Are Arctic Ocean ecosystems exceptionally vulnerable to global emissions of mercury? A call for emphasised research on methylation and the consequences of climate change
R. W. Macdonald A C and L. L. Loseto BA Department of Fisheries and Oceans, Institute of Ocean Sciences, PO Box 6000, Sidney, BC, V8L 4B2, Canada.
B Department of Fisheries and Oceans, Freshwater Institute, 501 University Crescent, Winnipeg, MB, R3T 2N6, Canada.
C Corresponding author. Email: robie.macdonald@dfo-mpo.gc.ca
Environmental Chemistry 7(2) 133-138 https://doi.org/10.1071/EN09127
Submitted: 8 October 2009 Accepted: 16 January 2010 Published: 22 April 2010
Environmental context. Mercury is a global contaminant that has entered Arctic food webs in sufficient quantity to put at risk the health of top predators and humans that consume them. Recent research has discovered a photochemical process unique to the Arctic that leads to mercury deposition on frozen surfaces after polar sunrise, but the connection between mercury deposition and entry into food webs remains tenuous and poorly understood. We propose here that the Arctic Ocean’s sensitivity to the global mercury cycle depends far more on neglected post-deposition processes that lead to methylation within the ice–ocean system, and the vulnerability of these processes to changes occurring in the cryosphere.
Abstract. Emissions, atmospheric transport and deposition have formed the emphasis of recent research to understand Hg trends in Arctic marine biota, with the expressed objective of predicting how biotic trends might respond to emission controls. To answer the question of whether the Arctic Ocean might be especially vulnerable to global mercury (Hg) contamination and how biota might respond to emission controls requires a distinction between the supply of Hg from source regions and the processes within the Arctic Ocean that sequester and convert mercury to monomethyl Hg (MeHg). Atmospheric Mercury Depletion Events (AMDEs) provide a unique Hg deposition process in the Arctic; however, AMDEs have yet to be linked quantitatively with Hg uptake in marine food webs. The difficulty in implicating AMDEs or emissions to biotic trends lie in the ocean where several poorly understood processes lead to MeHg production and biomagnification. We propose that sensitivity of the Arctic Ocean’s ecosystem to Hg lies not so much in the deposition process as in methylation processes within the ocean, Hg inputs from large drainage basins, and the vulnerability these to climate change. Future research needs to be better balanced across the entire Hg cycle.
Introduction
Research during the past fifteen years has shown Arctic biota to be highly exposed to semi-volatile contaminants like Hg.[1] In particular, northern aquatic ecosystems with long food webs, and long-lived top predators are susceptible to the biomagnification and bioaccumulation of Hg to levels of concern, both for the ecosystem and for the humans who depend on these species for much of their diet.[2,3]
In the mid 1990s, Schroeder et al.[4] discovered the unexpected deposition of atmospheric Hg to the frozen surface with the advent of polar sunrise. Given an atmospheric residence time of ~0.7 years for Hg, this finding was revolutionary, and immediately raised the question of whether such deposition might make the Arctic a receptor of atmospherically cycling Hg. