Single particle analysis of amines in ambient aerosol in Shanghai
Yuanlong Huang A , Hong Chen A , Lin Wang A , Xin Yang A B and Jianmin Chen AA Department of Environmental Science & Engineering, Fudan University, 220 Handan Road, Shanghai 200433, P. R. China.
B Corresponding author. Email: yangxin@fudan.edu.cn
Environmental Chemistry 9(3) 202-210 https://doi.org/10.1071/EN11145
Submitted: 28 November 2011 Accepted: 20 January 2012 Published: 26 March 2012
Journal Compilation © CSIRO Publishing 2012 Open Access CC BY-NC-ND
Environmental context. Amines, a group of basic organic compounds, play important roles in atmospheric chemistry. We studied their distribution in ambient aerosols at the single particle level, and found that high relative humidity and strong particle acidity can attract more amines from the gas phase to particles. Amines may account for a significant part of organic mass in aerosols in areas with high emissions of sulfur dioxide and nitrogen oxides.
Abstract. An aerosol time-of-flight mass spectrometer was deployed in urban Shanghai to analyse amine-containing particles during two separate sampling periods, 1–9 August 2007 and 22–27 December 2009. Amine-containing particles are identified by a mass spectrometric marker at m/z 86 [NCH2(C2H5)2+] and classified into six major particle types to explore their possible origins. The number fraction of amine-containing particles in winter was much higher than in summer (23.4 v. 4.4 %), which can be explained by preferred gas-to-particle partitioning of gaseous amines at lower temperatures. Mass spectrometric patterns show the strong acidity of particles collected in December 2009, suggesting the acid–base reaction pathway might also contribute to the high concentration of amine aerosol in winter. Two fog episodes and two after-rain episodes of amine-containing particle bursts were observed in August 2007. Tightly correlated number fractions of sulfate- and amine-containing particles in all these episodes reveal that high relative humidity greatly enhances particulate amine formation based on acid–base reaction and subsequent particle growth. Our observations suggest that amines may account for significant parts of secondary organic mass in heavily polluted areas.
Introduction
Aerosols in the atmosphere can cause the loss of visibility, affect global climate and impair human health.[1] Organic aerosols account for a considerable percentage of particulate matter, but their extensive sources and intricate chemical transformations, especially the formation mechanism of secondary organic aerosol (SOA), lead to great uncertainties in our current understanding.[2–4] The presence of amines, a group of nitrogen-containing organic compounds, as organic components in the atmosphere has recently generated significant interest. Because of the high vapour pressures of low-molecular-weight amines, over 150 gaseous amines[5,6] have been identified in the atmosphere from both natural and anthropogenic sources including animal husbandry, waste incineration and sewage treatment, marine sources and vehicle exhaust.[7–12] Gaseous amine concentrations are usually at least an order of magnitude lower than that of ammonia in the atmosphere,[13] but they can reach as high as 23 % of that of ammonia in animal husbandry environments.[14] The mass concentration of gaseous amines varies from 0.3–4.2 ng m–3 in the marine atmosphere[15] to 70.4–561.4 μg m–3 near a commercial dairy.[16]
Being strong gaseous bases and soluble in water, amines undergo acid–base reactions with nitric and sulfuric acid[13,17] to form low-volatility aminium salts. They can also undergo photooxidation and oxidation reactions with OH radicals, NO3 radicals and O3 in the ambient atmosphere to form non-volatile aerosol components.[13,18–20] De Haan et al.[21,22] observed that reactions between glyoxal and amines or amino acids can produce light-absorbing, nitrogen-containing imidazoles and oligomers. Particle phase amines have been extensively observed in many areas[9,17,23–25] especially in ultrafine particles during nucleation[26–28] and particle growth[29] events. Compared with ammonia, amines have higher molecular weights and present stronger alkalinity that make them perfect candidates in new particle formation and growth.[30] Many studies have been performed to explore the enhancement effect of amines in nucleation in competition with ammonia.[31–42]
In the past two decades, on-line single particle mass spectrometry has made significant contributions to the studies of ambient aerosols.[43] Using this technique, the size and composition of many individual particles can be determined simultaneously with good time resolution, allowing particle composition to be correlated with rapid changes in environmental conditions. Several studies have deployed single particle mass spectrometry to investigate particulate amines in laboratory and ambient environments.[17,29,44–46] Pratt et al. studied seasonal variations of aminium and ammonium salts with an aerosol time-of-flight mass spectrometer (ATOFMS) coupled with an automated thermodenuder, revealing the influence of particle acidity on determining the amine morphology in fine particles.[44] Creamean et al. employed an ultrafine aerosol time-of-flight mass spectrometer (UF-ATOFMS) to study the components of freshly formed particles (100 ≤ Dva ≤ 1000 nm), indirectly testifying to amine’s participation in new particle formation.[29] Rehbein et al. combined field and laboratory work to demonstrate that high relative humidity (RH) greatly enhances the gas-to-particle partitioning and subsequent aqueous acid–base reaction of amines.[45] In a field measurement in urban Shanghai, our group found that under low ambient oxidant levels of amines (or ammonia), formaldehyde and carbonyl-containing compounds, Mannich reactions occur to form high-molecular-weight aminiums, accounting for a substantial fraction of organic aerosol mass.[46]
In this article we utilise a commercial ATOFMS (TSI-3800) to investigate particulate amine distribution in the ambient atmosphere in urban Shanghai. To explore their origins, amine-containing particles are classified into six particle types based on the mass spectrometric patterns. Measurements during two sampling periods, 1–9 August 2007 and 22–27 December 2009, are compared to show the seasonal variations. Several amine-containing particle burst episodes are analysed based on the well time- and size-resolved spectra offered by aerosol time-of-flight mass spectrometry and the meteorological conditions.
Experimental
The detailed setup and mechanisms of the ATOFMS (TSI-3800) used in this study are available in previous publications.[46–48] A brief description is presented here. Aerosols in the size range of 0.2–2.0 μm were effectively drawn from the ambient atmosphere in a 0.1-L min–1 air flux through an aerodynamic focus lens (AFL). The expanded, attenuated air wrapped the particles into a vacuum region in a beam line and the particles were accelerated to a terminal size-dependent aerodynamic velocity. Two orthogonally oriented continuous lasers with a wavelength of 532 nm were fixed by a set distance in the size region. Scattered light caused by the transmitting particles passing through the size region was detected by two corresponding photomultiplier tubes to measure the velocity of the particles. By size calibration using monodisperse polystyrene latex spheres (Nanosphere Size Standards, Duke Scientific Corp., Palo Alto) with a fixed diameter (0.22–2.00 μm), the velocity was transformed to a particle’s aerodynamic diameter. Based on the time of flight, a pulsed desorption–ionisation laser (Q-switched Nd:YAG laser, 266 nm) fired exactly when a particle arrived at the right point. The laser pulse with ~1.0 mJ of energy vaporised and ionised the chemical species in a single particle, generating positive and negative ions. Both positive and negative ions were then analysed simultaneously by the time-of-flight mass spectrometer.
The ATOFMS was at the Department of Environmental Science and Engineering at Fudan University (31°14′N, 121°29′E) in urban Shanghai, close to residential, traffic and construction emissions sources. The inlet of the sampling tube, a 4-m copper pipe with a 10-mm inner diameter, was ~5 m above the ground and 0.5 m above the roof of the building. The ATOFMS was operated almost 24 h per day in two separate campaigns: one for 7 days during 1–5 and 8–9 August 2007 and one for 6 days from 22 to 27 December 2009. Local meteorological data including temperature, RH, atmospheric pressure, wind speed and direction were provided by the Shanghai Meteorological Bureau.
Original single particle mass spectra were converted into a list of peaks at each m/z using TSI MS-Analyse software with a limiting criteria of at least 30 arbitrary units above the baseline. They were then imported into YAADA (version 2.11, www.yaada.org), a software toolkit in Matlab (version R2010b) for further analysis of particle sizes and chemical components. Particles containing amines were identified by the mass spectrometric marker of alkylamines at m/z +86 [NCH2(C2H5)2+][17] with a threshold of an absolute peak area larger than 100 and a relative peak area greater than 0.05. Other aerosol time-of-flight mass spectrometry markers used were m/z +18 [NH4+] (absolute peak area greater than 30),[49] –97 [HSO4–] (absolute peak area greater than 100)[49] and –195 [H(HSO4)2–] (absolute peak area greater than 30), indicative of ammonium salts, sulfate compounds and particle acidity index[44] respectively. Particles were clustered into six major types using the ART-2a (adaptive resonance theory)[50] method with a vigilance factor of 0.7, a learning rate of 0.05 and 20 iterations based on the similarities of mass-to-charge ratio and peak intensity.
Results and discussion
Identification and classification of amine-containing particles
A total of 380 282 particles in August 2007 and 138 269 particles in December 2009 with both positive and negative mass spectra were recorded. Most of the particles with chemical composition information were in the size range of 0.2–2.0 μm. Using the amine marker (m/z +86 [NCH2(C2H5)2+]), 16 570 amine-containing particles were identified in August 2007, accounting for 4.4 % of the total particles. In December 2009, 32 312 amine-containing particles were distinguished, accounting for 23.4 % of the total particles.
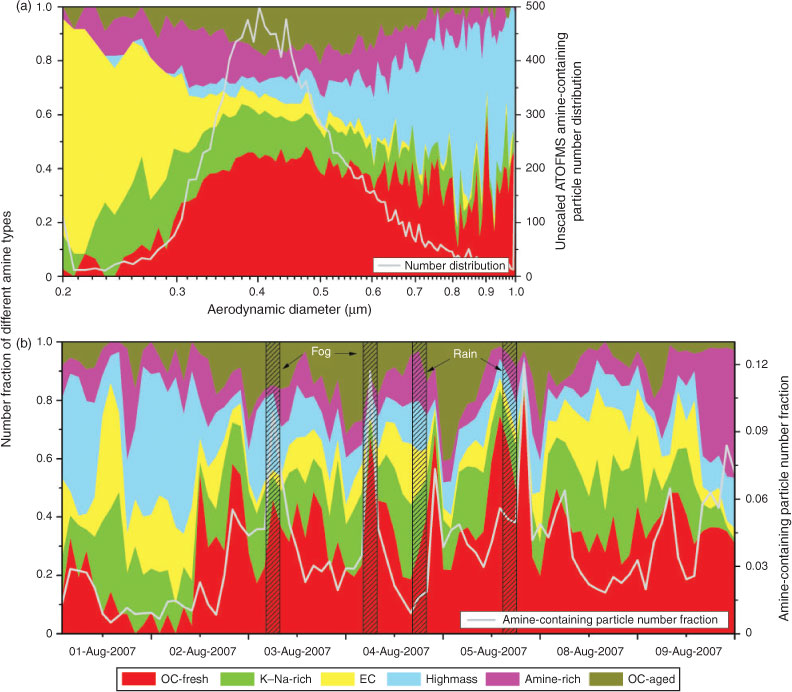
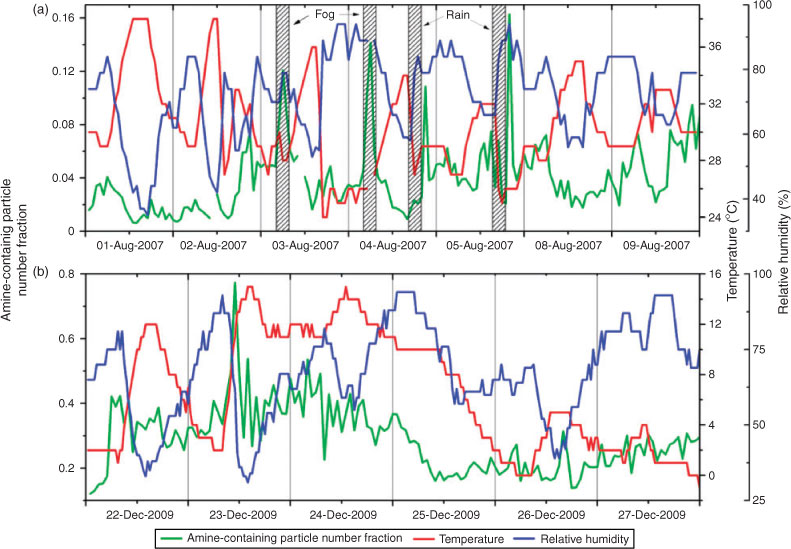
The temporal profiles of the number fraction of amine-containing particles, together with those of temperature and RH, are shown in Fig. 1 at a resolution of 1 h. During the campaign in August 2007, two fog events and two rain events occurred, as marked by the shadow in Fig. 1. A clear diurnal variation of temperature and RH can be drawn in August 2007 and a notable temperature decline was recorded during December 2009. The number fraction of amine-containing particles in August 2007 shows four sharp peaks in Fig. 1a, either during a fog event or after a rain. In Fig. 1b, the number fraction of amine-containing particle shows one prominent peak as high as nearly 80 %. Meteorological conditions were stable with a fairly low temperature and a high RH during amine burst episodes in summer 2007, whereas in December 2009 an amine peak emerged during a quick temperature-increasing and RH-decreasing process.
