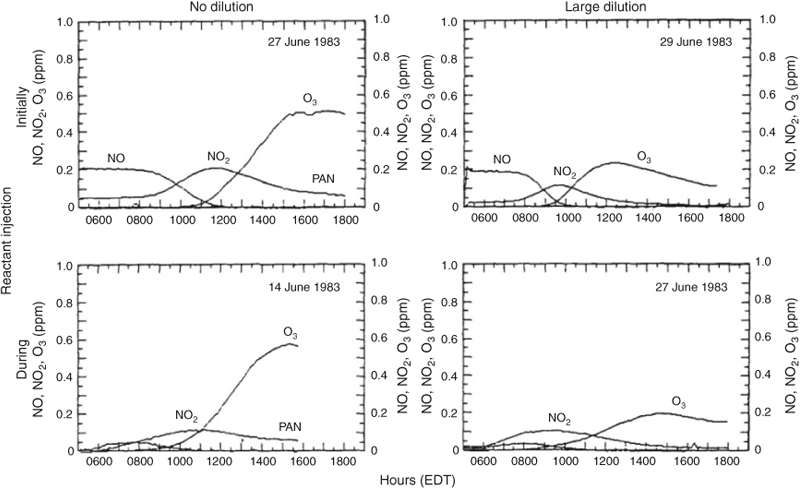
For example, Fig. 5 shows four experiments performed on three June 1983 days showing the same addition of NOx (0.25 ppm) and of a simple mix of propylene, butane, toluene (2.6 ppm C), but under variable injection and dilution conditions: (a) no dilution, initial injection only; (b) no dilution, slow injection during run; (c) large dilution, initial injection only and (d) large dilution, slow injection during run. The dilution was the same as the mixing height going from 250 to 1250 m in 10 h. These types of experiments were important to test various photochemical mechanisms that were being used in the Ozone Isopleth Plotting with Optional Mechanisms (OZIPM) and EKMA program. Sexton’s thesis described a dataset containing 20 dual experiments. What was demonstrated from this work was that the most difficult condition to simulate was the fully static case; the competing physical conditions and dilution produce conditions that are not as stressful on the chemistry as the static case. As a consequence, we lost interest in such dynamic experiments with difficulty to quantify time-varying physical conditions.
|
Beginning in 1984, Dr Sexton joined the research staff, became the Principal Site Manager and Operator and began to supervise students. Later Sexton became a Research Assistant Professor and has served on many M.Sc. and Ph.D. committees, as well as operating two chambers.
Experiments to test photochemical models
Much of the chamber experimental time after 1976 was devoted to work under Dodge’s program to expand and test new explicit and Carbon Bond-style photochemical mechanisms. Whitten’s team delivered monthly progress reports to Dodge. UNC funding was by co-operative grant agreement that required a final report. For three major overlapping projects, we ran experiments as weather permitted, performed calibrations and quality assurance tests, processed data and provided magnetic tapes of results to the SAI on a monthly basis, we monitored Whitten’s monthly reports, ran the models ourselves to see the modelling quality ourselves and provided feedback to Whitten’s team about mistakes in model inputs.
Subsequently, the UNC received chamber funding from the EPA to do experiments with real automobile exhaust and from the Coordinating Research Council and Department of Energy to investigate the photochemistry of alternative fuels. In later years, the EPA sponsored significant chamber work on aromatic chemistry experiments. Several small projects funded the exploration of formaldehyde measurements and modelling, including photolysis rates in the outdoor chamber and those in air quality models.
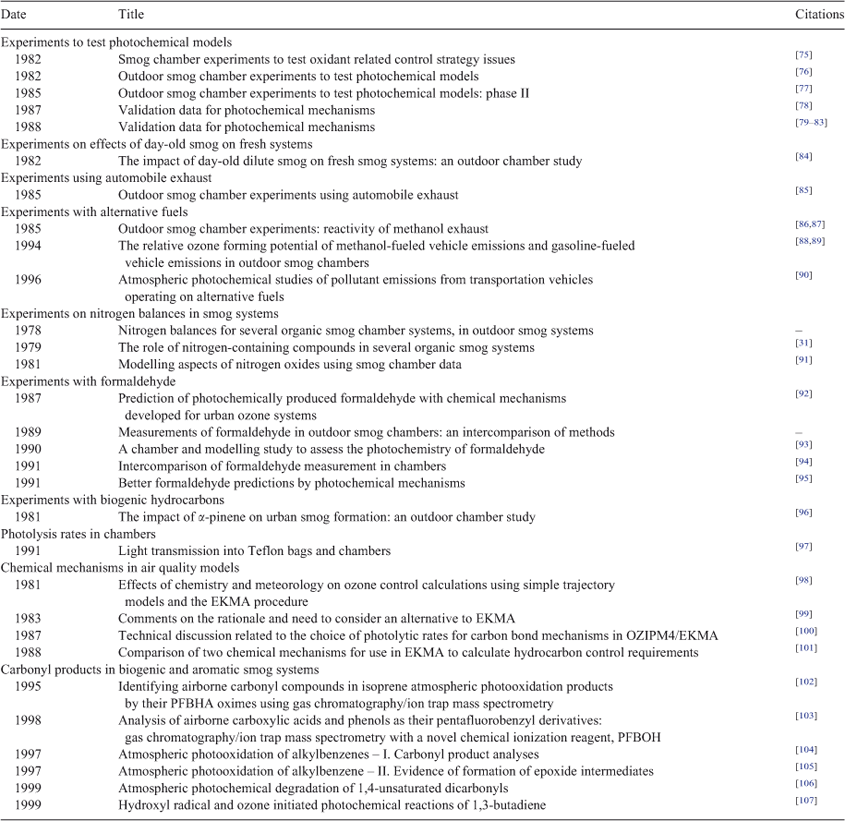
Table 1 summarises a series of projects from the late 1970s to the late 1990s that contributed to the chamber database. There are more than 1200 dual outdoor chamber experiments in the database. Sexton has modelled more than 300 of these; the ‘best’ 100 and 300 were shared with Whitten and with Dr William Carter at UC-Riverside.
|
Expansion of the research group and chambers to test combustion aerosols
In the early 1980s, the group also began to focus on atmospheric combustion aerosols. This led to the construction of two smaller 25-m3 chambers (Fig. 2) under the direction of Kamens.[33] For almost three decades, fine aerosol work at the UNC under the direction of Kamens has focussed on the chemical transformations that occur on atmospheric particles and have resulted in over 100 peer-reviewed journal articles. These studies initially addressed potentially toxic compounds like polycyclic aromatic hydrocarbons (PAH) and halogenated dibenzodioxins and furans, which are associated with different aerosol systems.
During the 1980s, the Kamens research group investigated the extent to which O3, NO2, N2O5 and sunlight influence chemical changes of organics on soot particles as these particles age in the atmosphere.[33–35] Kamens reported that sunlight[34] promotes the decay of PAH on soot particles as these particles age in the atmosphere, and is more important than oxidation by either O3 or NO2. Guo and Kamens[36] then developed techniques for studying heterogeneous reactions on soot surfaces. Bacterial mutagenic increases in aged wood smoke particles were also observed by Glen Rives and Douglas Bell in night-time systems with NO2 or O3 + NO2.[33] Much more recently student Weruka Rattanavaraha[37] and post-doc Qianfeng Li,[38] showed that the reactive oxidant species potential of dilute diesel exhaust dramatically increases as it ages with ozone in the dark and in sunlight in our chambers. In the mid-1980s from outdoor chamber experiments Kamens and his students were able to develop rate constants for these reactions as a function of sunlight, water vapour and temperature.[34] These rate constants and PAH source signatures were then used in chemical mass balance receptor models to estimate particle source apportionment by student Cheng Kang Li.[39] This rate constant work led to the integration of gas phase smog kinetics with particle PAH and nitro-PAH reactions and permitted the modelling of the daytime formation and decay of selected nitro-PAH (e.g. 2-nitrofluoranthene) in both the gas and particle phases by then student Zhihua Fan.[40,41] Much of this work is summarised in a 2002 text on aromatics by Cavert et al.[42] Kamens and his late faculty colleague M. Judith Charles, along with their students Parag Birla,[43] Chris Lutz[44] and David Penise[45] went on to employ these techniques to study the atmospheric stability of brominated and chlorinated dioxins and furans.
In the late 1980s, Dr Steven McDow[46] joined the Kamens’ research group and focussed interest on organic aerosols. Over the next 20 years the Kamens’ research group concentrated on semivolatile gas-particle partitioning, which was greatly influenced by the seminal work of Professor James Pankow.[47,48] UNC students Jay Odum, Jian Zhen Yu[49] and Michael Strommen[50] then implemented an inner particle radial diffusion model to illustrate uptake from the gas to the particle phase. In the late 1990s, Dr Myoseon Jang implemented an equilibrium-gas-particle partitioning technique which took advantage of activity coefficient calculations.[51,52] This provided a novel thermodynamic approach to estimate the partitioning of both polar and non-polar toxic semivolatiles. By the late 1990s, Kamens and his group were able to develop a kinetics model that used gas-particle partitioning to predict aerosol formation from biogenic HCs. Along with Mohamed Jaoui, Myoseon Jang, Michael Strommen and Kerri Leach, a gas-particle model was successfully developed to predict smog chamber secondary aerosol formation (SOA) from α-pinene smog systems.[53–55]
New outdoor aerosol chamber
Further aerosol work necessitated the building of a new 270-m3 outdoor aerosol smog chamber (Fig. 6) at the Pittsboro site.[56] The chamber provides a large volume with a low surface-to-volume ratio to permit long fine aerosol life-times. As with the 312-m3 dual gas chamber, its two sides afforded direct control comparisons. It has very short sampling lines for aerosol sampling, and has the unique ability to exchange contents of the chambers and to mix and react dilute reactants. Last, remote control of many of the chamber functions like venting, clean air purging and data acquisition permits remotely controlled experiments and provides a unique teaching tool for students as far away as Bangkok.

|
This new chamber was used to conduct experiments that were used to integrate gas and particle phase chemistry and equilibrium partitioning thermodynamics. Sirakarn Leungsakul extended the α-pinene approach to d-limonene + O3 dark systems and NOx + light systems.[57,58] This semi-explicit approach was then expanded to toluene by Di Hu et al.,[59] and was later adapted in much simpler form by Kamens, Yang Zhou and Elias P. Rosen et al. to toluene and xylene SOA systems,[60,61] with help from students Eric Chen, Rebecca Wilson and Katherine Galloway. The important discovery from the chamber experiments was that particle phase water could dramatically increase SOA formation from aromatics in the presence of background aerosols or ammonium sulfate seeds; the opposite was later observed for isoprene systems. Most recently, Dr Haofei Zhang[62] has shown how to kinetically predict isoprene SOA and at some time in the future mechanisms for mixtures of terpenes, isoprene and aromatics will be integrated. While all of this was going on, in the early 2000s Professor Myoseon Jang made her cornerstone discovery that acid catalysed reactions and along with Dr Michael Tolocka,[63] that subsequent oligomerisation could be very important in SOA formation.[64,65] This opened up an important vein of research that continues today.
Outdoor chamber to study gas and particulate matter toxicities on human lung cells
In 2004, on the UNC campus near the UNC Hospital and the UNC School of Public Health, the EPA Human Studies Division (HSD) constructed a laboratory called the Human Studies Facility (HSF) (see http://www.epa.gov/oaintrnt/facilities/rtp_chapel.htm, accessed 18 June 2013). The HSD is part of the National Health and Environmental Effects Research Laboratory (NHEERL) within EPA’s Office of Research and Development (ORD). The Laboratory conducts clinical research and studies on the causes and spread of diseases to improve the understanding of human health risks associated with environmental pollution. Prior to building the HSF, the EPA was conducting joint research with the UNC School of Medicine (SOM) researchers by cooperative agreements between SOM’s Center for Environmental Medicine, Asthma and Lung Biology (CEMALB) (see http://www.med.unc.edu/cemalb, accessed 18 June 2013) and EPA’s HSD researchers. Studies were carried out in the various departments of SOM with interest in air pollution health effects. The center was subsequently co-located in the EPA Human Studies Facility. The center provides a cooperative setting for UNC and EPA researchers to conduct research studies involving human volunteers that are aimed at understanding the negative health effects of air pollution on the lung and heart.
In 2002, Dr Phillip Bromberg, M.D. and founding member of CEMALB, who frequently explored collaborations between SOM and SPH, and Dr Ian Gilmour, a member of HDS and an adjunct faculty member in SPH and the Curriculum in Toxicology (see http://www.med.unc.edu/toxicology, accessed 18 June 2013), introduced our research group to Dr Ilona Jaspers, a faculty member of SOM’s Department of Paediatrics. In her laboratory, Jaspers had been exposing cultured human lung cells to ozone alone in air. Jaspers’ interest was to develop in vitro toxicity methods and to expand exposures from single air pollutants to more complex and realistic mixtures that characterise smog systems. We began to explore how to expose cultured lung cells to reacted urban-like HC–NOx mixtures in our smog chamber experiments. This involved creating an interface system between her gaseous in vitro exposure-chambers (that held and kept lung cells alive) and the contents of our smog chambers.
We built a twin incubator-based, gaseous in vitro exposure chamber connected to the dual outdoor smog chambers in Pittsboro, NC, as well as a humidified clean air source for a clean air exposure control and a holding system (Fig. 7). At that time, Jaspers, in her laboratory on campus, produced the cell-cultures and plated the cells on inserts for gaseous air–liquid-interface exposures. For each experiment, the plated inserts were transported by automobile to the Pittsboro Dual Chamber site.

|
In the first cellular exposure test with the dual chamber, we reacted in one chamber a standard complex SynUrban and NOx photochemical mixture leading to ozone and other reaction products and in the other chamber we injected into otherwise clean air only ozone equal to that produced in the photochemical system. Two sets of cultured lung cells were exposed, one to each chamber side. In a second cellular exposure test, cells were exposed, in the dark, to one chamber side in which just the injected reactants used in the photochemical mix were added to clean air. The results were very interesting in that the toxicological endpoints from the irradiated smog mixture were significantly larger than either the ozone-only or precursor-only exposure results.[66] This was an early example of the effect of atmospheric chemistry on the toxicity of air pollutant mixtures. It showed the enhanced toxicity effect, above that from ozone and initial primary reactants, due to the presence of secondary toxic compounds that were produced in the atmosphere. This paper was cited in the EPA’s revised ozone criteria document[67] as an approach to reconciling the so-called ‘disconnect’ between clinical and epidemiology ozone risk assessments.
These preliminary, yet compelling, joint studies allowed the chamber group and Dr Jaspers to propose to the EPA a cooperative agreement to build a new on-campus smog chamber. The design included a combined laboratory for atmospheric chemistry and human cellular effects of exposure to a ‘one-atmosphere’ environment that included ‘fresh’ and ‘aged’ exposures to gases and particulate matter (PM) from irradiated smog mixtures such as diesel exhaust, alternative fuels exhaust and a variety of urban-like gas and particle environments. The laboratory would be optimised for cellular exposures and subsequent on-site analysis of toxicity without having to transport biological samples from the existing remote chamber site or across campus. The EPA funded the proposal and it was extended to a 5-year period. In 2003, Dr Jaspers became a Joint Professor in the Department of ESE and has been an active student mentor and advisor on M.Sc. and Ph.D. committees. She also became Associate Director for CEMALB, and a Professor in the Curriculum of Toxicology.
While the new chamber and laboratory were being built, we continued the gas-only toxicity work at the Pittsboro dual outdoor gas chamber. We also received support in 2002 from the American Chemistry Council to begin applying the new exposure techniques to understand the modification of risk from atmospheric chemistry of industrial chemicals. It was decided that a series of simple system experiments (using 1,3-butadiene and isoprene) were needed to allow for direct side by side comparisons of: (a) toxicity of chamber gas from the photochemical oxidation of single HC and NOx experiments with (b) mixtures reproduced by injecting the same levels of the major explicit secondary products observed in the photochemically oxidised mixture; simple examples are shown in Fig. 8. These experiments demonstrate that secondary products can up-regulate and reproduce a significant amount of the toxicity endpoints observed in the full photochemical system. Results also suggested that there were even more unmeasured products contributing to the toxicity but these were detected toxicologically.[68,69] In addition, traditional photo-irradiation experiments of 1,3-butadiene and NOx were conducted and modelled to show that these experiments were useful for both chemical mechanism testing as well as toxicity testing, and gave validity to the realism of the exposure conditions for the toxicity testing.[70]
Meanwhile, the new chamber and laboratory were constructed (Fig. 9). The American Chemistry Council award also supported the expansion of the laboratory instrumentation.

|
With the EPA cooperative agreement, we began to develop a method for the direct exposure of PM at the air–liquid interface (ALI) of the human lung cells. The only effective method was use of a particle charging and electrostatic repelling field to gently deposit PM on the ALI of the cells in the in vitro exposure system. ESE faculty member Dr David Leith, with extensive experience in the use of electrostatic sampling of particles, began to collaborate on this project and to mentor students working on it. The M.Sc. and Ph.D. thesis work of Sandra Lake, Melanie Doyle, Kim Lichtveld (deBruijne), Seth Ebersviller and Jose Mendez have contributed greatly to the evolution and application of this method. This device proved to be useful in exposing diesel exhaust mixtures to cells in vitro.[71] Both fresh and photochemically aged mixtures were exposed for toxicity tests and it was shown that atmospheric chemistry could significantly alter the toxicity. Most of the published studies of in vitro aerosol and particulate toxicity studies have been performed by resuspension techniques, in which PM is collected on a filter, and then resuspended in liquid media and applied to cells. We compared the in vitro cellular effects of our direct deposition of PM at the ALI with the resuspended PM using diesel exhaust.[72] Resuspension, which has been widely used to assess particle in vitro effects, significantly underestimates the cellular toxicity.
Furthermore, the use of both gaseous and particle in vitro exposures conducted simultaneously was demonstrated to be very effective in disentangling the contributions of gas and particle toxicity. There was compelling evidence that there was an interaction between gas and particle contributions to the toxicity. A surrogate aerosol to diesel particles was designed to be non-toxic but of similar size and density (aerosolised mineral oil). By conducting a series of experiments where mixing in a dark chamber a non-toxic aerosol of mineral oil with a single toxic gas species, it was demonstrated that the non-toxic mineral oil could be made significantly toxic[73] (Fig. 10). In addition, it was demonstrated that a similar process happened in a photochemically aged complex urban mixture, in which gaseous reactions produced significant toxic gaseous products that would then cause the non-toxic mineral oil to become toxic from simple mixing and resulting gas-to-particle partitioning. In other words, the urban atmosphere itself is a significant source of gaseous and PM toxicity.[74]

|
The future of outdoor chamber studies at UNC
Professors Jeffries, Kamens and Sexton have retired (at the end of 2011 and, for Sexton, 2013) from full-time positions at UNC and have become emeritus faculty. All still serve on student committees and meet with students on research issues; Kamens is an active participant in leadership and travel in the UNC’s Study Abroad program.
We are pleased that the Department of ESE encouraged early recruitment of young ‘air faculty’ to maintain the vitality of the 50-year-long atmospheric research program in the ESE.
In the last several years, the Department has added three young and internationally recognised faculty members (see http://www2.sph.unc.edu/envr/our_faculty_and_staff_187_6352.html, accessed 18 June 2013). Dr Jason West’s area of expertise includes climate change, global scale modelling and its policy implications. Dr William Vizuete continues work on urban and regional scale photochemical modelling, advanced process analysis of results for regulatory issues, and health and environmental policy. He also leads the commercialisation of the cultured human lung cell exposure instrument (see http://www.quantaire.com, accessed 18 June 2013). Dr Jason Surratt’s research involves mechanisms for SOA formation, both in ambient atmospheric field studies and in experimental investigation of reactions and mechanisms using the outdoor chambers and advanced analytical instruments. These studies now extend to the toxic effects of these secondary produced aerosols.
Their combined academic trajectories will continue to position UNC as a centre of excellence for atmospheric research and policy.
References
[1] A. Stern, Air Pollution 1968 (Academic Press: New York).[2] Environmental Protection Agency Fed. Register 1971, 36, 8187.[30 April 1971]
[3] P. Leighton, Photochemistry of Air Pollution 1961 (Academic Press: New York).
[4] Environmental Protection Agency Fed. Register 1971, 36, 115 486-15 506.[14 August 1971]
[5] B. Dimitriades, Methodology in air pollution studies using irradiation chambers. J. Air Pollut. Control Assoc. 1967, 17, 460.
| Methodology in air pollution studies using irradiation chambers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2sXkslKisrk%3D&md5=eccc0385a5dbc059a319260b31030cdaCAS | 6042739PubMed |
[6] J. Worth, L. Ripperton, C. Berry, Ozone variability in mountainous terrain. J. Geophys. Res. 1967, 72, 2063.
| Ozone variability in mountainous terrain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2sXhtVOisr4%3D&md5=b5525a8ce92b4c06ed15cb69a3f903b6CAS |
[7] H. Jeffries, An experimental method for measuring the rate of synthesis, destruction, and transport of ozone in the lower atmosphere 1971, Ph.D. thesis, University of North Carolina at Chapel Hill, NC.
[8] M. Cazorla, W. H. Brune, Measurement of ozone production sensor. Atmos. Meas. Tech. 2010, 3, 545.
| Measurement of ozone production sensor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFSnur3P&md5=3e005b936f0458c330f6957544bb17f8CAS |
[9] R. M. Kamens, A. C. Stern, Methane in air quality and automobile exhaust emission standards. J. Air Pollut. Control Assoc. 1973, 23, 592.
| 1:CAS:528:DyaE3sXkvVarur0%3D&md5=252dbfa15e4d11cb1bb4c7c926bdfac1CAS | 4122975PubMed |
[10] H. Jeffries, Photochemical air pollution, in Composition, Chemistry, and Climate of the Atmosphere (Ed. H. B. Singh) 1995, pp. 308–348 (Van Nostand-Reinhold: New York).
[11] H. Jeffries, D. Fox, R. Kamens, Outdoor smog chamber studies: effect of hydrocarbon reduction on nitrogen dioxide. EPA-650/3-75-011 1975 (Environmental Protection Agency).
[12] H. Jeffries, D. Fox, R. Kamens, Outdoor smog chamber studies: light effects relative to indoor chambers. Environ. Sci. Technol. 1976, 10, 1006.
| Outdoor smog chamber studies: light effects relative to indoor chambers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXhvFCit78%3D&md5=9c2611a8b3f5ebaa8393a7d712d25ea0CAS |
[13] K. Demerjian, A. Kerr, J. Calvert, The mechanism of photochemical smog formation, in Advances in Environmental Sciences and Technology, Vol. 4 (Eds J. N. Pitts Jr and R. L. Metcaf) 1974 pp. 1–262. (Wiley: New York).
[14] N. R. Greiner, Hydroxyl‐radical kinetics by kinetic spectroscopy. II. Reactions with C2H6, C3H8, and iso‐C4H10 at 300 K. J. Chem. Phys. 1967, 46, 3389.
| Hydroxyl‐radical kinetics by kinetic spectroscopy. II. Reactions with C2H6, C3H8, and iso‐C4H10 at 300 K.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2sXktlKksbk%3D&md5=d48dfa3b8c06fc7f74e7253fcedeaf52CAS |
[15] D. Stedman, E. Morris, E. Daby, H. Niki, B. Weinstock, The role of OH radicals in photochemical smog reactions, in 160th National ACS Meeting, 13–18 September 1970, Chicago, IL, 1970 (American Chemical Society: Washington, DC).
[16] T. Hecht, J. Seinfeld, M. Dodge, Further development of generalized kinetic mechanism for photochemical smog. Environ. Sci. Technol. 1974, 8, 327.
| Further development of generalized kinetic mechanism for photochemical smog.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2cXhsVykt7w%3D&md5=80be4764c3dbd4300a3ecc4b3854ad7eCAS |
[17] G. Whitten, Modeling of Simulated Photochemical Smog with Kinetics Mechanisms. EPA-600/3-79-001a 1979 (Environmental Protection Agency).
[18] B. Dimitriades, (Ed.) International Conference on Photochemical Oxidant Pollution and its Control Proceedings: Vol. II, 12–17 September 1976, Raleigh, NC. EPA-600/3-77-001b 1977 (Environmental Protection Agency).
[19] B. Dimitriades, An alternative to the appendix-J method for calculating oxidant-and NO2-related control requirements, in International Conference on Photochemical Oxidant Pollution and its Control Proceedings: Vol. II, 12–17 September 1976, Raleigh, NC. EPA-600/3-77-001b (Ed. B. Dimitriades) 1977, pp. 871–880 (Environmental Protection Agency).
[20] B. Dimitriades, Effects of hydrocarbon and nitrogen oxides on photochemical smog formation. Environ. Sci. Technol. 1972, 6, 253.
| Effects of hydrocarbon and nitrogen oxides on photochemical smog formation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE38XhtVens74%3D&md5=ed1bca4312441af0b0b538547caa18f5CAS |
[21] B. Dimitriades, Oxidant control strategies. Part I. Urban oxidant control strategy derived from existing smog chamber data. Environ. Sci. Technol. 1977, 11, 80.
| Oxidant control strategies. Part I. Urban oxidant control strategy derived from existing smog chamber data.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2sXht1Kgtb4%3D&md5=d7bd0440d41da81d9a2848cc11a30287CAS |
[22] H. Jeffries, R. Kamens, D. L. Fox, B. Dimitriades, Outdoor smog chamber studies: effect of diurnal light, dilution, and continuous emission on oxidant precursor relationships, in International Conference on Photochemical Oxidant Pollution and its Control Proceedings: Vol. II, 12–17 September 1976, Raleigh, NC. EPA-600/3-77-001b (Ed. B. Dimitriades) 1977, pp. 891–902 (Environmental Protection Agency).
[23] M. Dodge, Combined use of modeling techniques and smog chamber data to derive ozone–precursor relationships, in International Conference on Photochemical Oxidant Pollution and its Control Proceedings: Vol. II, 12–17 September 1976, Raleigh, NC. EPA-600/3-77-001b (Ed. B. Dimitriades) 1977 (Environmental Protection Agency).
[24] B. Dimitriades, M. Dodge, Proceedings of the Empirical Kinetic Modeling Approach Validation Workshop, 15–16 December 1981, Research Triangle Park, NC. EPA-600/9-83-014 1983 (Environmental Protection Agency: Research Triangle Park, NC).
[25] M. Gery, D. Fox, H. Jeffries, L. Stockburger, W. Weathers, A continuous stirred tank reactor investigation of the gas-phase reaction of hydroxyl radicals and toluene. Int. J. Chem. Kinet. 1985, 17, 931.
| A continuous stirred tank reactor investigation of the gas-phase reaction of hydroxyl radicals and toluene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28XjvV2ntw%3D%3D&md5=c2cb76e50736de4ba87c5cf73b2f8e62CAS |
[26] M. Gery, G. Z. Whitten, J. P. Killus, Development and Testing of the CBM-IV (Carbon Bond Mechanism) for Urban and Regional Modeling. EPA-600/3-88/012 1985 (Environmental Protection Agency).
[27] M. Gery, G. Whitten, J. Killus, M. Dodge, A photochemical kinetics mechanism for urban and regional scale computer modeling. J. Geophys. Res. 1989, 94, 12 925.
| A photochemical kinetics mechanism for urban and regional scale computer modeling.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3cXlvFKrsL4%3D&md5=a25e6461dfd3fc71eb2a529016b7253eCAS |
[28] C. Feigley, H. Jeffries, Smog Chamber Validation using Lagrangian Atmospheric Data. EPA-600/3-79-050 1979 (Environmental Protection Agency).
[29] C. Feigley, H. Jeffries, Analysis of processes affecting oxidant and precursors in the Los Angeles Reactive Pollutant Program (LARPP) Operation 33. Atmos. Environ. 1979, 13, 1369.
| Analysis of processes affecting oxidant and precursors in the Los Angeles Reactive Pollutant Program (LARPP) Operation 33.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXotFGjug%3D%3D&md5=596cf0d1d9fe95c45e4215886db8247dCAS |
[30] C. Feigley, H. Jeffries, R. Kamens, An experimental simulation of Los Angeles Reactive Pollutant Program (LARPP) operation 33 – part I. Experimental simulation in an outdoor smog chamber. Atmos. Environ. 1982, 16, 1989.
| An experimental simulation of Los Angeles Reactive Pollutant Program (LARPP) operation 33 – part I. Experimental simulation in an outdoor smog chamber.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XlsV2rsrg%3D&md5=74f68af7902142dfc2c7890cc94416a1CAS |
[31] K. Sexton, The Role of Nitrogen-Containing Compounds in Several Organic Smog Systems. MSPH Technical Report 1979 (Department of Environmental Sciences and Engineering, School of Public Health, University of North Carolina: Chapel Hill).
[32] K. Sexton, Experimental simulation of urban-like smog systems for studying the chemistry of ozone formation. DAI Vol. 46–02B, Pub.# AAC8508621 1984, Ph.D. Dissertation, University of North Carolina, Chapel Hill, NC.
[33] R. Kamens, G. D. Rives, J. M. Perry, D. A. Bell, R. F. Paylor, L. D. Claxton, Mutagenic and chemical changes in dilute wood smoke as it ages and reacts with O3, NO2 in the dark – an outdoor chamber study. Environ. Sci. Technol. 1984, 18, 523.
| Mutagenic and chemical changes in dilute wood smoke as it ages and reacts with O3, NO2 in the dark – an outdoor chamber study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXktVWqs7c%3D&md5=b69296c61ee707c1b9729eba35579c4cCAS |
[34] R. Kamens, Z. Guo, J. N. Fulcher, D. A. Bell, The influence of humidity and temperature on the daytime decay of PAH on atmospheric soot particles. Environ. Sci. Technol. 1988, 22, 103.
| The influence of humidity and temperature on the daytime decay of PAH on atmospheric soot particles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXis1Citw%3D%3D&md5=de817964d1524057b9199bbc2025b200CAS | 22195517PubMed |
[35] R. Kamens, J. Guo, Z. Guo, PAH and N2O5 reactions on atmospheric soot particles. Atmos. Environ. 1990, 24A, 1161.
| 1:CAS:528:DyaK3cXks1Cnt70%3D&md5=7f82e85ccd341944ef2c1bc651612a6dCAS |
[36] Z. Guo, R. Kamens, An experimental technique for studying heterogeneous reactions of polyaromatic hydrocarbons on particle surfaces. J. Atmos. Chem. 1991, 12, 137.
| An experimental technique for studying heterogeneous reactions of polyaromatic hydrocarbons on particle surfaces.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhslShtrk%3D&md5=d663dcd606d11b327dcb0eddc783e9f7CAS |
[37] W. Rattanavaraha, E. Rosen, H. Zhang, Q. Li, K. Pantong, R. M. Kamens, The reactive oxidant potential of different types of aged atmospheric particles: an outdoor chamber. Atmos. Environ. 2011, 45, 3848.
| The reactive oxidant potential of different types of aged atmospheric particles: an outdoor chamber.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmvFOhu7Y%3D&md5=4c1845b8db71a13a2c00798da58519fdCAS |
[38] Q. Li, A. Wyatt, R. M. Kamens, Oxidant generation and toxicity enhancement of aged-diesel exhaust. Atmos. Environ. 2009, 43, 1037.
| Oxidant generation and toxicity enhancement of aged-diesel exhaust.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXps1agsQ%3D%3D&md5=b1543001f1a4744fe32db5a16b1f20d8CAS |
[39] C. Li, R. Kamens, The use of polycyclic aromatic hydrocarbons as source signatures in receptor modeling. Atmos. Environ. 1993, 27A, 523.
| 1:CAS:528:DyaK3sXitFyks7w%3D&md5=97e112fdaf4a853512ebee3eda7f9be1CAS |
[40] Z. Fan, D. Chen, P. Birla, R. M. Kamens, Modeling of nitro-polycyclic aromatic hydrocarbon formation and decay in the atmosphere. Atmos. Environ. 1995, 29, 1171.
| Modeling of nitro-polycyclic aromatic hydrocarbon formation and decay in the atmosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmsFSksbg%3D&md5=6114d9edc55475656f1c366d16b98377CAS |
[41] Z. Fan, R. M. Kamens, J. Hu, J. Zhang, S. McDow, Photostability of nitro-polycyclic aromatic hydrocarbons on combustion particles in sunlight. Environ. Sci. Technol. 1996, 30, 1358.
| Photostability of nitro-polycyclic aromatic hydrocarbons on combustion particles in sunlight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xht1ylsro%3D&md5=4af4fba56ee5d2e66f88fad37d0e771bCAS |
[42] J. Calvert, R. Atkinson, K. Becker, R. Kamens, J. Seinfeld, T. Wallington, G. Yarwood, The Mechanisms of Atmospheric Oxidation of Aromatic Hydrocarbons 2002 (Oxford University Press: New York).
[43] P. Birla, P. R. M. Kamens, Effects of combustion temperature on the atmospheric stability of polybrominated dibenzo-p-dioxins and dibenzofurans. Environ. Sci. Technol. 1994, 28, 1437.
| Effects of combustion temperature on the atmospheric stability of polybrominated dibenzo-p-dioxins and dibenzofurans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXks1CgsLs%3D&md5=f3038d8a9f702cf0537927349d2e306bCAS | 22165926PubMed |
[44] C. Lutes, M. J. Charles, J. R. Odum, R. M. Kamens, Chamber aging studies of the atmospheric stability of polybrominated dibenzo-p-dioxins and dibenzofurans. Environ. Sci. Technol. 1992, 26, 991.
| Chamber aging studies of the atmospheric stability of polybrominated dibenzo-p-dioxins and dibenzofurans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XitVSjtL8%3D&md5=f9c4b694ec28a6f670dda6792a2e2f75CAS |
[45] D. Pennise, R. M. Kamens, Effects of combustion temperature on the atmospheric stability of chlorinated dibenzo dioxins and furan. Environ. Sci. Technol. 1996, 30, 2832.
| Effects of combustion temperature on the atmospheric stability of chlorinated dibenzo dioxins and furan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XksFGrtro%3D&md5=1263dcc9fdc201654f32eced9926d82aCAS |
[46] S. McDow, M. Jang, Y. Hong, R. M. Kamens, An approach to studying the effect of organic composition on atmospheric aerosol photochemistry. J. Geophys. Res. 1996, 101, 19 593.
| An approach to studying the effect of organic composition on atmospheric aerosol photochemistry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XlvVKitbw%3D&md5=62737d5aeb82d655d6bc31652c20b1e9CAS |
[47] J. Pankow, Review and comparative analysis of the theories on partitioning between the gas and aerosol particulate phases in the atmosphere. Atmos. Environ. 1987, 21, 2275.
| Review and comparative analysis of the theories on partitioning between the gas and aerosol particulate phases in the atmosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXhtVGmtw%3D%3D&md5=65fda9734779624fc7060dc5cedd274fCAS |
[48] J. Pankow, An absorption model of gas/particle partitioning of organic compounds in the atmosphere. Atmos. Environ. 1994, 28, 185.
| An absorption model of gas/particle partitioning of organic compounds in the atmosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXisFajs78%3D&md5=c57a2c978502e6cc9c92a9ebca46e43aCAS |
[49] J. Odum, J. Yu, R. M. Kamens, Modeling the mass transfer of semi-volatile organics in combustion aerosols. Environ. Sci. Technol. 1994, 28, 2278.
| Modeling the mass transfer of semi-volatile organics in combustion aerosols.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXms1agurs%3D&md5=779d794ac430d63cb0bff6f95f44ac03CAS | 22176045PubMed |
[50] M. Strommen, R. M. Kamens, Development and application of a dual-impedance radial diffusion model to simulate the partitioning of semivolatle organic compounds in combustion aerosols. Environ. Sci. Technol. 1997, 31, 2983.
| Development and application of a dual-impedance radial diffusion model to simulate the partitioning of semivolatle organic compounds in combustion aerosols.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlslGhtbo%3D&md5=d2b2a7350a04424b15c27d3bc0480db6CAS |
[51] M. Jang, R. M. Kamens, B. K. Leach, M. R. Strommen, A thermodynamic approach using group contribution methods to model: the partitioning of semi-volatile organic compounds on atmospheric particulate matter. Environ. Sci. Technol. 1997, 31, 2805.
| A thermodynamic approach using group contribution methods to model: the partitioning of semi-volatile organic compounds on atmospheric particulate matter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlsFymtLw%3D&md5=98cef71f3f1db9184e8e18a03835a0e0CAS |
[52] M. Jang, R. M. Kamens, A thermodynamic approach for modeling partitioning of semivolatile organic compounds on atmospheric particulate matter: humidity effects. Environ. Sci. Technol. 1998, 32, 1237.
| A thermodynamic approach for modeling partitioning of semivolatile organic compounds on atmospheric particulate matter: humidity effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXitVyruro%3D&md5=3b74eaca100e0e179f60e35a2ebbaa5eCAS |
[53] R. Kamens, M. Jang, B. K. Leach, M. R. Strommen, C. Chien, Aerosol formation from the reaction of α-pinene and ozone using a gas phase kinetics-aerosol partitioning model. Environ. Sci. Technol. 1999, 33, 1430.
| Aerosol formation from the reaction of α-pinene and ozone using a gas phase kinetics-aerosol partitioning model.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhvFOmtbg%3D&md5=b14f70b6996a3e3edb4e2921f9049480CAS |
[54] R. Kamens, M. Jaoui, Modeling aerosol formation from α-pinene + NOx in the presence of natural sunlight using gas phase kinetics and gas-particle partitioning theory. Environ. Sci. Technol. 2001, 35, 1394.
| Modeling aerosol formation from α-pinene + NOx in the presence of natural sunlight using gas phase kinetics and gas-particle partitioning theory.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXhsFKisb4%3D&md5=3a7d40f431accc009459b782e1046763CAS | 11348073PubMed |
[55] M. Jaoui, R. M. Kamens, Mass gaseous and particulate oxidation products of the reaction from a mixture of α-pinene + β-pinene/O3/air in the absence of light and α-pinene + β-pinene/NOx/air in the presence of natural sunlight. J. Atmos. Chem. 2003, 44, 259.
| Mass gaseous and particulate oxidation products of the reaction from a mixture of α-pinene + β-pinene/O3/air in the absence of light and α-pinene + β-pinene/NOx/air in the presence of natural sunlight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXitl2it7w%3D&md5=44c242d294dc2ac9751cea473ace6d75CAS |
[56] S. Lee, M. Jang, R. Kamens, SOA formation from the photooxidation of α-pinene in the presence of freshly emitted diesel soot exhaust. Atmos. Environ. 2004, 38, 2597.
| SOA formation from the photooxidation of α-pinene in the presence of freshly emitted diesel soot exhaust.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXivFGgs7c%3D&md5=019ca7516310c6965edfdc3ed371d61eCAS |
[57] S. Leungsakul, H. E. Jeffries, R. M. Kamens, A kinetic mechanism for predicting secondary aerosol formation from the reaction of d-limonene with NOx and natural sunlight. Atmos. Environ. 2005, 39, 7063.
| A kinetic mechanism for predicting secondary aerosol formation from the reaction of d-limonene with NOx and natural sunlight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFGht7jI&md5=016cc7af52550e7e11085ae0a75e1fbfCAS |
[58] S. Leungsakul, M. Jaoui, R. M. Kamens, A kinetic mechanism for predicting secondary aerosol formation from the reaction of d-limonene with Ozone. Environ. Sci. Technol. 2005, 39, 9583.
| A kinetic mechanism for predicting secondary aerosol formation from the reaction of d-limonene with Ozone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFymsL3M&md5=d3325ef0483a02a830e5d4e5b1725393CAS | 16475339PubMed |
[59] D. Hu, M. Tolocka, Q. L, D. Hu, M. Tolocka, Q. L, A kinetic mechanism for predicting secondary organic aerosol formation from toluene oxidation in the presence of NOx and natural sunlight. Atmos. Environ. 2007, 41, 6478.
| A kinetic mechanism for predicting secondary organic aerosol formation from toluene oxidation in the presence of NOx and natural sunlight.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpsl2jtr8%3D&md5=370737349981ac802faddbd115d3bd18CAS |
[60] R. M. Kamens, H. Zhang, E. H. Chen, Y. Zhou, H. M. Parikh, R. Wilson, K. Galloway, E. P. Rosen, Secondary organic aerosol formation from toluene in an atmospheric hydrocarbon mixture: water and particle seed effects. Atmos. Environ. 2011, 45, 2324.
| Secondary organic aerosol formation from toluene in an atmospheric hydrocarbon mixture: water and particle seed effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvVWktbs%3D&md5=70dcfaa60ebb5bdf1b9cec81061bbb5dCAS |
[61] Y. Zhou, H. Zhang, H. M. Parikh, E. H. Chen, W. Rattanavaraha, E. P. Rosen, W. Wang, R. M. Kamens, Secondary organic aerosol formation from xylenes and mixtures of toluene and xylenes in an atmospheric urban hydrocarbon mixture: water and particle seed effects (II). Atmos. Environ. 2011, 45, 3882.
| Secondary organic aerosol formation from xylenes and mixtures of toluene and xylenes in an atmospheric urban hydrocarbon mixture: water and particle seed effects (II).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmvFOgsrw%3D&md5=c9e1cc03e5d99df2c1fe20023a695aebCAS |
[62] H. F. Zhang, H. M. Parikh, J. Bapat, Y.-H. Lin, J. D. Surratt, R. M. Kamens, Modeling of SOA formation from isoprene photooxidation chamber studies using different approaches. Environ. Chem. 2013, 10, 194.
| Modeling of SOA formation from isoprene photooxidation chamber studies using different approaches.Crossref | GoogleScholarGoogle Scholar |
[63] M. Tolocka, M. Jang, J. M. Ginter, F. J. Cox, R. M. Kamens, M. Johnston, Formation of oligomers in secondary organic aerosol. Environ. Sci. Technol. 2004, 38, 1428.
| Formation of oligomers in secondary organic aerosol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXntVaksw%3D%3D&md5=db598c55b8b61241fd9ee7fb51d488cbCAS | 15046344PubMed |
[64] M. Jang, N. M. Czoschke, S. Lee, R. M. Kamens, Heterogeneous atmospheric organic aerosol production by inorganic acid-catalyzed particle-phase reactions. Science 2002, 298, 814.
| Heterogeneous atmospheric organic aerosol production by inorganic acid-catalyzed particle-phase reactions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XotFSntLk%3D&md5=f347a4de506d13b874097c68a4f53a39CAS | 12399587PubMed |
[65] M. Jang, R. M. Kamens, Atmospheric secondary aerosol formation by heterogeneous reactions of aldehydes in the presence of a sulfuric acid aerosol catalyst. Environ. Sci. Technol. 2001, 35, 4758.
| Atmospheric secondary aerosol formation by heterogeneous reactions of aldehydes in the presence of a sulfuric acid aerosol catalyst.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotFOnsLk%3D&md5=a11ee754ffe67dbeaa5ed2039a1ac7c0CAS | 11775150PubMed |
[66] K. Sexton, H. Jeffries, M. Jang, R. Kamens, M. Doyle, I. Voicu, I. Jaspers, Photochemical products in urban mixtures enhance inflammatory responses in lung cells. Inhal. Toxicol. 2004, 16, 107.
| Photochemical products in urban mixtures enhance inflammatory responses in lung cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXksVKls70%3D&md5=6e261ae4c5706412fa9a0f29600005f3CAS | 15204799PubMed |
[67] Air Quality Criteria for Ozone and Related Photochemical Oxidants (Final). Volumes I, II, and III, EPA 600/R-05/004aF-cF 2006 (Environmental Protection Agency: Research Triangle Park, NC).
[68] M. Doyle, K. Sexton, H. E. Jeffries, K. Bridge, I. Jaspers, Effects of 1,3-butadiene, isoprene, and their photochemical degradation products on human lung cells. Environ. Health Perspect. 2004, 112, 1488.
| Effects of 1,3-butadiene, isoprene, and their photochemical degradation products on human lung cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhtVGmur7N&md5=678a8984597786e6af0f6a42b1009e5aCAS | 15531432PubMed |
[69] M. Doyle, K. G. Sexton, H. E. Jeffries, I. Jaspers, Atmospheric photochemical transformations enhance 1,3-butadiene-induced inflammatory responses in human epithelial cells: the role of ozone and other photochemical degradation products. Chem. Biol. Interact. 2007, 166, 163.
| Atmospheric photochemical transformations enhance 1,3-butadiene-induced inflammatory responses in human epithelial cells: the role of ozone and other photochemical degradation products.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjtFGkt7g%3D&md5=3cff74ee2efc57067dffd79f922c03d8CAS | 16860297PubMed |
[70] K. G. Sexton, M. L. Doyle, H. E. Jeffries, S. Ebersviller, Development and testing of a chemical mechanism for atmospheric photochemical transformations of 1,3-butadiene. Chem. Biol. Interact. 2007, 166, 156.
| Development and testing of a chemical mechanism for atmospheric photochemical transformations of 1,3-butadiene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjtFGkt7s%3D&md5=ad061f8abb8a6fe4b63f2550f973b766CAS | 17328875PubMed |
[71] K. de Bruijne, S. Ebersviller, K. G. Sexton, S. Lake, D. Leith, R. Goodman, J. Jetters, G. W. Walters, M. Doyle-Eisele, R. Woodside, H. E. Jeffries, I. Jaspers, Design and testing of electrostatic aerosol in vitro exposure system (EAVES): an alternative exposure system for particles. Inhal. Toxicol. 2009, 21, 91.
| Design and testing of electrostatic aerosol in vitro exposure system (EAVES): an alternative exposure system for particles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXksFyhtA%3D%3D&md5=44bb81649e27e8ef1597cc6761c5fc8dCAS | 18800273PubMed |
[72] K. M. Lichtveld, S. M. Ebersviller, K. G. Sexton, W. Vizuete, I. Jaspers, H. E. Jeffries, In vitro exposures in diesel exhaust atmospheres: resuspension of PM from filters versus direct deposition of PM from air. Environ. Sci. Technol. 2012, 46, 9062.
| In vitro exposures in diesel exhaust atmospheres: resuspension of PM from filters versus direct deposition of PM from air.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFSjurfO&md5=c064aec7b9eb527216c544d158b5fff7CAS | 22834915PubMed |
[73] S. Ebersviller, K. Lichtveld, K. G. Sexton, J. Zavala, Y. H. Lin, I. Jaspers, H. E. Jeffries, Gaseous VOCs rapidly modify particulate matter and its biological effects – Part 1. Simple VOCs and model PM. Atmos. Chem. Phys. 2012, 12, 12 277.
| Gaseous VOCs rapidly modify particulate matter and its biological effects – Part 1. Simple VOCs and model PM.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltVOjsLY%3D&md5=74c1f7f1b47ea7ba1498b740d38b9c4bCAS |
[74] S. Ebersviller, K. Lichtveld, K. G. Sexton, J. Zavala, Y. H. Lin, I. Jaspers, H. E. Jeffries, Gaseous VOCs rapidly modify particulate matter and its biological effects – Part 2. Complex urban VOCs and model PM. Atmos. Chem. Phys. 2012, 12, 12 293.
| Gaseous VOCs rapidly modify particulate matter and its biological effects – Part 2. Complex urban VOCs and model PM.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXltVOjsLc%3D&md5=c7400ea1dc2cca3df58d7e97bfd58a31CAS |
[75] R. Kamens, H. E. Jeffries, K. G. Sexton, A. A. Gerhardt, Smog Chamber Experiments to Test Oxidant Related Control Strategy Issues, EPA/600/3-82/014 (NTIS PB82227695) 1982 (US Environmental Protection Agency: Washington, DC).
[76] H. Jeffries, R. M. Kamens, K. G. Sexton, A. A. Gerhardt, Outdoor Smog Chamber Experiments to Test Photochemical Models. EPA-600/3-82-016a 1982 (Environmental Protection Agency).
[77] H. Jeffries, K. G. Sexton, R. M. Kamens, M. S. Holleman, Outdoor Smog Chamber Experiments to Test Photochemical Models: Phase II. EPA-600/3-85/029 1985 (Environmental Protection Agency).
[78] K. Sexton, H. E. Jeffries, J. R. Arnold, T. L. Kale, R. M. Kamens, Validation Data for Photochemical Mechanisms. EPA-600/3-87/003 1987 (Environmental Protection Agency).
[79] K. G. Sexton, H. E. Jeffries, J. R. Arnold, T. L. Kale, R. M. Kamens, Validation Data for Photochemical Mechanisms: Experimental Result. EPA-600/3-88/000 1988 (Environmental Protection Agency).
[80] H. E. Jeffries, K. G. Sexton, J. R. Arnold, J. L. Li, Validation Testing of New Mechanisms with Outdoor Chamber Data, Vol. 1. Comparison of CB4 and CAL Mechanisms. EPA-600/3-88/000a 1988 (Environmental Protection Agency).
[81] H. E. Jeffries, K. G. Sexton, J. R. Arnold, Validation Testing of New Mechanisms with Outdoor Chamber Data, Vol. 2. Analysis of VOC Data for the CB4 and CAL Photochemical Mechanisms. EPA-600/3-88/000b 1988 (Environmental Protection Agency).
[82] H. E. Jeffries, K. G. Sexton, J. R. Arnold, T. L. Kale, Validation Testing of New Mechanisms with Outdoor Chamber Data, Vol. 3. Calculation of Photochemical Reaction Photolysis Rates in the UNC Chamber. EPA-600/3-88/000c 1988 (Environmental Protection Agency).
[83] H. E. Jeffries, K. G. Sexton, J. R. Arnold, T. L. Kale, Validation Testing of New Mechanisms with Outdoor Chamber Data, Vol. 4. Appendices to Photochemical Reaction Photolysis Rates in the UNC Chamber. EPA-600/3-88/000d 1988 (Environmental Protection Agency).
[84] R. M. Kamens, H. E. Jeffries, K. G. Sexton, R. W. Wiener, The impact of day-old dilute smog on fresh smog systems: an outdoor chamber study. Atmos. Environ. 1982, 16, 1027.
| The impact of day-old dilute smog on fresh smog systems: an outdoor chamber study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38XksVens7k%3D&md5=9b76eccb5434a8b1a7ac106dc3528769CAS |
[85] H. E. Jeffries, K. G. Sexton, T. P. Morris, M. Jackson, R. G. Goodman, R. M. Kamens, M. S. Holleman, Outdoor Smog Chamber Experiments Using Automobile Exhaust. EPA-600/3-85/032 1985 (Environmental Protection Agency).
[86] H. E. Jeffries, K. G. Sexton, M. S. Holleman, Outdoor Smog Chamber Experiments: Reactivity of Methanol Exhaust. EPA-460/3-85/009a 1985 (Environmental Protection Agency).
[87] H. E. Jeffries, K. G. Sexton, M. S. Holleman, Outdoor Smog Chamber Experiments: Reactivity of Methanol Exhaust, Part II: Quality Assurance and Data Processing System Description. EPA-460/3-85/009b 1985 (Environmental Protection Agency).
