Chemical characterisation of humic-like substances from urban, rural and tropical biomass burning environments using liquid chromatography with UV/vis photodiode array detection and electrospray ionisation mass spectrometry
Magda Claeys A F , Reinhilde Vermeylen A , Farhat Yasmeen A D , Yadian Gómez-González A , Xuguang Chi B E , Willy Maenhaut B , Tímea Mészáros C and Imre Salma CA Department of Pharmaceutical Sciences, University of Antwerp, Universiteitsplein 1, BE-2610 Antwerp, Belgium.
B Department of Analytical Chemistry, Ghent University, Proeftuinstraat 86, Gent, BE-9000 Belgium.
C Institute of Chemistry, Eötvös University, H-1518 Budapest, PO Box 32, Hungary.
D Present address: Chemistry Department, University of Engineering and Technology, G.T. Road, Lahore 54840, Pakistan.
E Present address: Biogeochemistry Department, Max Planck Institute for Chemistry, PO Box 3060, D-55020 Mainz, Germany.
F Corresponding author. Email: magda.claeys@ua.ac.be
Environmental Chemistry 9(3) 273-284 https://doi.org/10.1071/EN11163
Submitted: 18 December 2011 Accepted: 14 April 2012 Published: 20 June 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Environmental context. One of the most important classes of water-soluble organic compounds in continental fine and tropical biomass burning aerosol is humic-like substances (HULIS), which contain components with strong polar, acidic and chromophoric properties. We focus on the chemical characterisation of HULIS and provide evidence that nitro-aromatic catecholic compounds are among the major species of HULIS. This indicates that volatile aromatic hydrocarbons emitted during biomass burning are important gas-phase precursors for HULIS.
Abstract. Humic-like substances (HULIS) are ubiquitously present in the troposphere and make up a major fraction of continental fine-sized water-soluble organic compounds. They are regarded as material with strong polar, acidic and chromophoric properties; however, structural information at the individual component level is rather limited. In the present study, we have characterised HULIS from different locations using liquid chromatography coupled to photodiode array detection and negative ion electrospray ionisation mass spectrometry. Aerosol samples with particles less than 2.5 μm in diameter (PM2.5) were collected in Budapest and K-puszta, Hungary, during 2007 and 2008 spring and summer periods, and in Rondônia, Brazil, during a 2002 biomass burning experiment. Major components of the Budapest 2007 and Brazil 2002 HULIS corresponded to chromophoric substances, of which 4-nitrocatechol (molecular weight (MW) 155) was identified as the most abundant organic species and less abundant ones were attributed to mono- and dimethyl nitrocatechols (MWs 169 and 183). The mass concentrations of 4-nitrocatechol in the water-soluble organic carbon (WSOC) of the Budapest 2007 and day- and night-time Brazil 2002 HULIS were 0.46, 0.50 and 1.80 %. Abundant components of K-puszta 2008 HULIS were assigned to α-pinene secondary organic aerosol (SOA) tracers, i.e. 3-methyl-1,2,3-butanetricarboxylic acid and terpenylic acid; their mass concentrations in the HULIS WSOC were 0.75 and 0.40 %. Tere- and ortho-phthalic acids (MW 166) were major components of the Budapest and K-puszta HULIS, but only minor ones of the Brazil 2002 biomass burning HULIS, consistent with a source that is different from biomass burning and likely related to open waste burning of phthalate ester-containing material such as plastic.
Additional keywords: α-pinene, secondary organic aerosol, tracer, volatile organic compound.
Introduction
Humic-like substances (HULIS) are a complex mixture of organic compounds from ambient atmospheric aerosols or isolated from fog and cloud samples. They are operationally defined and have also been denoted as organic macromolecules[1,2] or macromolecular polycarboxylic acids.[3] The term HULIS was derived from their close similarities in structural properties to terrestrial and aquatic humic and fulvic acids.[1,4] Our understanding of the chemical composition of HULIS at the individual component level is still rather limited. However, in recent years substantial progress has been made owing to instrumental developments in analytical organic mass spectrometry, in particular, electrospray ionisation in combination with tandem and high-resolution mass spectrometry (MS). At present, HULIS are believed to comprise aromatic and aliphatic compounds containing polar functional groups such as hydroxy, carboxy, carbonyl, nitrate, nitrooxy and sulfate groups,[5–10] and to contain organosulfates and nitrooxy organosulfates that only have been recently reported in biogenic secondary organic aerosol (SOA).[11–15]
Of the recent studies dealing with molecular characterisation of HULIS using MS approaches,[5–10] only two[6,9] resort to liquid chromatography (LC), a technique which offers additional analytical power to MS. Furthermore, it is worth noting that the latter LC/MS studies employ either size exclusion[6] or hydrophilic interaction chromatography.[9] LC/MS has also been shown to be a suitable tool for the analysis of polar organic compounds present in biogenic SOA and ambient fine aerosol, including polar terpenoic acids[16,17] and organosulfates,[6,9,11–15] thereby employing a reversed-phase stationary phase with di- or trifunctional C18 chains that allow for polar analyte retention.
Previous studies have documented both similarities and differences between terrestrial humic and fulvic acids, and atmospheric HULIS (for a review, see Graber and Rudich[18]). With respect to molecular weight (MW), several studies demonstrated that atmospheric HULIS have much lower MWs than terrestrial humic and fulvic acids, with an upper limit of 600.[9,19,20]
The objectives of the current study were to characterise HULIS at the individual component level using high-performance LC with UV/vis photodiode array detection (PAD) and electrospray ionisation mass spectrometry in the negative ion mode ((–)ESI-MS) with a focus on tracers for biomass burning (BB), biogenic and anthropogenic organic aerosols, including primary and secondary tracers. The rationale for including UV/vis detection was that HULIS show strong UV/vis absorption and are yellow-coloured,[18] whereas that for using (–)ESI-MS was that HULIS contain acidic compounds that can be deprotonated and are thus suitable for (–)ESI detection.[5–10] Moreover, the (–)ESI-MS technique in combination with MS/MS allows for a detailed characterisation of individual molecules, including functional groups, such as hydroxy, carbonyl, carboxy, nitrooxy and sulfate groups.[5–10] Data analysis in the present study was mainly focussed on the comparison of HULIS in different locations, i.e. an urban (Budapest, Hungary), a rural (K-puszta, Hungary) and a tropical BB location (Rondônia, Brazil), characterised by different primary and secondary organic aerosol sources.
Field studies have demonstrated that BB is a significant primary source of HULIS.[3,21–25] In addition, evidence has been obtained that: (1) SOA formed from BB emissions contributes to HULIS[25]; (2) urban and suburban environments are affected by BB[3,25]; (3) vehicular exhaust is not a significant primary emission source of HULIS[25] and (4) SOA contributes significantly to urban HULIS.[26] Significant correlation between photochemical activity and the atmospheric HULIS concentration in summer is also consistent with their secondary formation.[2]
Secondary BB aerosol contains methyl nitrocatechols,[27] which are strong absorbers of UV and visible radiation, and, therefore, could be linked to the chromophoric properties of HULIS. Methyl nitrocatechols are formed through photooxidation, in the presence of NOx, of m-cresol, which is emitted at significant levels during BB and originates from the degradation of lignins.[27] They also arise from the photooxidation, in the presence of NOx, of anthropogenic volatile organic compounds (VOCs), i.e. toluene,[28] which accounts for ~6 % of the observed non-methane hydrocarbons, making it the most abundant aromatic VOC in urban air.[29] In addition, nitrocatechols should be considered as target SOA tracers as they are formed through the same formation mechanism from benzene,[30] which is also an abundant anthropogenic VOC in urban air.[29]
Experimental
Samples and sample preparation
The rural–continental background aerosol samples were collected at K-puszta (latitude 46°58.3′N, longitude 19°32.5′E, altitude 125 m above sea level) on the Great Hungarian Plain, ~80 km south-east of Budapest and 15 km north-west of the nearest town Kecskemét. The site is located in the clearing of a mixed forest (62 % coniferous and 28 % deciduous trees) including 10 % grassland. Two samples were taken from 5 to 7 June 2008 for 23 h each. Each 23-h sampling started at 0730 hours coordinated universal time (UTC). The sampling was done with a KS-300 high volume aerosol sampler equipped with a particles less than 2.5 μm in diameter (PM2.5) pre-separator (Kalman System, Budapest, Hungary) and operated at an air flow rate of 530 L min–1. Whatman QM-A quartz fibre filters were used as the collection substrate. The filters were pre-baked at 650 °C for 8 h before sampling to remove organic contaminants. The exposed area of the filters was 154 cm2.
Urban aerosol samples were collected in central Budapest (at Széna Square, latitude 47°30.5′N, longitude 19°1.7′E, altitude 114 m above sea level) from 7 to 14 March 2007, and from 3 to 10 June 2008 for 1 week each. The site is heavily influenced by vehicular traffic. The aerosol samples and field blank filters were taken with a DHA-80 high-volume aerosol sampler equipped with a PM2.5 pre-separator (Digitel Elektronik, Hegnau, Switzerland) and operated at an air flow rate of 500 L min–1. Whatman QM-A quartz fibre filters were used as the collection substrate. The filters had been pre-baked at 550 °C for 12 h before sampling. The exposed area of the filters was 154 cm2.
The BB aerosol samples were collected at a pasture site (latitude 10°45′44″S, longitude 62°21′27″E, altitude 315 m above sea level) in the Amazon Rainforest, state of Rondônia, Brazil, during the LBA-SMOCC field campaign.[31] The samples selected for the present study were taken from 18 to 22 September 2002 separately over daylight periods (~0745–1745 hours local time (LT) with LT = UTC – 4) and at night (~1830–0700 hours LT). The BB activity was reported to be the most intensive during this period of the campaign.[31] The samples were collected with a high-volume dichotomous virtual impactor[32] on Gelman Pallflex front and back quartz fibre filters in series. The filters were pre-baked at 550 °C for 24 h before sampling. The exposed area of the filters was 61.5 cm2. PM2.5-size fraction aerosol samples, collected on the front filters in the high-flow air stream at a flow rate of 300 L min–1, were used for the present research. Altogether, five samples for the daylight periods, five samples for nights and two field blank filters were obtained during the days specified above.
The aerosol samples were placed in pre-baked Al foils and were kept in a freezer until further treatment. The two samples collected at the K-puszta site were treated together. Of the aerosol samples collected at the BB-affected site, one-quarter of each filter was processed for the present study. The filter sections for the daylight periods were treated together, and those for the nights were also processed together. The samples were utilised for isolation of water-soluble HULIS by a one-step solid-phase extraction (SPE) method.[4,24,33] In short, the filters were cut into pieces and the extraction was carried out with high-purity reagent Milli-Q water with occasional stirring and manual shaking at room temperature for 36 h. The volume of water used for the rural, urban and BB filters was 40, 50 and 70 mL. The filter-water system was allowed to stand for 30 min at the end of the extraction procedure. The aqueous extracts were filtered through a 0.22-μm syringe PVDF membrane filter (Millipore, Billerica, MA, USA) to remove the filter debris and suspended insoluble particles. For part of each filtered extract, the pH of the filtrate was adjusted to 2 with HCl, and HULIS were separated by pre-conditioned SPE columns (Oasis HLB, Waters, Milford, MA). The retained organics were eluted with methanol, and the eluents were first combined and then subdivided into several aliquots. The aliquots were evaporated to dryness under a nitrogen stream. The mass of the resulting HULIS aliquot samples was measured by gravimetry at a stabilised temperature (20 °C) and relative humidity (50 %). The samples were pre-equilibrated at these conditions for several hours. The weighing was repeated one day later after the samples had been placed into a drying chamber at a temperature of 40 °C for 1 h. The unprocessed parts of the aqueous extracts and the isolated HULIS samples were kept in a refrigerator until further analyses.
Measurements for water-soluble organic carbon
The unprocessed parts of the aqueous aerosol extracts and the HULIS samples were analysed for water-soluble organic carbon (WSOC) using a commercial Total Organic Carbon analyser (TOC-V CPH, Shimadzu, Kyoto, Japan). A two-step procedure consisting of separately measuring water-soluble total carbon (WSTC) and water-soluble inorganic carbon (WSIC) was thereby applied, after which WSOC was obtained as WSOC = WSTC – WSIC. As indicated above, for the HULIS samples, mass data were available from gravimetry. As a consequence, it could also be estimated how much HULIS was present in the unprocessed parts of the aqueous aerosol extracts. Based on data provided in Salma et al.[34] it was assumed that the organic mass-to-organic carbon ratio for HULIS is ~2. The HULIS samples were dissolved in 2 or 3 mL of Millipore Simplicity water. The dissolved HULIS samples and the unprocessed aerosol extracts were diluted with Millipore Simplicity water so that the estimated organic carbon concentration from HULIS in the final solution used for analysis would be of the order of 3–10 μg mL–1 for the actual samples. For the field blank samples, similar dilutions were done as for the actual samples. Volumes of 150 μL of the final solutions were injected in triplicate for the analyses for WSTC and WSIC, with the difference representing WSOC. The WSOC data obtained for the HULIS samples are further denoted as HULIS-C.
Liquid chromatography–UV/vis and –electrospray mass spectrometry
Both the unprocessed aqueous aerosol extracts and the dissolved HULIS samples were subjected to detailed organic analyses. The separation of the HULIS components was achieved using a T3 Atlantis C18 (Waters) column, which contains trifunctionally bonded C18 alkyl chains and shows polar analyte retention. The mobile phase consisted of acetic acid 0.1 % (v/v) (A) and methanol (B). The applied gradient elution program for the analyses performed was as follows: the concentration of eluent B was kept at 3 % for 2 min, and then increased to 90 % in 18 min, kept at 90 % for 43 min, and then decreased to 3 % in 5 min and kept at 3 % for 12 min.
The LC/MS instrument consisted of a Surveyor Plus LC system (pump and autosampler) and an LXQ linear ion trap mass spectrometer (Thermo Scientific, San Jose, CA). The liquid chromatograph was equipped with a photodiode array detector (Thermo Scientific). Electrospray was used as the ionisation technique in the negative ion mode. The operating conditions of the LXQ instrument were as follows: sheath gas flow (nitrogen), 50 arbitrary units (0.75 L min–1); auxiliary gas flow (nitrogen), 5 arbitrary units (1.5 L min–1); source voltage, –4.5 kV; capillary temperature, 350 °C; and maximum ion injection time, 200 ms. The [M–H]– signal optimisation was done by introducing a 50 μg mL–1 malic acid standard solution. Data were acquired and processed using Xcalibur 2.0 software (Thermo Scientific). Base peak chromatograms (BPCs) were obtained in the m/z 122–500 range because of an interference at m/z 119. This ion corresponds to an acetic acid cluster ion (CH3COOH:CH3COO–) due to acetic acid being present in the mobile phase. The (–)ESI-MS data recorded for unknown acidic and nitro-aromatic compounds were compared with data available from previous studies obtained under the same LC/MS conditions[16,17,35]; based on retention time (RT) and (–)ESI-MS data, azelaic acid and several terpenoic acids and nitro-aromatic compounds could be assigned.
The quantification of selected compounds (i.e. 4-nitrocatechol, terpenylic acid and 3-methyl-1,2,3-butanetricarboxylic acid, MBTCA) was based on an internal standard calibration procedure employing sebacic acid (Sigma–Aldrich, St Louis, MI) in the case of the terpenoic acids and 4-tert-butylcatechol (Sigma–Aldrich) in the case of 4-nitrocatechol as internal recovery standards, and reference compounds. The reference compounds, MBTCA and terpenylic acid, were available from previous studies,[36,37] whereas 4-nitrocatechol was purchased from Sigma–Aldrich. Extracted ion chromatography using [M–H]– ions was utilised to obtain clear chromatographic peaks and to derive the peak areas used as input for quantitative determinations. The concentrations in the extracts of the actual samples were obtained by relating the peak area (analyte-to-internal recovery standard) ratio data for the extracts to linear calibration lines, which were obtained with unweighted regression from the calibration curve data. For performing the unweighted regressions and the calculations of the concentration, use was made of the software Excel (Microsoft, USA). Field blanks were prepared and analysed in the same way as the samples; they proved to be free of the compounds of interest, except for the BB field blanks where some contamination was observed.
Results and discussion
Contribution of HULIS-C to WSOC
The WSOC analyses of the unprocessed aqueous aerosol extracts and the HULIS samples enabled us to assess the contribution of HULIS-C to WSOC. This fraction was 0.31 for the Budapest 2007 sample, 0.35 for the Budapest 2008 sample, 0.33 for the K-puszta samples, 0.40 for the BB day-time samples and 0.61 for the BB night-time samples. The results confirm that HULIS-C has a substantial contribution to WSOC for all HULIS types, and that carbon in HULIS from tropical BB represents the major chemical mass fraction of WSOC.[34] The higher contribution of HULIS-C to WSOC for the BB night-time samples compared with the day-time samples can be explained by a different combustion stage with flaming combustion taking place during the day-time when fires are started and smouldering combustion of biomass prevailing at night.[31] In this respect, it is worth mentioning that a diel variation in the contribution of levoglucosan, a tracer for biomass burning, to WSOC has been reported for day- and night-time PM2.5 samples collected during the dry period of the 2002 LBA/SMOCC BB experiment, with the highest contributions at night.[38]
Characterisation of HULIS isolated from urban PM2.5 aerosol
In order to determine which type of acidic organic compounds were absent in the HULIS samples following the SPE procedure, we first compared LC/MS data obtained for HULIS samples with those for aqueous aerosol extracts that had not undergone SPE. Fig. 1 illustrates the results obtained for 2007 urban PM2.5 aerosol. Similar results were obtained for samples from the other locations. The BPCs demonstrate that the polar compounds eluting in the first 10 min are not present in the HULIS sample. These compounds include polar organosulfates, for example, the 2-methyltetrol sulfates (RTs at 2.0 and 2.6 min), which were identified on the basis of available (–)ESI-MS data obtained in our laboratory under comparable experimental conditions (not shown).[15] The results also reveal that the BPC profiles for the compounds eluting between 15 and 30 min are rather similar. This region contains aromatic and nitro-aromatic compounds, as will be discussed in more detail below, as well as terpenoic acids, as found, for example, for rural summer PM2.5 aerosol from forested sites such as K-puszta, Hungary.[17] The other chromatograms shown in Fig. 1 are extracted ion chromatograms (EICs) for the deprotonated forms of major aromatic and nitro-aromatic compounds (m/z 154, 165 and 168), azelaic acid (m/z 187) or selected terpenoic acids (m/z 187, 171 and 203).