|
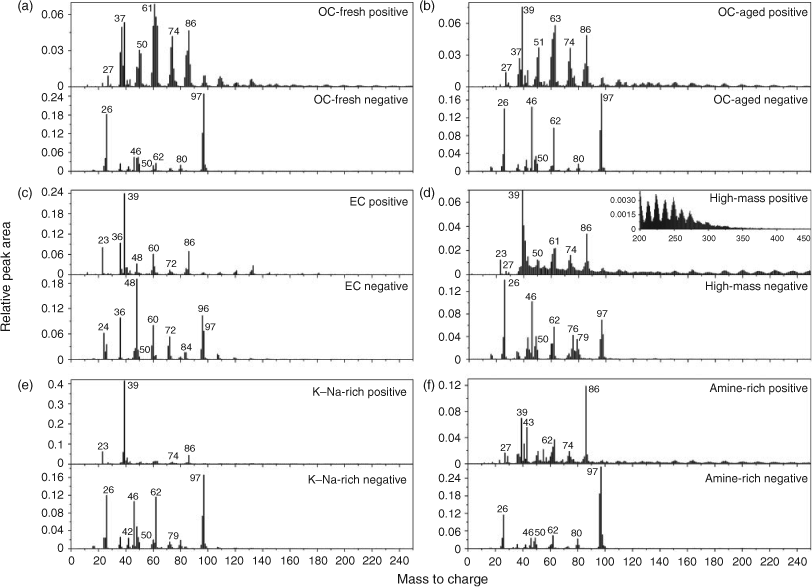
To explore the origins, these amine-containing particles are classified into six groups by ART-2a, representing six major particle types. Particles fall into these types accounting for ~91 % of all amine-containing particles. The name of each particle type and its number fraction in the total amine-containing particles are listed in Table 1. Since the particle types in December 2009 are almost identical to those in August 2007, here we only present the average mass spectra for each particle type in August 2007 in Fig. 2. Seasonal variations in the mass spectrometric pattern will be discussed later.
|
Two organic carbon (OC)-type groups account for nearly half of the total amine particles (as shown in Table 1). As shown in Fig. 2, mass spectra patterns of the OC-fresh-type and OC-aged-type particles are quite similar, both showing a series of carbon hydrate signals such as m/z +27 [C2H3+] and +37 [C3H+]. The major difference between these two types is the signal intensities of nitrite and nitrate (m/z –46 [NO2–], –62 [NO3–]) in the negative spectrum in Fig. 2b, indicating the chemical transformation of OC-aged particles. Usually, particles are regarded as ‘aged’ when a high intensity of secondary species (ammonium, nitrate and sulfate) occurs or when nitrate signals are more abundant than sulfate ones in the mass spectrum.[49] Elementary carbon (EC) particles are characterised by a series of elementary carbon clusters signals [Cn+/–] in both positive and negative spectra. This group of particles were fairly fresh showing weak signals of secondary species like [NO2–] (m/z –46), [NO3–] (m/z –62) and [HSO4–] (m/z –97). We assign these amine-containing EC particles to fresh mobile emissions, similar to the observation in Riverside, CA, 1998.[17] High-mass-type particles were characterised by high-mass ions with m/z up to 400 Da. These species are likely nitrogen-containing organic salts derived from particle-phase reactions, such as Mannich reactions. Major ingredients of the reactions include amines (or ammonia), formaldehyde and carbonyl-containing compounds. The nature and formation mechanism of these secondary organic species are discussed in our recently published paper.[46] K–Na-rich particles show strong [K+] (m/z +39) and [Na+] (m/z +23) signals in the positive mass spectrum together with [CN–] (m/z –26), [NO2–] (m/z –46), [NO3–] (m/z –62) and [HSO4–] (m/z –97) in the negative mass spectrum. The amine signal (m/z +86 [NCH2(C2H5)2+]) in K–Na-rich particles is relatively weaker compared with other particle types. The combination of [K+] and [CN–] suggests that this group of amine particles might be from biomass burning emissions.[51] The positive mass spectra of amine-rich particles show the strongest m/z +86 [NCH2(C2H5)2+] and +74 [NH2(C2H5)2+] signals and fewer other organic peaks compared with other particle types, quite similar with the aerosol time-of-flight mass spectrometry measurement of pure diethylamine particles.[17] No such type of amine aerosol has been reported so far in ambient measurements. We note that all the six types have [CN–] (m/z –26) and [C3N–] (m/z –50) signals in negative spectra, which confirms the existence of carbon–nitrogen bonding in aerosol components.[46]
Fig. 3a presents the size-resolved number fraction of the six particle types in August 2007 in the size range of 0.2–1.0 μm. Fig. 3b shows the temporal profile of the number fraction of each type in the total amine-containing particles at a resolution of 2 h. OC-fresh and OC-aged particles basically share the same size distribution in the aerosol time-of-flight mass spectrometry sampling range, peaking at 0.4–0.5 μm. We notice that the number fraction of OC-aged particles often increased after a burst of OC-fresh particles (as shown in Fig. 3b) with a couple of hours delay. Considering the similar mass spectrometric patterns between these two groups, we may conclude that these two amine-containing OC groups share the same origins, representing different stages of aging processes. The formation process of these amine-containing OC particles will be discussed later. K–Na-rich and amine-rich particles show nearly equal distributions along the presented size range. Their number fractions remained stable throughout the sampling period, indicating possible steady background sources in the sampling environment. Most of the EC-type particles were smaller than 500 nm and contributed to nearly 80 % of the total amine particles at 200 nm. The average size of the high-mass-type particles was larger than any other types and they constituted most of the amine particles larger than 600 nm. The polarised size distributions of the EC and high-mass types are consistent with the nature of these particles. As discussed above, amine-containing EC particles were mostly from fresh mobile emissions. The organic salts in high-mass particles were produced from Mannich reactions between amines, formaldehyde and carbonyl-containing compounds.[46] The incorporation of non- and semi-volatile organic compounds to the surface of pre-existing particles helped to yield large size particles.[3]
Seasonal variation
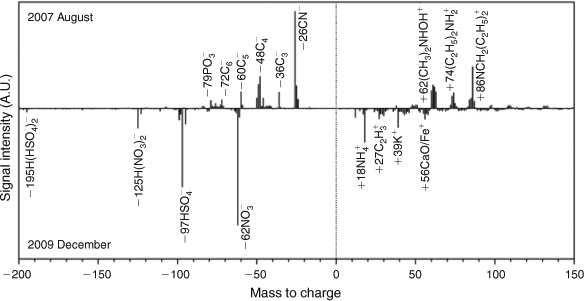
Fig. 4 shows the differential mass spectrum between the average mass spectrum of particles collected in August 2007 and that in December 2009. The positive and negative going peaks represent net signal intensities of chemical species that appeared in both summer and winter. In summer 2007, stronger amine related signals such as m/z +86 [NCH2(C2H5)2+], +74 [NH2(C2H5)2+], +62 [NHOH(CH3)2+] and –26 [CN–] were observed. A series of carbon clusters at m/z –36 [C3–], –48 [C4–], –60 [C6–] and –72 [C6–] also appear in the positive going spectrum. These strong signals suggest larger portions of amines and carbon hydrates in the summer aerosol, thus the freshness of these amine-containing particles. In winter 2009, net negative going signals are mostly secondary species including sulfate (m/z –97 [HSO4–]), nitrate (m/z –62 [NO3–]) and ammonium (m/z +18 [NH4+]); all markers for aged particles. Signals of a prononated bisulfate dimer m/z –195 [H(HSO4)2–] and the nitrate dimer m/z –125 [H(NO3)2–] were observed, which are the indicators of strong particle acidity.[44] The appearance of m/z +39 [K+] and +56 [CaO/Fe+] in the negative going spectrum suggest that dust particles contributed to the amine-containing particles in winter.