[88] H. E. Jeffries, K. G. Sexton, The relative ozone forming potential of methanol-fueled vehicle emissions and gasoline-fueled vehicle emissions in outdoor smog chambers, Final Report, Coordinating Research Council Project ME-1, NTIS Accession number PB 93 206902 1994 (University of North Carolina: Atlanta, GA).
[89] H. E. Jeffries, K. G. Sexton, Outdoor smog chamber studies of alternative fuel vehicle emissions, in Preprints of the Annual Automotive Technology Development Contractors’ Coordination Meeting, 24–27 October 1994, Dearborn, MI 1994 (US Department of Energy).
[90] H. E. Jeffries, K. G. Sexton, Yu. Jianzhen, Atmospheric Photochemical Studies of Pollutant Emissions from Transportation Vehicles Operating on Alternative Fuels 1996 (National Renewable Energy Laboratory: Golden, CO).
[92] H. E. Jeffries, K. G. Sexton, Modeling aspects of nitrogen oxides using smog chamber data, in Workshop Proceedings on Formation and Fate of Atmospheric Nitrates, 22–23 October 1979, Research Triangle Park, NC. EPA-600/9-81-025 1981 (Environmental Protection Agency: Research Triangle Park, NC).
[93] K. G. Sexton, Prediction of photochemically produced formaldehyde with chemical mechanisms developed for urban ozone systems, in Proceedings of the 1987 EPA/APCA Symposium on Measurement of Toxic and Related Air Pollutants, 3–6 May 1987, Research Triangle Park, NC 1987 (Air Pollution Control Association: Pittsburgh, PA).
[95] H. E. Jeffries, K. G. Sexton, J. Arnold, Y. Bai, J. L. Li, R. Crouse, A Chamber and Modeling Study to Assess the Photochemistry of Formaldehyde. EPA-600/3–90/052 1990 (Environmental Protection Agency).
[96] K. G. Sexton, H. E. Jeffries, J. Arnold, Intercomparison of formaldehyde measurement in chambers, in Proceedings of the 1991 US EPA/A&WMA International Symposium, Measurement of Toxic and Related Air Pollutants, 6–10 May 1991, Durham, NC. Vol. 2, number VIP-21 1991, pp. 1147–1152 (Air and Waste Management Association: Pittsburgh, PA).
[97] H. E. Jeffries, K. G. Sexton, J. Arnold, Better formaldehyde predictions by photochemical mechanisms, in Proceedings of the 1991 US EPA/A&WMA International Symposium, Measurement of Toxic and Related Air Pollutants, 6–10 May 1991, Durham, NC. Vol. 1, number VIP-21 1991, pp. 97–103 (Air and Waste Management Association: Pittsburgh, PA).
[98] R. M. Kamens, H. E. Jeffries, M. W. Gery, R. W. Wiener, K. G. Sexton, G. B. Howe, The impact of α-pinene on urban smog formation: an outdoor chamber study. Atmos. Environ. 1981, 15, 969.
| The impact of α-pinene on urban smog formation: an outdoor chamber study.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXlsFGmurc%3D&md5=d03a77fd870aab4df560d94cd6886f57CAS |
[99] H. E. Jeffries, R. Crouse, K. G. Sexton, Light transmission into Teflon bags and chambers, in Measurement of Toxic and Related Air Pollutants. Vol. 1, number VIP-21 1991, pp. 97–103 (Air and Waste Management Association: Pittsburgh, PA).
[100] H. E. Jeffries, K. G. Sexton, C. H. Salmi, Effects of Chemistry and Meteorology on Ozone Control Calculations Using Simple Trajectory Models and the EKMA Procedure. EPA-450/4-81-034 1981 (Environmental Protection Agency: Research Triangle Park, NC).
[101] H. E. Jeffries, K. G. Sexton, Comments on the rationale and need to consider an alternative to EKMA. J. Air Pollut. Control Assoc. 1983, 33, 1087.
| Comments on the rationale and need to consider an alternative to EKMA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXisFGluw%3D%3D&md5=862ba89c8ad41ef8c2189dcb2d49b875CAS |
[102] H. E. Jeffries, K. G. Sexton, Technical Discussion Related to the Choice of Photolytic Rates for Carbon Bond Mechanisms in OZIPM4/EKMA. EPA-450/4-87/003 1987 (Environmental Protection Agency).
[103] H. E. Jeffries, K. G. Sexton, Comparison of two chemical mechanisms for use in EKMA to calculate hydrocarbon control requirements, in Proceedings of the APCA International Specialty Conference on The Scientific and Technical Issues Facing Post 1987 Ozone Control Strategies, 16–19 November 1987, Hartford, CT. APCA Transactions Series, number 12 (Eds G. T. Wolff, J. L. Hanisch, K. Schere) 1988 (Air and Waste Management Association: Pittsburgh, PA).
[104] J. Yu, H. E. Jeffries, R. M. Le Lacheur, Identifying airborne carbonyl compounds in isoprene atmospheric photooxidation products by their PFBHA oximes using gas chromatography/ion trap mass spectrometry. Environ. Sci. Technol. 1995, 29, 1923.
| Identifying airborne carbonyl compounds in isoprene atmospheric photooxidation products by their PFBHA oximes using gas chromatography/ion trap mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmsFClu7c%3D&md5=76b013e472e9912c5006647b207eea42CAS | 22191338PubMed |
[105] C.-J. Chien, M. J. Charles, K. G. Sexton, H. E. Jeffries, Analysis of airborne carboxylic acids and phenols as their pentafluorobenzyl derivatives: gas chromatography/ion trap mass spectrometry with a novel chemical ionization reagent, PFBOH. Environ. Sci. Technol. 1998, 32, 299.
| Analysis of airborne carboxylic acids and phenols as their pentafluorobenzyl derivatives: gas chromatography/ion trap mass spectrometry with a novel chemical ionization reagent, PFBOH.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjtFKlsA%3D%3D&md5=f5a38c9a7c5563fb42ff5a4166074d40CAS |
[106] J. Yu, H. Jeffries, K. Sexton, Atmospheric photooxidation of alkylbenzenes – I. Carbonyl product analyses. Atmos. Environ. 1997, 31, 2261.
| Atmospheric photooxidation of alkylbenzenes – I. Carbonyl product analyses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjslyhtrk%3D&md5=2342e92fc3ea4f59f47196339a4d3d8aCAS |
[107] J. Yu, H. Jeffries, Atmospheric photooxidation of alkylbenzene – II. Evidence of formation of epoxide intermediates. Atmos. Environ. 1997, 31, 2281.
| Atmospheric photooxidation of alkylbenzene – II. Evidence of formation of epoxide intermediates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjslyhtrY%3D&md5=f55f95828ce9c1c21c39741207419276CAS |
[108] X. Liu, H. Jeffries, K. Sexton, Atmospheric photochemical degradation of 1,4-unsaturated dicarbonyls. Environ. Sci. Technol. 1999, 33, 4212.
| Atmospheric photochemical degradation of 1,4-unsaturated dicarbonyls.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmsV2nu7k%3D&md5=93743800aef473d0ee1f58ed0a841127CAS |
[109] X. Liu, H. Jeffries, K. Sexton, Hydroxyl radical and ozone initiated photochemical reactions of 1,3-butadiene. Atmos. Environ. 1999, 33, 3005.
| Hydroxyl radical and ozone initiated photochemical reactions of 1,3-butadiene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXjtFSrt7Y%3D&md5=f71b8f4acd67384ac25517ba5fcbdf65CAS |