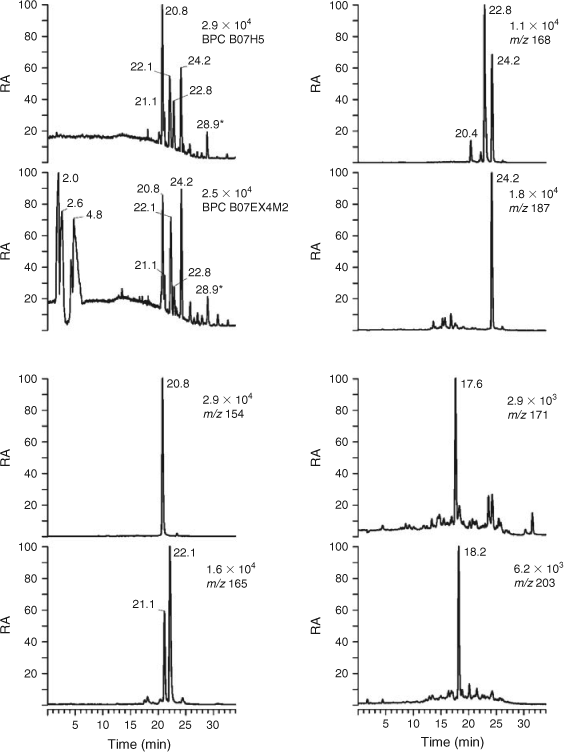
|
The BPC for the HULIS sample in Fig. 1 is dominated by a m/z 154 compound with RT 20.8 min, which was identified as 4-nitrocatechol. This nitro-aromatic compound has been suggested as a tracer for both BB[39] and the photooxidation of benzene.[30] It was found to exhibit maximum concentrations in PM3, PM >3 and total suspended particles size fractions at Mainz, Germany, in summer.[40] Other abundant aromatic compounds were m/z 165 compounds with RTs 21.1 and 22.1 min, which were identified as tere- and ortho-phthalic acid. Isomeric phthalic acids are known to have many sources, both primary and secondary.[41–43] The origin of these compounds has not been fully investigated; however, formation from phthalic esters emitted during open waste burning of plastic material is a likely source.[43] The major m/z 168 compounds were identified as isomers of methyl nitrocatechols, based on comparison with literature data; i.e. the compounds eluting at RTs 22.8 and 24.2 min were assigned to 4-methyl-5-nitrocatechol and 3-methyl-5-nitrocatechol.[35] These compounds have recently been suggested as BB SOA tracers, formed by photooxidation of m-cresol which is released during fires as a degradation product of lignins,[27] as well as anthropogenic SOA tracers, formed by photooxidation of toluene.[28] The weak compound with RT 20.4 min was attributed to a nitroguaiacol (likely 6-nitroguaicol, for which a reference is not available), a compound which has recently been reported in urban winter PM2.5 aerosol from Maribor, Slovenia,[35] and may point to the combustion of softwood.[39] In addition, higher methyl homologues of the m/z 168 methyl nitrocatechols, i.e. isomeric dimethyl nitrocatechols eluting at RTs 19.5, 20.0 and 20.6 min, could be detected at m/z 182 with low intensity (not shown). The major m/z 187 compound eluting at RT 24.2 min was identified as azelaic acid, a well known oxidation product of biogenic unsaturated fatty acids,[44] which has been reported in ambient aerosol from forested, urban and remote sites.[44–48] Furthermore, the EICs shown in Fig. 1 also illustrate that the HULIS extract contains terpenoic acids as minor compounds, i.e. terpenylic acid (m/z 171) at RT 17.6 min and MBTCA (m/z 203) at RT 18.2 min. Of these, terpenylic acid, a C8-lactone-containing terpenoic acid, originates from the photooxidation of α-pinene,[37] whereas MBTCA, a C8-tricarboxylic acid, is a higher-generation photooxidation product of α-pinene formed in the presence of NOx.[36] It should be noted that the peak intensities of the [M–H]– signals of the terpenoic acids are lower than those of the aromatic compounds and azelaic acid.
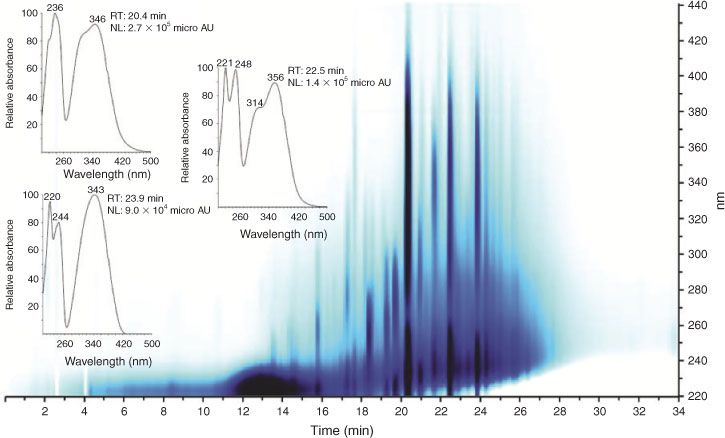
Selected LC-PAD array data for 2007 urban HULIS are shown in Fig. 2. The LC-PAD density map reveals a complex mixture of UV/vis absorbing compounds in the 220–440-nm region, with 4-nitrocatechol being the dominant one. It is noted that this compound elutes at 20.5 min (~0.3 min earlier than in (–)ESI-MS because of the lag-time between the PAD and the ESI source). Other intense peaks (visible in dark blue) could be attributed to the aromatic compounds discussed above, i.e. tere- and ortho-phthalic acid, and methyl nitrocatechols. Close examination of the LC-PAD data for this urban HULIS sample reveals that there is absorption in the 420–430-nm region at RTs where 4-nitrocatechol and the other nitro-aromatic compounds elute, thus is consistent with its yellow colour.
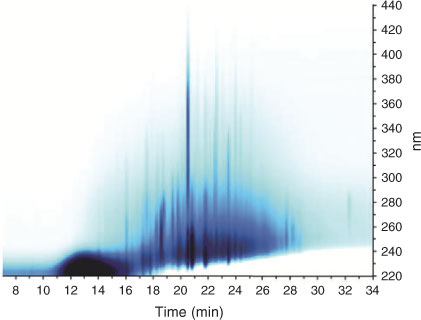
|
Characterisation of HULIS isolated from rural PM2.5 aerosol
Selected LC/MS data for the second urban HULIS sample, isolated from PM2.5 aerosol collected in Budapest during summer 2008, and for a rural HULIS sample, obtained from PM2.5 aerosol collected at K-puszta during spring 2008, are given in Figs 3 and 4. It was noted during the isolation procedure that the appearance of 2008 urban HULIS was not as intense a yellow as that of 2007 urban HULIS but much paler, more resembling 2008 rural HULIS. It can indeed be seen that the BPC profile of 2008 urban HULIS (Fig. 3) is quite different from the 2007 sample (Fig. 1) and resembles 2008 rural HULIS (Fig. 4). Therefore, we will focus here on the main qualitative differences between 2007 urban and 2008 rural HULIS. In a later section, quantitative data for three major SOA tracers, i.e. 4-nitrocatechol, terpenylic acid and MBTCA, will be presented, allowing for a more quantitative assessment of HULIS from the different locations. It is noted that the abundance of 4-nitrocatechol eluting at RT 20.4 min and detected at m/z 154 is much lower (~20 times) in the rural sample, thereby taking into account the amount of HULIS (expressed as WSOC) analysed. With respect to the m/z 168 compounds, the pattern is quite different and is dominated by a nitroguaiacol (likely 6-nitroguaicol[35]) eluting at RT 19.9 min, suggesting that there is mainly combustion of softwood. The tere- and ortho-phthalic acids, on the other hand, eluting at RTs 20.7 and 21.7 min, and detected at m/z 165, have an increased abundance. The m/z 187 EIC also shows a very different profile; whereas azelaic acid was dominant in the urban HULIS sample, the EIC is now characterised by an abundant compound eluting at RT 14.9 min. This compound has been partially characterised in previous work as a C8-hydroxydicarboxylic acid formed by photooxidation of α-pinene but still remains to be fully elucidated.[17] In a recent field study conducted at a Belgian polluted forest site (i.e. Brasschaat) during a 2007 summer period,[49] the concentrations of this unknown MW 188 compound were found to be well correlated with those of MBTCA, suggesting that it could serve, as MBTCA, as a useful tracer for aged biogenic SOA. With respect to the terpenoic acids, it can be seen that the signals for the deprotonated forms of terpenylic acid (eluting at RT 17.3 min and detected at m/z 171) and MBTCA (eluting at RT 17.7 min and detected at m/z 203) are much more intense (a factor of 10 or larger) than those in 2007 urban HULIS. The LC/MS data also include m/z 157 EICs; the compound eluting at 16.0 min was identified as terebic acid, which corresponds to a higher oxidation product of terpenylic acid.[16] Furthermore, the (–)ESI-MS data for the m/z 171 compound eluting at 20.6 min show that it corresponds to norpinic acid.[16,17] The LC/MS results thus demonstrate that the rural HULIS sample contains terpenoic acids originating from the photooxidation of α-pinene as major compounds, and nitro-aromatic compounds as minor ones, in contrast to 2007 urban HULIS where the nitro-aromatic compounds are prevalent and the terpenoic acids are only minor compounds. With respect to tere- and ortho-phthalic acid, the intensity of the signals in the two HULIS extracts was comparable. The overall LC/MS results for 2008 urban HULIS reveal that it resembles more the 2008 rural HULIS, which is consistent with its paler visual appearance. The LC-PAD density maps for 2008 urban and rural HULIS samples are provided in the Supplementary material (Figs S1, S2, see http://www.publish.csiro.au/?act=view_file &file_id=EN11163_AC.pdf); compared with 2007 urban HULIS, a much lower intensity of the UV-absorbing compounds could be noted, taking into account the amount of WSOC analysed. The differences between the 2007 and 2008 urban HULIS can possibly be explained by more intense regional BB in the surroundings of Budapest during the 2007 sampling episode. In this context, a recent field study by Lin et al.[25] conducted in the Pearl River Delta Region, China, demonstrated that urban and suburban environments are affected by BB and that vehicular exhaust is not a significant primary emission source for HULIS.
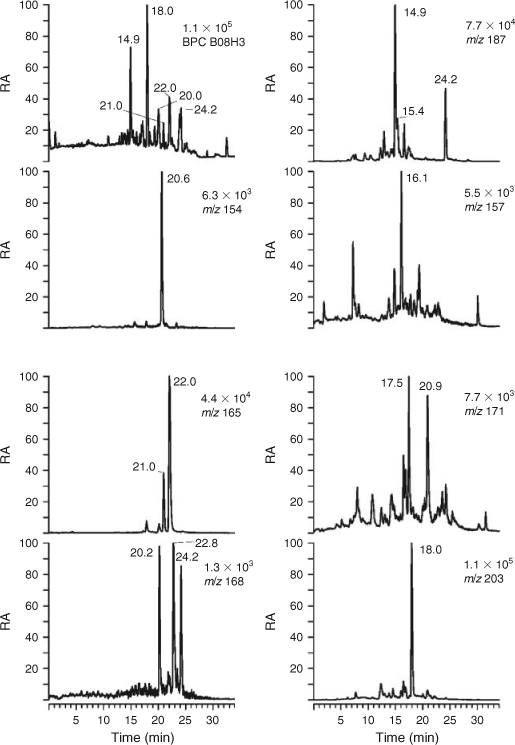
|
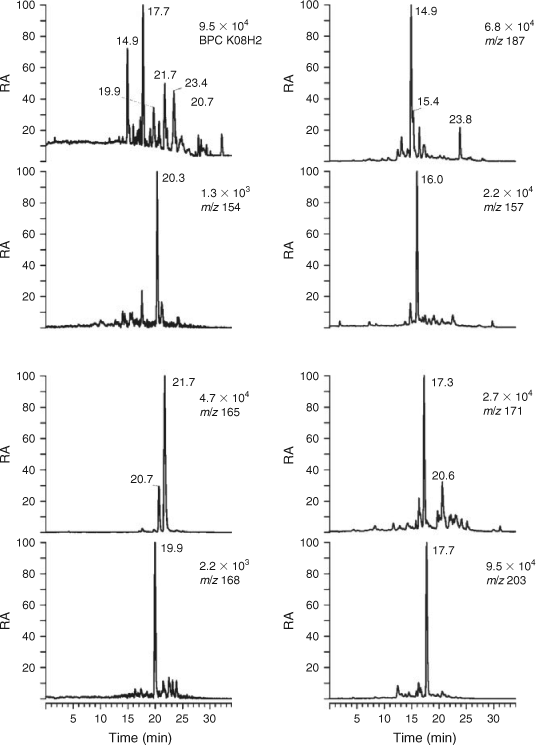
|
Characterisation of HULIS isolated from tropical BB PM2.5 aerosol
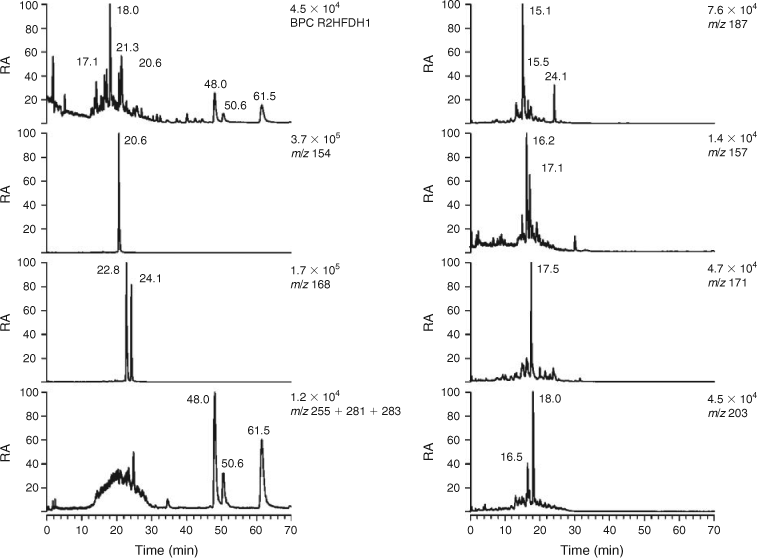
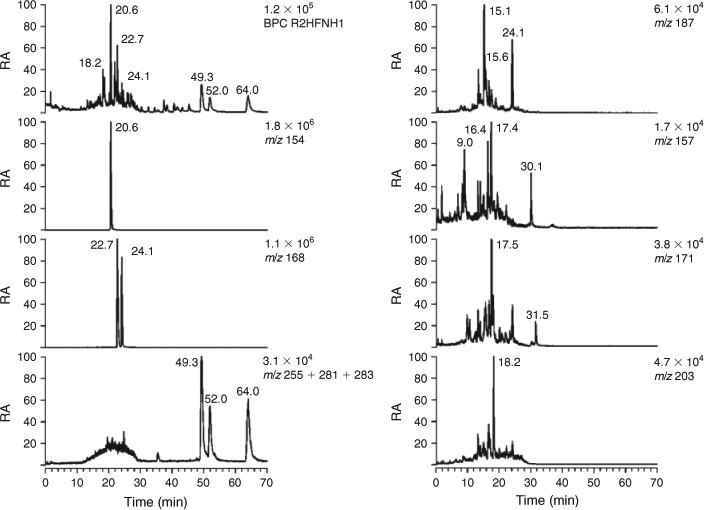
Selected LC/MS data for day- and night-time tropical BB HULIS are shown in Figs 5 and 6. It is known from earlier studies carried out in the framework of the 2002 LBA-SMOCC BB experiment that there are pronounced diel differences with respect to the concentrations of the WSOC and the BB primary tracer levoglucosan, which are much higher at night when smouldering combustion takes place.[31,38] Hence, differences in the LC/MS profiles for day- and night-time BB HULIS could be expected. Initially, quantitative differences between day- and night-time BB HULIS are discussed; subsequently, substantial qualitative differences with urban and rural HULIS are highlighted. The BPCs of both day- and night-time BB HULIS (Figs 5, 6) are dominated by 4-nitrocatechol (m/z 154) and methyl nitrocatechols (m/z 168). The absence of nitroguaiacol can be related to the combustion of tropical hardwood. As expected, the BB SOA tracers 4-nitrocatechol (m/z 154) and the isomeric methyl nitrocatechols (m/z 168) are more abundant at night (Fig. 6) than during the day-time (Fig. 5), thereby taking into account the amount of WSOC analysed. With respect to the biogenic–terpenoic SOA tracers, most tracers noted for rural HULIS could also be observed, i.e. the unknown C8-hydroxydicarboxylic acid (m/z 187; RT 15.1 min), terebic acid (m/z 157; RT 15.8 min (day), 15.7 min (night)), terpenylic acid (m/z 171; RT 18.1 min (day), 18.0 min (night)) and MBTCA (m/z 203, RT 18.9 min (day), 18.6 min (night)). Furthermore, azelaic acid (m/z 187; RT 24.1 min) could be detected with an abundance comparable to that of the terpenoic acids. However, some differences with rural HULIS should also be addressed. The m/z 157 EICs are more complex and contain compounds other than terebic acid, which were not further examined in the present study. Furthermore, the m/z 203 EICs also show more complexity and reveal, in addition to MBTCA, a compound eluting at an earlier RT (16.5 min (day), 16.7 min (night)). This compound could be assigned to an isomer of MBTCA, i.e. 3-carboxyheptanedioic acid, and is thought to originate from the photooxidation of d-limonene.[17,50] The m/z 165 EICs (not shown) were distinctly different from those of urban and rural HULIS discussed above; in contrast, the m/z 165 EICs of BB HULIS reveal that ortho-phthalic acid (RT 22.1 min (day), 21.8 min (night)) is only a minor constituent with an [M–H]– signal intensity of 1.1 % (day) and 0.5 % (night) of that of 4-nitrocatechol. Tere- and ortho-phthalic acid likely originate from open waste burning of material containing phthalate esters,[43] which are known to be widely used for various purposes (e.g. plasticisers, fabrics, adhesives, coatings, etc.). The compounds with RTs 48.0, 50.6 and 61.5 min were identified as fatty acids, i.e. palmitic (m/z 255), oleic (m/z 281) and stearic (m/z 283) acid. The latter fatty acids have been previously reported in Amazonian PM2.5 aerosol[51]; the presence of the unsaturated fatty acid, oleic acid, suggests that the aerosol was relatively fresh or non-aged.
The LC-PAD density map for night-time BB HULIS is given in Fig. 7. As expected, the bands for the nitro-aromatic compounds, 4-nitrocatechol and the methyl nitrocatechols, are very intense in the case of this night-time sample, which is known to be highly affected by smouldering biomass combustion.[31,38] It can also be clearly seen that there is absorption in the 420–430-nm region at RTs where 4-nitrocatechol and the methyl nitrocatechols elute, thus consistent with its fairly strong yellow colour. The LC-PAD density map for corresponding day-time BB HULIS is shown in Fig. S3; compared with night-time BB HULIS, a lower intensity of the UV-absorbing compounds could be noted, taking into account the amount of WSOC analysed.
The LC/MS results thus demonstrate that BB HULIS contain nitro-aromatic compounds as major tracers, including 4-nitrocatechol and methyl nitrocatechols, and reveal diel differences for these tracers.
Quantitation of major SOA tracers and their contribution to the WSOC
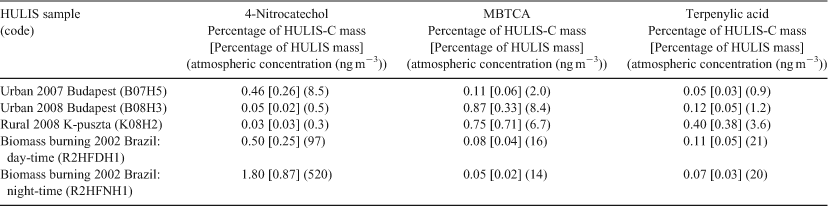
In order to make a quantitative assessment between the HULIS samples from the different locations, selected tracers, for which reference standards were available, i.e. 4-nitrocatechol, terpenylic acid and MBTCA, were quantified, and their contributions to the HULIS WSOC mass (i.e. to the HULIS-C) were calculated. The percentage contributions to the HULIS-C mass of the selected tracers are summarised in Table 1. Also included in Table 1 are the percentage concentrations of the tracers in the HULIS itself and the atmospheric concentrations of the tracers; the percentage concentrations of the tracers in the K-puszta 2008 HULIS should be considered as semiquantitative, as the HULIS mass for this sample was only 0.5 mg and had therefore a large associated uncertainty. The nitro-aromatic tracer 4-nitrocatechol contributes 0.46 % to the WSOC mass in 2007 urban HULIS, whereas this value is only 0.03 and 0.04 % for 2008 rural and 2008 urban HULIS. The latter sample had, as already discussed above, a much paler appearance compared with 2007 urban HULIS and resembled 2008 rural HULIS in both its pale yellow appearance and LC/MS organic tracer profile. In contrast, the biogenic SOA tracer MBTCA accounts for 0.75 % of the WSOC mass in 2008 rural HULIS, whereas this value is only 0.11 % for 2007 urban HULIS. Interestingly, the contribution of MBTCA in urban 2008 Budapest HULIS (0.87 %) is even somewhat higher than for rural HULIS, which is consistent with MBTCA being a higher-generation photooxidation product of α-pinene formed in the presence of NOx.[36,40,52,53] As to the biogenic SOA tracer terpenylic acid, the contributions to the WSOC mass are as expected, with the highest values for 2008 rural (0.05 %) and 2008 urban (0.12 %) HULIS. With respect to BB HULIS, 4-nitrocatechol accounts for 0.50 and 1.80 % of the WSOC in the day- and night-time samples. These values may be compared with those of levoglucosan, an established primary BB tracer,[54] which contributed, on average, 4.0 % to the WSOC for the dry (BB) period of the LBA-SMOCC campaign.[38] The percentage contributions of the biogenic SOA tracers MBTCA and terpenylic acid to the WSOC mass in BB HULIS are very low (i.e. ≤0.11 %), as could be expected as the biogenic SOA tracers at the Amazonian pasture site are mainly related to isoprene.[38,51]
Conclusions and perspectives
Our results demonstrate that abundant tracers in all the examined HULIS samples belong to the class of nitro-aromatic catecholic compounds with 4-nitrocatechol being the most prevalent, suggesting that aromatic hydrocarbons emitted during BB are important gas-phase precursors for HULIS. However, it remains to be demonstrated whether aromatic hydrocarbons present in traffic exhaust serve as precursors for nitro-aromatic catecholic compounds; to address this issue samples from other urban locations that are less influenced by regional BB need to be examined. In contrast to BB HULIS, where ortho-phthalic acid was only a minor compound, tere- and ortho-phthalic acids were prevalent in urban Budapest and rural K-puszta HULIS. However, it remains to be seen whether these tracers, which point to open waste burning of phthalate ester-containing material, are specific to urban and rural HULIS; therefore, it would be warranted to examine more samples from different urban and rural locations.
The issue was raised as to how humic HULIS are because of substantial differences with humic and fulvic acids, such as, for example, their MW range.[18] Our results show that the prevalent acidic compounds characterised in the examined HULIS samples from different locations all fall within the MW range of 150–210. It thus follows that an earlier proposed definition of HULIS being compounds with a MW >300, based on size-exclusion chromatography and laser desorption ionisation (LDI) MS, is hard to hold.[23] Future work should focus on the characterisation of organosulfates and nitrooxy organosulfates and higher-MW oligomers in HULIS from different locations. In this respect, we have evidence that the MW 295 α-pinene-related nitrooxy organosulfates[14,15] and the MW 358 di-ester formed between pinic acid and diaterpenylic acid[16] are present in rural PM2.5 aerosol. Hence, considering that the HULIS fraction contains the same constituents as the original aqueous aerosol extract from which it was prepared, except the very polar isoprene-related organosulfates (as documented in the present study), such higher-MW constituents are expected to be present in HULIS. This would extend the MW range of the acidic HULIS constituents to ~400, consistent with literature data based on ultrafiltration, vapour pressure osmometry and direct infusion ESI-MS,[19] as well as data based on size-exclusion chromatography and LDI-MS.[23] The characterised compounds, i.e. nitro-aromatic catechols, aromatic carboxylic acids and terpenoic acids, cannot be regarded as ‘macromolecules’ in a strict chemical sense. They are rather low-MW molecules, which all correspond to SOA tracers formed through photooxidation of anthropogenic and biogenic VOCs. Furthermore, the yellow appearance of the HULIS samples can be explained by nitro-aromatic and aromatic compounds, i.e. nitro-aromatic catechols and phthalic acids, which show featureless UV/vis spectra and a tailing absorption in the yellow region (400–420 nm), consistent with literature data (for a review see ref.[18]).
Acknowledgements
Research at the Universities of Antwerp and Ghent was supported by the Belgian Federal Science Policy Office (contracts SD/AT/02 and SD/CS/05A), the Research Foundation–Flanders (FWO) and the Special Research Funds of the Universities of Antwerp and Ghent. Research at Eötvös University was supported the Hungarian Scientific Research Fund (contract K84091). The authors thank A. Hoffer and G. Kiss of the University of Pannonia (Vezprém, Hungary) for providing the aerosol samples for K-puszta.