|
Although the differential mass spectrum shows stronger amine signals in summer, the number fraction of amine particles in summer 2007 was much lower than that in winter 2009. As shown in Fig. 1, the number fraction of amine-containing particles ranged from 0.20 to 0.77 in December 2009 whereas this ratio decreased to 0.02–0.16 in August 2007. The same trend was observed for the distribution of ammonia (ammonium); ~69 % of particles in December 2009 contained a [NH4+] signal whereas only 3 % of particles in August contained a [NH4+] signal. The enhanced particulate ammonium and ammine production could be explained by a favoured gas-to-particle partitioning of gaseous ammonia and amines at lower temperatures.[6,44] The ambient temperature during the sampling period in December 2009 varied from –1 to 15 °C, much lower than the temperature (24–38 °C) in summer 2007.
Gaseous amines are stronger bases than ammonia. They can compete with ammonia to react with nitric acid and sulfuric acid to form non-volatile aminium salts in the particle phase. An acidic particle environment can increase its affinity to amines, leading to protonation of amines and subsequent salt formation. Apparently, the increased amount of ammonium had not fully neutralised the particles in December 2009. The presence of [H(NO3)2–] and [H(HSO4)2–], proxies of non-neutralised acids, indicate strong particle acidity in December, strongly suggesting that acid–base reactions also contributed to the high yield of particulate amine in December. Hence, the higher amine-containing particle number fractions in December 2009 can be explained by both lower ambient temperature and stronger particle acidity. The important roles of meteorological conditions including ambient temperature and RH for amine distribution in the gas phase and particulate matters will be further discussed below.
Particulate amine burst episodes
Five amine-containing particle burst episodes were observed during the sampling periods, four in August 2007 and one in December 2009. As shown in Fig. 1, two fog events and two heavy rain events occurred during the sampling period in August 2007. The number fractions of amine-containing particles were strongly associated with the meteorological conditions. Two amine burst episodes occurred during fog events and two bursts occurred right after heavy rains. Here we take the fog event in the morning of 4 August 2007 and the after-rain episode in the evening of 4 August 2007 as examples to examine in detail how meteorological conditions can affect the amine distribution in the particle phase.
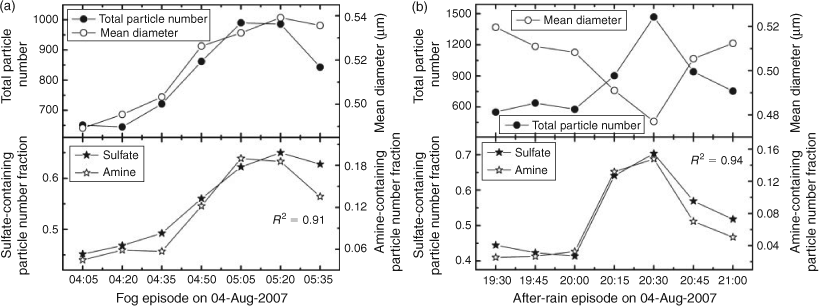
Both laboratory and field studies have revealed that high RH helps gas-phase amines partition to the particle phase.[17,45] Angelino et al. reported that the frequency of amine-containing particles correlated well with the fluctuation of ambient RH during the 1999 EPA Supersite experiment in Atlanta, GA.[17] In our experiment, no tight correlation between the number fraction of amine-containing particles and the diurnal pattern of RH was observed for the entire sampling period in August 2007. However, all the amine-containing particle burst episodes occurred with high RH values. In the fog event in the morning of 4 August 2007, ambient RH reached ~90 %. Fig. 5a shows the temporal profiles of the total particle number, mean particle size and sulfate- and amine-containing particle number fraction at a resolution of 15 min measured by aerosol time-of-flight mass spectrometry in this fog event. Both the total particle counts and the mean particle size reached their peak values in a fog event. Meanwhile, the number fractions of sulfate- and amine-containing particles tightly correlated with each other during the event (R2 = 0.91, R2 for the entire sampling period equals 0.08) and also synchronised with the total particle counts. Apparently, a high water content in particles during the fog event greatly enhanced the acid–base reaction by dissolving ammonium salts into their ionic forms and shifting the gas/particle equilibrium of amines to the particle phase.[17,45] A high percentage of OC-fresh-type amine particles during the burst episode further confirms the presence of newly incorporated alkylamines in the particle phase (Fig. 3b).
The heavy rain in the evening of 4 August 2007 started at ~1700 hours and lasted for 1 h. An amine-containing particle burst episode was observed 2.5 h after the rain (~2030 hours) together with elevated total particle counts (as shown in Fig. 5b). Again, the number fractions of sulfate-containing particles tightly correlated with the amine particles (R2 = 0.94). Unlike the fog episodes, the mean particle size reached a minimum when the particle counts peaked. Creamean et al. reported an amine-OC particle burst episode with decreased mean particle sizes after a precipitation in Northern California, which was explained by amine-assisted particle growth after a particle nucleation event.[29] Precipitation can effectively remove airborne particles and leave a pristine ambient environment with less surface area, which is favoured for particle nucleation. However, new particle formation processes cannot explain our observation. The growth rates of new particles were measured in previous studies to be ~1–20 nm h–1,[52] implying that newly formed particles can grow into the smallest aerosol time-of-flight mass spectrometry detectable size (~200 nm, Dva) only after tens of hours of time. In our experiment, the amine burst episode was observed only two and a half hours after the rain, too short for newly formed particles to grow. Apparently, heavy rain effectively scavenged large size particles. After the rain, enhanced by the high ambient RH and low temperature, amines entered the particle phase by coating, or dissolving in, pre-existing ultrafine particles, forming aminium salts with inorganic acids and contributing to the growth of fine particles into the lower end of the detectable size range of aerosol time-of-flight mass spectrometry. Hence, increased particle counts and decreased mean diameters were observed. The tight correlation between sulfate- and amine-containing particles illustrates the importance of the aqueous amine reaction in particle growth. OC-fresh particles dominated other particle types in all the four burst episodes (Fig. 3b), suggesting their similarity in chemistry.