References
[1] S. Zappoli, A. Andracchio, S. Fuzzi, M. C. Facchini, A. Gelencsér, G. Kiss, Z. Krivácsy, A. Molnár, E. Mészáros, H. C. Hansson, K. Rosman, Inorganic, organic and macromolecular components of fine aerosol in different areas of Europe in relation to their water solubility. Atmos. Environ. 1999, 33, 2733.| Inorganic, organic and macromolecular components of fine aerosol in different areas of Europe in relation to their water solubility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXivFKntL0%3D&md5=59ae1c03b01464943f036e27afc84eafCAS |
[2] V. Samburova, S. Szidat, C. Hueglin, R. Fisseha, U. Baltensperger, R. Zenobi, M. Kalberer, Seasonal variation of high-molecular-weight compounds in the water-soluble fraction of organic urban aerosols. J. Geophys. Res. 2005, 110, D23210.
| Seasonal variation of high-molecular-weight compounds in the water-soluble fraction of organic urban aerosols.Crossref | GoogleScholarGoogle Scholar |
[3] S. Decesari, M. C. Facchini, S. Fuzzi, E. Tagliavini, Characterisation of water-soluble organic compounds in atmospheric aerosol: a new approach. J. Geophys. Res. 2000, 105, 1481.
| Characterisation of water-soluble organic compounds in atmospheric aerosol: a new approach.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhtlKgtrg%3D&md5=57b2502fbef023822e41d0d9c5d35812CAS |
[4] G. Kiss, B. Varga, I. Galambos, I. Ganszky, Characterization of water-soluble organic matter isolated from atmospheric fine aerosol. J. Geophys. Res. 2002, 107, 8339.
| Characterization of water-soluble organic matter isolated from atmospheric fine aerosol.Crossref | GoogleScholarGoogle Scholar |
[5] F. Romero, M. Oehme, Organosulfates – a new component of humic-like substances in atmospheric aerosols? J. Atmos. Chem. 2005, 52, 283.
| Organosulfates – a new component of humic-like substances in atmospheric aerosols?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1KntL7M&md5=a35fd0eeea4648fae6a7992da5dab652CAS |
[6] T. Reemtsma, A. These, P. Venkatachari, X. Xia, P. Hopke, A. Springer, M. Linscheid, Identification of fulvic acids and sulfated and nitrated analogues in atmospheric aerosol by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 2006, 78, 8299.
| Identification of fulvic acids and sulfated and nitrated analogues in atmospheric aerosol by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1Sjtb7I&md5=5a1eabc2bc82904c1740c9d3e7e20768CAS |
[7] A. S. Wozniak, J. E. Bauer, R. L. Sleighter, R. M. Dickhut, P. G. Hatcher, Technical Note: Molecular characterization of aerosol-derived water soluble organic carbon using ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Atmos. Chem. Phys. 2008, 8, 5099.
| Technical Note: Molecular characterization of aerosol-derived water soluble organic carbon using ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlCntbbF&md5=b7c276c2bc6dfaf81e28aa390a128fd4CAS |
[8] K. E. Altieri, B. J. Turpin, S. P. Seitzinger, Oligomers, organosulfates, and nitrooxy organosulfates in rainwater identified by ultra-high resolution electrospray ionization FTICR mass spectrometry. Atmos. Chem. Phys. 2009, 9, 2533.
| Oligomers, organosulfates, and nitrooxy organosulfates in rainwater identified by ultra-high resolution electrospray ionization FTICR mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmvVGjtbc%3D&md5=b23507f2b8bca3c1b667f4d129b43782CAS |
[9] E. A. Stone, C. J. Hedman, R. J. Sheesley, M. M. Shafer, J. J. Schauer, Investigating the chemical nature of humic-like substances (HULIS) in North American atmospheric aerosols by liquid chromatography tandem mass spectrometry. Atmos. Environ. 2009, 43, 4205.
| Investigating the chemical nature of humic-like substances (HULIS) in North American atmospheric aerosols by liquid chromatography tandem mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpt1Cltrg%3D&md5=03e8ffe409b2316ec0bccad092f7a39aCAS |
[10] L. R. Mazzoleni, B. M. Ehrmann, X. H. Shen, A. G. Marshall, J.L. Collett, Water-soluble atmospheric organic matter in fog: exact masses and chemical formula identification by ultrahigh-resolution Fourier transform ion cyclotron resonance mass spectrometry. Environ. Sci. Technol. 2010, 44, 3690.
| Water-soluble atmospheric organic matter in fog: exact masses and chemical formula identification by ultrahigh-resolution Fourier transform ion cyclotron resonance mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXkvVaiu7o%3D&md5=f2df57988217a3fa1940176ccaa4f8dfCAS |
[11] J. D. Surratt, J. H. Kroll, T. E. Kleindienst, E. O. Edney, M. Claeys, A. Sorooshian, N. L. Ng, J. H. Offenberg, M. Lewandowski, M. Jaoui, R. C. Flagan, J. H. Seinfeld, Evidence for organosulfates in secondary organic aerosol. Environ. Sci. Technol. 2007, 41, 517.
| Evidence for organosulfates in secondary organic aerosol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xht1OmsLzM&md5=3ba1496dcab5f18f744e84387c36ab24CAS |
[12] Y. Iinuma, C. Müller, O. Böge, T. Gnauk, H. Herrmann, The formation of organic sulfate esters in limonene ozonolysis secondary organic aerosol (SOA) under acidic conditions. Atmos. Environ. 2007, 41, 5571.
| The formation of organic sulfate esters in limonene ozonolysis secondary organic aerosol (SOA) under acidic conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXot1Ghs7Y%3D&md5=aa77a2a7637ca319239540a9d81d34b2CAS |
[13] Y. Iinuma, C. Müller, T. Berndt, O. Böge, M. Claeys, H. Herrmann, Evidence for the existence of organosulfates from beta-pinene ozonolysis in ambient secondary organic aerosol. Environ. Sci. Technol. 2007, 41, 6678.
| Evidence for the existence of organosulfates from beta-pinene ozonolysis in ambient secondary organic aerosol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpvVCntLo%3D&md5=ee5b0887594bf9e8f467cab9f1b8db9bCAS |
[14] J. D. Surratt, Y. Gómez-González, A. W. H. Chan, R. Vermeylen, M. Shahgholi, T. E. Kleindienst, E. O. Edney, J. H. Offenberg, M. Lewandowski, M. Jaoui, W. Maenhaut, M. Claeys, R. C. Flagan, J. H. Seinfeld, Organosulfate formation in biogenic secondary organic aerosol. J. Phys. Chem. A 2008, 112, 8345.
| Organosulfate formation in biogenic secondary organic aerosol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXpvFOgsrw%3D&md5=8ed1709bc00dac5ed4673e4b498bad19CAS |
[15] Y. Gómez-González, J. D. Surratt, F. Cuyckens, R. Szmigielski, R. Vermeylen, M. Jaoui, M. Lewandowski, J. H. Offenberg, T. E. Kleindienst, E. O. Edney, F. Blockhuys, C. Van Alsenoy, W. Maenhaut, M. Claeys, Characterization of organosulfates from the photooxidation of isoprene and unsaturated fatty acids in ambient aerosol using liquid chromatography/(–)electrospray ionization mass spectrometry. J. Mass Spectrom. 2008, 43, 371.
| Characterization of organosulfates from the photooxidation of isoprene and unsaturated fatty acids in ambient aerosol using liquid chromatography/(–)electrospray ionization mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[16] F. Yasmeen, R. Vermeylen, R. Szmigielski, Y. Iinuma, O. Böge, H. Herrmann, W. Maenhaut, M. Claeys, Terpenylic acid and related compounds: precursors for dimers in secondary organic aerosol from the ozonolysis of α- and β-pinene. Atmos. Chem. Phys. 2010, 10, 9383.
| Terpenylic acid and related compounds: precursors for dimers in secondary organic aerosol from the ozonolysis of α- and β-pinene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhs1ait7jM&md5=b1d18dbae61484d25a937b3e8c8def2eCAS |
[17] F. Yasmeen, R. Szmigielski, R. Vermeylen, Y. Gómez-González, J. D. Surratt, A. W. H. Chan, J. H. Seinfeld, W. Maenhaut, M. Claeys, Mass spectrometric characterization of isomeric terpenoic acids from the oxidation of α-pinene, β-pinene, d-limonene, and Δ3-carene in fine forest aerosol. J. Mass Spectrom. 2011, 46, 425.
| Mass spectrometric characterization of isomeric terpenoic acids from the oxidation of α-pinene, β-pinene, d-limonene, and Δ3-carene in fine forest aerosol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjvVWmsbc%3D&md5=56ccfd37a3596772b619d7fbac668451CAS |
[18] E. R. Graber, Y. Rudich, Atmospheric HULIS: how humic-like are they? A comprehensive and critical review. Atmos. Chem. Phys. 2006, 6, 729.