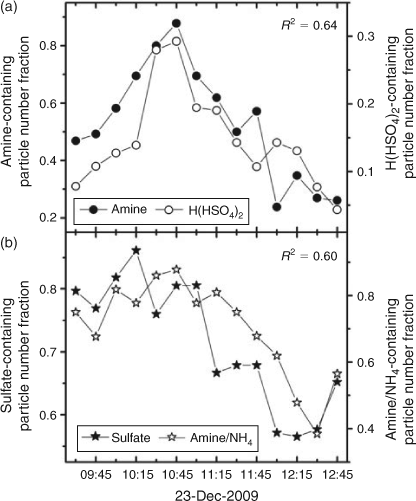
The amine burst episode on 23 December 2009 occurred during a haze event with a daily averaged PM10 (fine particulate matter with aerodynamic diameter <10.0 μm) value of 192 μg m–3. Different from the burst episodes in August 2007, amine-containing particles peaked at a fairly low RH (40 %). Mass spectra patterns of amine-containing particles were analysed to identify major particle types. No new particle types emerged during the episode. The number fractions of sulfate-containing particles ([H(HSO4)2–], marker for particle acidity) were also examined. As shown in Fig. 6, the number fraction of particles containing amine or ammonium signals reached almost 90 % in the episode and agreed well with sulfate-containing particles (R2 = 0.60). With such high number concentrations of ammonium- and amine-containing particles, particles remained surprisingly acidic. Particles containing the H(HSO4)2– signal showed the same temporal profile as amine-containing particles. Yao et al. argued that the hit ratio of ion m/z –195 [H(HSO4)2–] to ion m/z –97 [HSO4–] should be a better indicator of the particle acidity than the [H(HSO4)2–] signal alone.[53] Here, we calculated the hit ratio of [H(HSO4)2–] to [HSO4–] and found that it shared the same temporal trend as the [H(HSO4)2–] signal during the episode. We thus tentatively assign this amine burst episode to the prolonged aging of particles with strong acidity in the stagnant air parcel in the haze event.
|
By taking advantage of online single particle mass spectrometry, individual amine-containing particles are identified in the ambient atmosphere in Shanghai. Well time-resolved chemical information of these amine particles reveals that high RH and strong particle acidity can greatly enhance gas-phase amines’ partitioning to the particle phase. Aminium salts formed between amines and inorganic acids like sulfuric acid and nitric acid can play important roles in the particle growth. Acidic aerosol particles can be produced in areas with high SO2 and NOx emissions. In Shanghai, the lowest pH value of the aerosol sample filtrates was reported to be as low as 2.81.[54] Our study suggests that amines may account for a substantial mass fraction of atmospheric aerosols in heavily polluted urban areas.
Acknowledgements
This work was supported by The National Natural Science Foundation of China (20937001, 21177027, 40875074) and the Science & Technology Commission of Shanghai Municipality (10JC1402000).
References
[1] B. J. Finlayson-Pitts, J. N. Pitts, Particles in the troposphere, in Chemistry of the Upper and Lower Atmosphere 2000 (Academic Press: San Diego, CA).[2] M. Kanakidou, J. H. Seinfeld, S. N. Pandis, I. Barnes, F. J. Dentener, M. C. Facchini, R. Van Dingenen, B. Ervens, A. Nenes, C. J. Nielsen, E. Swietlicki, J. P. Putaud, Y. Balkanski, S. Fuzzi, J. Horth, G. K. Moortgat, R. Winterhalter, C. E. L. Myhre, K. Tsigaridis, E. Vignati, E. G. Stephanou, J. Wilson, Organic aerosol and global climate modelling: a review. Atmos. Chem. Phys. 2005, 5, 1053.
| Organic aerosol and global climate modelling: a review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXktlyrtbw%3D&md5=0b28a8d31bcdc89aefbae127bbbe1c32CAS |
[3] J. H. Kroll, J. H. Seinfeld, Chemistry of secondary organic aerosol: formation and evolution of low-volatility organics in the atmosphere. Atmos. Environ. 2008, 42, 3593.
| Chemistry of secondary organic aerosol: formation and evolution of low-volatility organics in the atmosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXls1Kksbs%3D&md5=e4081486255a7e6de6be34deb1620aecCAS |
[4] M. Hallquist, J. C. Wenger, U. Baltensperger, Y. Rudich, D. Simpson, M. Claeys, J. Dommen, N. M. Donahue, C. George, A. H. Goldstein, J. F. Hamilton, H. Herrmann, T. Hoffmann, Y. Iinuma, M. Jang, M. E. Jenkin, J. L. Jimenez, A. Kiendler-Scharr, W. Maenhaut, G. McFiggans, Th. F. Mentel, A. Monod, A. S. H. Prévôt, J. H. Seinfeld, J. D. Surratt, R. Szmigielski, J. Wildt, The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155.
| The formation, properties and impact of secondary organic aerosol: current and emerging issues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFGhs77M&md5=0437ae491e64eca2afaa26d3b70e085eCAS |
[5] X. Ge, A. S. Wexler, S. L. Clegg, Atmospheric amines – Part I. A review. Atmos. Environ. 2011, 45, 524.[Published online ahead of print 15 October 2010]
| Atmospheric amines – Part I. A review.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXovVGktg%3D%3D&md5=888536eedd7314339eabbbb3179e07f7CAS |
[6] X. Ge, A. S. Wexler, S. L. Clegg, Atmospheric amines – Part II. Thermodynamic properties and gas/particle partitioning. Atmos. Environ. 2011, 45, 561.[Published online ahead of print 15 October 2010]
| Atmospheric amines – Part II. Thermodynamic properties and gas/particle partitioning.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXovVGkug%3D%3D&md5=c49caa6c447f16e5ef61b2a298b562a3CAS |
[7] G. W. Schade, P. J. Crutzen, Emission of aliphatic amines from animal husbandry and their reactions: potential source of N2O and HCN. J. Atmos. Chem. 1995, 22, 319.
| Emission of aliphatic amines from animal husbandry and their reactions: potential source of N2O and HCN.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXpslCqt7w%3D&md5=5db1c774a625e7240b4d1dc8991f1a41CAS |
[8] J. Leach, A. Blanch, A. C. Bianchi, Volatile organic compounds in an urban airborne environment adjacent to a municipal incinerator, waste collection centre and sewage treatment plant. Atmos. Environ. 1999, 33, 4309.
| Volatile organic compounds in an urban airborne environment adjacent to a municipal incinerator, waste collection centre and sewage treatment plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlvFyit70%3D&md5=98f143b8e74fa8359419591b6aa662b6CAS |
[9] M. C. Facchini, S. Decesari, M. Rinaldi, C. Carbone, E. Finessi, M. Mircea, S. Fuzzi, F. Moretti, E. Tagliavini, D. Ceburnis, C. D. O'Dowd, Important source of marine secondary organic aerosol from biogenic amines. Environ. Sci. Technol. 2008, 42, 9116.
| Important source of marine secondary organic aerosol from biogenic amines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlCgtbfL&md5=2c4e553e7f4cd0d2a7c0f6a6b51bd33eCAS |
[10] S. H. Cadle, P. A. Mulawa, Low-molecular-weight aliphatic amines in exhaust from catalyst-equipped cars. Environ. Sci. Technol. 1980, 14, 718.
| Low-molecular-weight aliphatic amines in exhaust from catalyst-equipped cars.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXlvFWhs7s%3D&md5=9b1f0503de58b84623a48bf4a147329aCAS |
[11] Y. Miyazaki, K. Kawamura, M. Sawano, Size distributions of organic nitrogen and carbon in remote marine aerosols: evidence of marine biological origin based on their isotopic ratios. Geophys. Res. Lett. 2010, 37, L06803.
| Size distributions of organic nitrogen and carbon in remote marine aerosols: evidence of marine biological origin based on their isotopic ratios.Crossref | GoogleScholarGoogle Scholar |
[12] A. Sorooshian, L. T. Padró, A. Nenes, G. Feingold, A. McComiskey, S. P. Hersey, H. Gates, H. H. Jonsson, S. D. Miller, G. L. Stephens, R. C. Flagan, J. H. Seinfeld, On the link between ocean biota emissions, aerosol, and maritime clouds: airborne, ground, and satellite measurements off the coast of California. Global Biogeochem. Cycles 2009, 23, GB4007.
| On the link between ocean biota emissions, aerosol, and maritime clouds: airborne, ground, and satellite measurements off the coast of California.Crossref | GoogleScholarGoogle Scholar |
[13] S. M. Murphy, A. Sorooshian, J. H. Kroll, N. L. Ng, P. Chhabra, C. Tong, J. D. Surratt, E. Knipping, R. C. Flagan, J. H. Seinfeld, Secondary aerosol formation from atmospheric reactions of aliphatic amines. Atmos. Chem. Phys. 2007, 7, 2313.
| Secondary aerosol formation from atmospheric reactions of aliphatic amines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXms1ygsLg%3D&md5=03ebfe57d8d4e98dd0f347792fc450b7CAS |
[14] A. Sorooshian, S. M. Murphy, S. Hersey, H. Gates, L. T. Padro, A. Nenes, F. J. Brechtel, H. Jonsson, R. C. Flagan, J. H. Seinfeld, Comprehensive airborne characterization of aerosol from a major bovine source. Atmos. Chem. Phys. 2008, 8, 5489.
| Comprehensive airborne characterization of aerosol from a major bovine source.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlCnur%2FI&md5=a8b83ed820132bf354ee301c8aaa92d6CAS |
[15] A. Van Neste, R. A. Duce, C. Lee, Methylamines in the marine atmosphere. Geophys. Res. Lett. 1987, 14, 711.
| Methylamines in the marine atmosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXlvVSmurk%3D&md5=ba96dcf87d82cfeb1ac6901a4bbc8d3aCAS |
[16] N. E. Rabaud, S. E. Ebeler, L. L. Ashbaugh, R. G. Flocchini, Characterization and quantification of odorous and non-odorous volatile organic compounds near a commercial dairy in California. Atmos. Environ. 2003, 37, 933.
| Characterization and quantification of odorous and non-odorous volatile organic compounds near a commercial dairy in California.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXht1Wguro%3D&md5=a3b21f03b6c2ba53e08d2e1050c72376CAS |
[17] S. Angelino, D. T. Suess, K. A. Prather, Formation of aerosol particles from reactions of secondary and tertiary alkylamines: characterization by aerosol time-of-flight mass spectrometry. Environ. Sci. Technol. 2001, 35, 3130.
| Formation of aerosol particles from reactions of secondary and tertiary alkylamines: characterization by aerosol time-of-flight mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXksFSis7Y%3D&md5=ea86c39ce213d4aec8e2ca75455a51dcCAS |
[18] T. B. Nguyen, J. Laskin, A. Laskin, S. A. Nizkorodov, Nitrogen-containing organic cmopounds and oligomers in secondary organic aerosol formed by photooxidation of isoprene. Environ. Sci. Technol. 2011, 45, 6908.
| 1:CAS:528:DC%2BC3MXpt1Cjsr8%3D&md5=b1d210602638999a949c998a5e63fad8CAS |
[19] P. J. Silva, M. E. Erupe, D. Price, J. Elias, Q. G. J. Malloy, Q. Li, B. Warren, D. R. Cocker, Trimethylamine as precursor to secondary organic aerosol formation via nitrate radical reaction in the atmosphere. Environ. Sci. Technol. 2008, 42, 4689.
| Trimethylamine as precursor to secondary organic aerosol formation via nitrate radical reaction in the atmosphere.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmt1Sqs78%3D&md5=546a16bf6a32c8d9c41c311e0e9279caCAS |
[20] Q. G. J. Malloy, L. Qi, B. Warren, D. R. Cocker, M. E. Erupe, P. J. Silva, Secondary organic aerosol formation from primary aliphatic amines with NO3 radical. Atmos. Chem. Phys. 2009, 9, 2051.
| Secondary organic aerosol formation from primary aliphatic amines with NO3 radical.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlt1yisrc%3D&md5=d62bdc32457486717daaacff0b9b67d0CAS |
[21] D. O. De Haan, A. L. Corrigan, K. W. Smith, D. R. Stroik, J. J. Turley, F. E. Lee, M. A. Tolbert, J. L. Jimenez, K. E. Cordova, G. R. Ferrell, Secondary organic aerosol-forming reactions of glyoxal with amino acids. Environ. Sci. Technol. 2009, 43, 2818.
| Secondary organic aerosol-forming reactions of glyoxal with amino acids.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjtFyru7Y%3D&md5=5d710d9662123302670b77e5d8d8a4a8CAS |
[22] D. O. De Haan, L. N. Hawkins, J. A. Kononenko, J. J. Turley, A. L. Corrigan, M. A. Tolbert, J. L. Jimenez, Formation of nitrogen-containing oligomers by methylglyoxal and amines in simulated evaporating cloud droplets. Environ. Sci. Technol. 2011, 45, 984.
| Formation of nitrogen-containing oligomers by methylglyoxal and amines in simulated evaporating cloud droplets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFygtL7J&md5=c17ba561d56e5147bf1048b3e21c4e70CAS |
[23] P. V. Tan, G. J. Evans, J. Tsai, S. Owega, M. S. Fila, O. Malpica, J. R. Brook, On-line analysis of urban particulate matter focusing on elevated wintertime aerosol concentrations. Environ. Sci. Technol. 2002, 36, 3512.
| On-line analysis of urban particulate matter focusing on elevated wintertime aerosol concentrations.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlsVShu7k%3D&md5=6997ab965b69c15f9b94363065f88024CAS |
[24] H. Yang, J. Xu, W. Wu, C. Wan, J. Yu, Chemical characterization of water-soluble organic aerosols at Jeju Island collected during ACE-Asia. Environ. Chem. 2004, 1, 13.