| Atmospheric HULIS: how humic-like are they? A comprehensive and critical review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XltV2jtLg%3D&md5=0074ab99e5455ae9389439b055531c2dCAS |
[19] G. Kiss, E. Tombácz, B. Varga, T. Alsberg, L. Persson, Estimation of the average molecular weight of humic-like substances isolated from fine atmospheric aerosol. Atmos. Environ. 2003, 37, 3783.
| Estimation of the average molecular weight of humic-like substances isolated from fine atmospheric aerosol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXlsleku7g%3D&md5=00b3dcae9e51130edc55d6d76b8d0a9aCAS |
[20] V. Samburova, R. Zenobi, M. Kalberer, Characterization of high molecular weight compounds in urban atmospheric particles. Atmos. Chem. Phys. 2005, 5, 2163.
| Characterization of high molecular weight compounds in urban atmospheric particles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXht1Kgs7jI&md5=21fc80d598ac4bdf3dc0f8b48d82c86cCAS |
[21] H. Mukai, Y. Ambe, Characterization of a humic acid-like brown substance in airborne particulate matter and tentative identification of its origin. Atmos. Environ. 1986, 20, 813.
| Characterization of a humic acid-like brown substance in airborne particulate matter and tentative identification of its origin.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL28Xkt12jsL8%3D&md5=4c095e04b7db99123f346511c8bfe4d1CAS |
[22] O. L. Mayol-Bracero, P. Guyon, B. Graham, G. Roberts, M. O. Andreae, S. Decesari, M. C. Facchini, S. Fuzzi, P. Artaxo, Water-soluble organic compounds in biomass burning aerosols over Amazonia, 2. Apportionment of the chemical composition and importance of the polyacidic fraction. J. Geophys. Res. 2002, 107, 8091.
| Water-soluble organic compounds in biomass burning aerosols over Amazonia, 2. Apportionment of the chemical composition and importance of the polyacidic fraction.Crossref | GoogleScholarGoogle Scholar |
[23] T. Feczko, H. Puxbaum, A. Kasper-Giebl, M. Handler, A. Limbeck, A. Gelencsér, C. Pio, S. Preunkert, M. Legrand, Determination of water and alkaline extractable atmospheric humic-like substances with the TU Vienna HULIS analyzer in samples from six background sites in Europe. J. Geophys. Res. 2007, 112, D23S10.
| Determination of water and alkaline extractable atmospheric humic-like substances with the TU Vienna HULIS analyzer in samples from six background sites in Europe.Crossref | GoogleScholarGoogle Scholar |
[24] I. Salma, R. Ocskay, G. G. Láng, Properties of atmospheric humic-like substances – water system. Atmos. Chem. Phys. 2008, 8, 2243.
| Properties of atmospheric humic-like substances – water system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvVSksb0%3D&md5=aacc79b5eefddae7bdfcd36780eb98ebCAS |
[25] P. Lin, G. Engling, J. Z. Yu, Humic-like substances in fresh emissions of rice straw burning and in ambient aerosols in the Pearl River Delta Region, China. Atmos. Chem. Phys. 2010, 10, 6487.
| Humic-like substances in fresh emissions of rice straw burning and in ambient aerosols in the Pearl River Delta Region, China.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFKlt7zI&md5=7b5455ac7f32a52e1be3d273c60b3967CAS |
[26] I. Salma, R. Ocskay, X. Chi, W. Maenhaut, Sampling artefacts, concentrations and chemical composition of fine water-soluble organic carbon and humic-like substances in a continental urban atmospheric environment. Atmos. Environ. 2007, 41, 4106.
| Sampling artefacts, concentrations and chemical composition of fine water-soluble organic carbon and humic-like substances in a continental urban atmospheric environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltFyltLc%3D&md5=b7bc27eeffa868f03aa3ec6b6bae94f9CAS |
[27] Y. Iinuma, O. Böge, R. Gräfe, H. Herrmann, Methyl-nitrocatechols: atmospheric tracer compounds for biomass burning secondary organic aerosols. Environ. Sci. Technol. 2010, 44, 8453.
| Methyl-nitrocatechols: atmospheric tracer compounds for biomass burning secondary organic aerosols.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlansLvE&md5=04971605dc40b1f8478963642e93d5d9CAS |
[28] J. L. Kelly, D. V. Michelangeli, P. A. Makar, D. R. Hastie, M. Mozurkewich, J. Auld, Aerosol speciation and mass prediction from toluene oxidation under high NOx conditions. Atmos. Environ. 2010, 44, 361.
| Aerosol speciation and mass prediction from toluene oxidation under high NOx conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtVWrtQ%3D%3D&md5=915084177a9353c620f748c0c90441e2CAS |
[29] H. E. Jeffries, Photochemical air pollution, in Composition, Chemistry and Climate of the Atmosphere (Ed. H. B. Singh) 1995, pp. 308–348 (Van Nostrand Reinhold: New York).
[30] E. Borrás, L. A. Tortajada-Genaro, Secondary organic aerosol formation from the photo-oxidation of benzene. Atmos. Environ. 2012, 47, 154.
| Secondary organic aerosol formation from the photo-oxidation of benzene.Crossref | GoogleScholarGoogle Scholar |
[31] S. Decesari, S. Fuzzi, M. C. Facchini, M. Mircea, L. Emblico, F. Cavalli, W. Maenhaut, X. Chi, G. Schkolnik, A. Falkovich, Y. Rudich, M. Claeys, V. Pashynska, G. Vas, I. Kourtchev, R. Vermeylen, A. Hoffer, M. O. Andreae, E. Tagliavini, F. Moretti, P. Artaxo, Characterization of the organic composition of aerosols from Rondônia, Brazil, during the LBA-SMOCC 2002 experiment and its representation through model compounds. Atmos. Chem. Phys. 2006, 6, 375.
| Characterization of the organic composition of aerosols from Rondônia, Brazil, during the LBA-SMOCC 2002 experiment and its representation through model compounds.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xks1Kqur0%3D&md5=41020614c4a0c28c73d9a545e1c0592eCAS |
[32] P. A. Solomon, J. L. Moyers, R. A. Fletcher, High-volume dichotomous virtual impactor for the fractionation and collection of particles according to aerodynamic size. Aerosol Sci. Technol. 1983, 2, 455.
| High-volume dichotomous virtual impactor for the fractionation and collection of particles according to aerodynamic size.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXjvVymuw%3D%3D&md5=6b732060d09f6b0d4fb5ee441f157b00CAS |
[33] B. Varga, G. Kiss, I. Ganszky, A. Gelencsér, Z. Krivácsy, Isolation of water-soluble organic matter from atmospheric aerosol. Talanta 2001, 55, 561.
| Isolation of water-soluble organic matter from atmospheric aerosol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmsFSiu7Y%3D&md5=1bd55dd89ac43a4b1d3df5f1fe6365dcCAS |
[34] I. Salma, T. Mészáros, W. Maenhaut, E. Vass, Z. Majer, Chirality and the origin of atmospheric humic-like substances. Atmos. Chem. Phys. 2010, 10, 1315.
| Chirality and the origin of atmospheric humic-like substances.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjsl2rur0%3D&md5=1a9f61b262a49f2f4234961b1ca6b5d6CAS |
[35] Z. Kitanovski, I. Grgić, F. Yasmeen, M. Claeys, A. Čusak, Development of a liquid chromatographic method based on UV/Vis and electrospray mass spectrometric detection for the identification of nitrocatechols and related tracers in biomass burning atmospheric organic aerosol. Rapid Commun. Mass Spectrom. 2012, 26, 793.
| Development of a liquid chromatographic method based on UV/Vis and electrospray mass spectrometric detection for the identification of nitrocatechols and related tracers in biomass burning atmospheric organic aerosol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XivVyitr8%3D&md5=fa04db5ae9ea59423cb41f3fa26fbb36CAS |
[36] R. Szmigielski, J. D. Surratt, Y. Gómez-González, P. Van der Veken, I. Kourtchev, R. Vermeylen, F. Blockhuys, M. Jaoui, T. E. Kleindienst, M. Lewandowski, J. H. Offenberg, E. O. Edney, J. H. Seinfeld, W. Maenhaut, M. Claeys, 3-methyl-1,2,3-butanetricarboxylic acid: an atmospheric tracer for terpene secondary organic aerosol. Geophys. Res. Lett. 2007, 34, L24811.
| 3-methyl-1,2,3-butanetricarboxylic acid: an atmospheric tracer for terpene secondary organic aerosol.Crossref | GoogleScholarGoogle Scholar |
[37] M. Claeys, Y. Iinuma, R. Szmigielski, J. D. Surratt, F. Blockhuys, C. Van Alsenoy, O. Böge, B. Sierau, Y. Gómez-González, R. Vermeylen, P. Van der Veken, M. Shahgholi, A. W. H. Chan, H. Herrmann, J. H. Seinfeld, W. Maenhaut, Terpenylic acid and related compounds from the oxidation of α-pinene: implications for new particle formation and growth above forest. Environ. Sci. Technol. 2009, 43, 6976.
| Terpenylic acid and related compounds from the oxidation of α-pinene: implications for new particle formation and growth above forest.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVSis7nK&md5=d924fad936ba357434e464138d5e8905CAS |
[38] M. Claeys, I. Kourtchev, V. Pashynska, G. Vas, R. Vermeylen, W. Wang, J. Cafmeyer, X. Chi, P. Artaxo, M. O. Andreae, W. Maenhaut, Polar organic marker compounds in atmospheric aerosols during the LBA-SMOCC 2002 biomass burning experiment in Rondônia, Brazil: sources and source processes, time series, diel variations and size distributions. Atmos. Chem. Phys. 2010, 10, 9319.
| Polar organic marker compounds in atmospheric aerosols during the LBA-SMOCC 2002 biomass burning experiment in Rondônia, Brazil: sources and source processes, time series, diel variations and size distributions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhs1ait7vK&md5=a241fdee8f6bbe3fa6086f99c5b13d27CAS |
[39] D. Hoffmann, Y. Iinuma, H. Herrmann, Development of a method for fast analysis of phenolic molecular markers in biomass burning particles using high performance liquid chromatography/atmospheric pressure chemical ionisation mass spectrometry. J. Chromatogr. A 2007, 1143, 168.
| Development of a method for fast analysis of phenolic molecular markers in biomass burning particles using high performance liquid chromatography/atmospheric pressure chemical ionisation mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhs1ChsL4%3D&md5=0474aa0fc7545ea8248dc5bdb7415355CAS |
[40] Y. Y. Zhang, L. Müller, R. Winterhalter, G. K. Moortgat, T. Hoffmann, U. Pöschl, Seasonal cycle and temperature dependence of pinene oxidation products, dicarboxylic acids and nitrophenols in fine and coarse air particulate matter. Atmos. Chem. Phys. 2010, 10, 7859.
| Seasonal cycle and temperature dependence of pinene oxidation products, dicarboxylic acids and nitrophenols in fine and coarse air particulate matter.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtlWls7nN&md5=98e7b8c58cf9949e4e19be9222480710CAS |
[41] K. Kawamura, I. R. Kaplan, Motor exhaust emissions as a primary source for dicarboxylic acids in Los Angeles ambient air. Environ. Sci. Technol. 1987, 21, 105.
| Motor exhaust emissions as a primary source for dicarboxylic acids in Los Angeles ambient air.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXit1Giuw%3D%3D&md5=06d5c9eea66524c35d5a76fbd52e253cCAS |
[42] W. F. Rogge, M. A. Mazurek, L. M. Hildeman, C. G. Cass, B. R. T. Simoneit, Quantification of urban organic aerosols at a molecular level: identification, abundance and seasonal variation. Atmos. Environ. 1993, 27A, 1309.
| 1:CAS:528:DyaK3sXlvVOntrw%3D&md5=86c47fcba48953ee9b950ed949eef327CAS |
[43] B. R. T. Simoneit, P. M. Medeiros, B. M. Didyk, Combustion products of plastics as indicators for refuse burning in the atmosphere. Environ. Sci. Technol. 2005, 39, 6961.
| Combustion products of plastics as indicators for refuse burning in the atmosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXmvFymsrw%3D&md5=843da12c481648c54fa85a2a680bd765CAS |
[44] E. G. Stephanou, N. Stratigakis, Oxocarboxylic and α,ω-dicarboxylic acids – photooxidation products of biogenic unsaturated fatty acids present in urban aerosols. Environ. Sci. Technol. 1993, 27, 1403.
| Oxocarboxylic and α,ω-dicarboxylic acids – photooxidation products of biogenic unsaturated fatty acids present in urban aerosols.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXktFCgt7g%3D&md5=9ecfc42647365bfb9ff22c05b5a056b2CAS |
[45] S. Gao, J. D. Surratt, E. M. Knipping, E. S. Edgerton, M. Shahgholi, J. H. Seinfeld, Characterization of polar organic components in fine aerosols in the southeastern United States: identity, origin, and evolution. J. Geophys. Res. 2006, 111, D14314.
| Characterization of polar organic components in fine aerosols in the southeastern United States: identity, origin, and evolution.Crossref | GoogleScholarGoogle Scholar |
[46] A. Kubátová, R. Vermeylen, M. Claeys, J. Cafmeyer, M. Maenhaut, G. Roberts, P. Artaxo, Carbonaceous aerosol characterization in the Amazon basin, Brasil: novel dicarboxylic acids and related compounds. Atmos. Environ. 2000, 34, 5037.
| Carbonaceous aerosol characterization in the Amazon basin, Brasil: novel dicarboxylic acids and related compounds.Crossref | GoogleScholarGoogle Scholar |
[47] A. Kubátová, R. Vermeylen, M. Claeys, J. Cafmeyer, W. Maenhaut, Organic compounds in urban aerosols from Gent, Belgium: characterization, sources, and seasonal differences. J. Geophys. Res. 2002, 107, 8343.
| Organic compounds in urban aerosols from Gent, Belgium: characterization, sources, and seasonal differences.Crossref | GoogleScholarGoogle Scholar |
[48] K. Kawamura, H. Kasukabe, L. A. Barrie, Source and reaction pathways of dicarboxylic acids, ketoacids and dicarbonyls in arctic aerosols: one year of observations. Atmos. Environ. 1996, 30, 1709.
| Source and reaction pathways of dicarboxylic acids, ketoacids and dicarbonyls in arctic aerosols: one year of observations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XivVKmtbw%3D&md5=6d44f120652d4cdcbb48a7c68d0157a4CAS |
[49] Y. Gómez-González, W. Wang, R. Vermeylen, X. Chi, J. Neirynck, I. A. Janssens, W. Maenhaut, M. Claeys, Chemical characterization of atmospheric aerosols during a 2007 summer field campaign at Brasschaat, Belgium: sources and source processes of biogenic secondary organic aerosol. Atmos. Chem. Phys. 2012, 12, 125.
| Chemical characterization of atmospheric aerosols during a 2007 summer field campaign at Brasschaat, Belgium: sources and source processes of biogenic secondary organic aerosol.Crossref | GoogleScholarGoogle Scholar |
[50] M. Jaoui, E. Corse, T. E. Kleindienst, J. H. Offenberg, M. Lewandowski, E. O. Edney, Analysis of secondary organic aerosol from the photooxidation of d-limonene and their detection in ambient PM2.5 aerosol. Environ. Sci. Technol. 2006, 40, 3819.
| Analysis of secondary organic aerosol from the photooxidation of d-limonene and their detection in ambient PM2.5 aerosol.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XksFKku7o%3D&md5=f30c59165decded2166d42bcfa062125CAS |
[51] M. Claeys, B. Graham, G. Vas, W. Wang, R. Vermeylen, V. Pashynska, J. Cafmeyer, P. Guyon, M. O. Andreae, P. Artaxo, W. Maenhaut, Formation of secondary organic aerosols through photooxidation of isoprene. Science 2004, 303, 1173.
| Formation of secondary organic aerosols through photooxidation of isoprene.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhsVWgtb4%3D&md5=eef442b73dfc7608ee1529dcde8b7e37CAS |
[52] N. L. Ng, P. S. Chhabra, A. W. H. Chan, J. D. Surratt, J. H. Kroll, A. J. Kwan, D. C. McCabe, P. O. Wennberg, A. Sorooshian, S. M. Murphy, N. F. Dalleska, R. C. Flagan, J. H. Seinfeld, Effect of NOx level on secondary organic aerosol (SOA) formation from the photooxidation of terpenes. Atmos. Chem. Phys. 2007, 7, 5159.
| Effect of NOx level on secondary organic aerosol (SOA) formation from the photooxidation of terpenes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlOnsrvL&md5=da8699ff1c75702b18eda2c0655cd695CAS |
[53] L. Müller, M.-C. Reinnig, K. H. Naumann, H. Saathoff, T. F. Mentel, N. Donahue, T. Hoffmann, Formation of 3-methyl-1,2,3-butanetricarboxylic acid via gas phase oxidation of pinonic acid – a mass spectrometric study of SOA aging. Atmos. Chem. Phys. 2012, 12, 1483.
| Formation of 3-methyl-1,2,3-butanetricarboxylic acid via gas phase oxidation of pinonic acid – a mass spectrometric study of SOA aging.Crossref | GoogleScholarGoogle Scholar |
[54] B. R. T. Simoneit, J. J. Schauer, C. G. Nolte, D. R. Oros, V. O. Elias, M. Fraser, W. F. Rogge, G. R. Cass, Levoglucosan, a tracer for cellulose in biomass burning and atmospheric particles. Atmos. Environ. 1999, 33, 173.
| Levoglucosan, a tracer for cellulose in biomass burning and atmospheric particles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXhvVagtw%3D%3D&md5=b0c46e6c032e9e385e6c431afac2b010CAS |