| Chemical characterization of water-soluble organic aerosols at Jeju Island collected during ACE-Asia.Crossref | GoogleScholarGoogle Scholar |
[25] D. C. S. Beddows, R. J. Donovan, R. M. Harrison, M. R. Heal, R. P. Kinnersley, M. D. King, D. H. Nicholson, K. C. Thompson, Correlations in the chemical composition of rural background atmospheric aerosol in the UK determined in real time using time-of-flight mass spectrometry. J. Environ. Monit. 2004, 6, 124.
| Correlations in the chemical composition of rural background atmospheric aerosol in the UK determined in real time using time-of-flight mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovVCmtg%3D%3D&md5=74acf90a01f01ee6c9ce38e1750d35fbCAS |
[26] J. M. Mäkelä, S. Yli-Koivisto, V. Hiltunen, W. Seidl, E. Swietlicki, K. Teinilä, M. Sillanpää, I. K. Koponen, J. Paatero, K. Rosman, K. Hämeri, Chemical composition of aerosol during particle formation events in boreal forest. Tellus B Chem. Phys. Meterol. 2001, 53, 380.
| Chemical composition of aerosol during particle formation events in boreal forest.Crossref | GoogleScholarGoogle Scholar |
[27] J. N. Smith, M. J. Dunn, T. M. VanReken, K. Iida, M. R. Stolzenburg, P. H. McMurry, L. G. Huey, Chemical composition of atmospheric nanoparticles formed from nucleation in Tecamac, Mexico: evidence for an important role for organic species in nanoparticle growth. Geophys. Res. Lett. 2008, 35, L04808.
| Chemical composition of atmospheric nanoparticles formed from nucleation in Tecamac, Mexico: evidence for an important role for organic species in nanoparticle growth.Crossref | GoogleScholarGoogle Scholar |
[28] J. N. Smith, K. C. Barsanti, H. R. Friedli, M. Ehn, M. Kulmala, D. R. Collins, J. H. Scheckman, B. J. Williams, P. H. McMurry, Observations of aminium salts in atmospheric nanoparticles and possible climatic implications. Proc. Natl. Acad. Sci. USA 2010, 107, 6634.
| Observations of aminium salts in atmospheric nanoparticles and possible climatic implications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXltFSjsLw%3D&md5=b7e5b71e1d39e5e5dd60570e03d3523dCAS |
[29] J. M. Creamean, A. P. Ault, J. E. Ten Hoeve, M. Z. Jacobson, G. C. Roberts, K. A. Prather, Measurements of aerosol chemistry during new particle formation events at a remote rural mountainsite. Environ. Sci. Technol. 2011, 45, 8208.
| Measurements of aerosol chemistry during new particle formation events at a remote rural mountainsite.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFWmu73P&md5=f5e4d46009fd13e432be267f8b88c287CAS |
[30] T. Kurtén, V. Loukonen, H. Vehkamäki, M. Kulmala, Amines are likely to enhance neutral and ion-induced sulfuric acid-water nucleation in the atmosphere more effectively than ammonia. Atmos. Chem. Phys. 2008, 8, 4095.
| Amines are likely to enhance neutral and ion-induced sulfuric acid-water nucleation in the atmosphere more effectively than ammonia.Crossref | GoogleScholarGoogle Scholar |
[31] C. A. Zordan, S. Wang, M. V. Johnston, Time-resolved chemical composition of individual nanoparticles in urban air. Environ. Sci. Technol. 2008, 42, 6631.
| Time-resolved chemical composition of individual nanoparticles in urban air.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovFSktLo%3D&md5=cab70b8dfaa849c1727881c67db78e0aCAS |
[32] K. C. Barsanti, P. H. McMurry, J. N. Smith, The potential contribution of organic salts to new particle growth. Atmos. Chem. Phys. 2009, 9, 2949.
| The potential contribution of organic salts to new particle growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXosFKku70%3D&md5=e94d2d83641ea5c2e61951039b1e3cbcCAS |
[33] J. A. Lloyd, K. J. Heaton, M. V. Johnston, Reactive uptake of trimethylamine into ammonium nitrate particles. J. Phys. Chem. A 2009, 113, 4840.
| Reactive uptake of trimethylamine into ammonium nitrate particles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjvFKlur8%3D&md5=e99f82ca303085988da3d2aaa3c98628CAS |
[34] B. R. Bzdek, D. P. Ridge, M. V. Johnston, Size-dependent reactions of ammonium bisulfate clusters with dimethylamine. J. Phys. Chem. A 2010, 114, 11 638.
| Size-dependent reactions of ammonium bisulfate clusters with dimethylamine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1OgsLrP&md5=803efbd6e71c6efedfa3e4d89e8f2776CAS |
[35] B. R. Bzdek, D. P. Ridge, M. V. Johnston, Amine exchange into ammonium bisulphate and ammonium nitrate nuclei. Atmos. Chem. Phys. 2010, 10, 3495.
| Amine exchange into ammonium bisulphate and ammonium nitrate nuclei.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptVeit7w%3D&md5=628dbc11f21f4432ce2f27c5458f4e94CAS |
[36] T. Berndt, F. Stratmann, M. Sipilä, J. Vanhanen, T. Petäjä, J. Mikkilä, A. Grüner, G. Spindler, R. Lee Mauldin, J. Curtius, M. Kulmala, J. Heintzenberg, Laboratory study on new particle formation from the reaction OH + SO2: influence of experimental conditions, H2O vapour, NH3 and the amine tert-butylamine on the overall process. Atmos. Chem. Phys. 2010, 10, 7101.
| Laboratory study on new particle formation from the reaction OH + SO2: influence of experimental conditions, H2O vapour, NH3 and the amine tert-butylamine on the overall process.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVSkt7rK&md5=82ab441085847869330926c273460424CAS |
[37] L. Wang, V. Lal, A. F. Khalizov, R. Zhang, Heterogeneous chemistry of alkylamines with sulfuric acid: implications for atmospheric formation of alkylaminium sulphates. Environ. Sci. Technol. 2010, 44, 2461.
| Heterogeneous chemistry of alkylamines with sulfuric acid: implications for atmospheric formation of alkylaminium sulphates.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXisFalsbw%3D&md5=eb25da528ac6da641c5c02fe08220b23CAS |
[38] L. Wang, A. F. Khalizov, J. Khalizov, W. Xu, W. Ma, V. La, R. Zhang, Atmospheric nanoparticles formed from heterogeneous reactions of organics. Nat. Geosci. 2010, 3, 238.
| Atmospheric nanoparticles formed from heterogeneous reactions of organics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktVWru7s%3D&md5=ee03a7e071cdd37ad5be2a8aee79e467CAS |
[39] M. E. Erupe, A. A. Viggiano, S.-H. Lee, The effect of trimethylamine on atmospheric nucleation involving H2SO4. Atmos. Chem. Phys. 2011, 11, 4767.
| The effect of trimethylamine on atmospheric nucleation involving H2SO4.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVWlt7%2FL&md5=60b6acf23465b82646ff986f90edd8b4CAS |
[40] B. R. Bzdek, D. P. Ridge, M. V. Johnston, Amine reactivity with charged sulfuric acid clusters. Atmos. Chem. Phys. 2011, 11, 8735.
| Amine reactivity with charged sulfuric acid clusters.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVyju7nN&md5=44ab650c71165c950eb0d0a8d0568b30CAS |
[41] J. Zhao, J. N. Smith, F. L. Eisele, M. Chen, C. Kuang, P. H. McMurry, Observation of neutral sulfuric acid-amine containing clusters in laboratory and ambient measurements. Atmos. Chem. Phys. 2011, 11, 10 823.
| Observation of neutral sulfuric acid-amine containing clusters in laboratory and ambient measurements.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XisFOisrg%3D&md5=84e8579af733163a1a4d300f4d689a2dCAS |
[42] C. Qiu, L. Wang, V. Lal, A. F. Khalizov, R. Zhang, Heterogeneous reactions of alkylamines with ammonium sulfate and ammonium bisulfate. Environ. Sci. Technol. 2011, 45, 4748.
| Heterogeneous reactions of alkylamines with ammonium sulfate and ammonium bisulfate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlsFWnur4%3D&md5=96354752f151f9027ae2e4107e42eb9dCAS |
[43] R. C. Sullivan, K. A. Prather, Recent advances in our understanding of atmospheric chemistry and climate made possible by on-line aerosol analysis instrumentation. Anal. Chem. 2005, 77, 3861.
| Recent advances in our understanding of atmospheric chemistry and climate made possible by on-line aerosol analysis instrumentation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXktlaksLY%3D&md5=e00c689506a440bb327d5b1539097313CAS |
[44] K. A. Pratt, L. E. Hatch, K. A. Prather, Seasonal volatility dependence of ambient particle phase amines. Environ. Sci. Technol. 2009, 43, 5276.
| Seasonal volatility dependence of ambient particle phase amines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXmvVCitr0%3D&md5=4a1ccecaaf2e523f76cc7c18dcccbef6CAS |
[45] P. J. G. Rehbein, C.-H. Jeong, M. L. McGuire, X. Yao, J. C. Corbin, G. J. Evans, Cloud and fog processing enhanced gas-to-particle partitioning of trimethylamine. Environ. Sci. Technol. 2011, 45, 4346.
| Cloud and fog processing enhanced gas-to-particle partitioning of trimethylamine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFGgurs%3D&md5=6e180e9156abcef3d5eb38ed1dae372eCAS |
[46] X. Wang, S. Gao, X. Yang, H. Chen, J. Chen, G. Zhuang, J. D. Surratt, M. N. Chan, J. H. Seinfeld, Evidence for high molecular weight nitrogen-containing organic salts in urban aerosols. Environ. Sci. Technol. 2010, 44, 4441.
| Evidence for high molecular weight nitrogen-containing organic salts in urban aerosols.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmtFWjurc%3D&md5=0200d2e92d5491703123aab9bfac7f59CAS |
[47] E. Gard, J. E. Mayer, B. D. Morrical, T. Dienes, D. P. Fergenson, K. A. Prather, Real-time analysis of individual atmospheric aerosol particles: design and performance of a portable ATOFMS. Anal. Chem. 1997, 69, 4083.
| Real-time analysis of individual atmospheric aerosol particles: design and performance of a portable ATOFMS.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXlvFyitLc%3D&md5=97998b1df316c31aa2c41db852bb7e0fCAS |
[48] X. Wang, Y. Zhang, H. Chen, X. Yang, J. Chen, Particle nitrate formation in a highly polluted urban area: a case study by single-particle mass spectrometry in Shanghai. Environ. Sci. Technol. 2009, 43, 3061.
| Particle nitrate formation in a highly polluted urban area: a case study by single-particle mass spectrometry in Shanghai.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjvVOmur8%3D&md5=a7bbde9c46f1b13e8477350fdb738c8cCAS |
[49] D. Liu, R. J. Wenzel, K. A. Prather, Aerosol time-of-flight mass spectrometry during the Atlanta Supersite Experiment. 1. Measurements. J. Geophys. Res. 2003, 108, 8426.
| Aerosol time-of-flight mass spectrometry during the Atlanta Supersite Experiment. 1. Measurements.Crossref | GoogleScholarGoogle Scholar |
[50] X. Song, P. K. Hopke, D. P. Fergenson, K. A. Prather, Classification of single particle analyzed by ATOFMS using an artificial neural network, ART-2A. Anal. Chem. 1999, 71, 860.
| Classification of single particle analyzed by ATOFMS using an artificial neural network, ART-2A.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXis1Grsw%3D%3D&md5=75c85589418952a4c9ffdf4201c715dbCAS |
[51] P. J. Silva, D.-Y. Liu, C. A. Noble, K. A. Prather, Size and chemical characterization of individual particles resulting from biomass burning of local southern California species. Environ. Sci. Technol. 1999, 33, 3068.
| Size and chemical characterization of individual particles resulting from biomass burning of local southern California species.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXkvVCrsrY%3D&md5=3f81d9980011fbb906c551f9e1a30d0aCAS |
[52] R. Zhang, A. Khalizov, L. Wang, M. Hu, W. Xu, Nucleation and growth of nanoparticles in the atmosphere. Chem. Rev. 2011, [Published online ahead of print 1 November 2011]
| Nucleation and growth of nanoparticles in the atmosphere.Crossref | GoogleScholarGoogle Scholar |
[53] X. Yao, P. J. G. Rehbein, C. J. Lee, G. J. Evans, J. Corbin, C.-H. Jeong, A study on the extent of neutralization of sulphate aerosol through laboratory and field experiments using an ATOFMS and a GPIC. Atmos. Environ. 2011, 45, 6251.
| A study on the extent of neutralization of sulphate aerosol through laboratory and field experiments using an ATOFMS and a GPIC.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFCgtbrK&md5=fd46b2efce52a7e2371054d2e0702287CAS |
[54] K. Huang, G. Zhuang, J. Li, Q. Wang, Y. Sun, Y. Lin, J. S. Fu, Mixing of Asian dust with pollution aerosol and the transformation of aerosol components during the dust storm over China in spring 2007. J. Geophys. Res. 2010, 115, D00K13.
| Mixing of Asian dust with pollution aerosol and the transformation of aerosol components during the dust storm over China in spring 2007.Crossref | GoogleScholarGoogle Scholar |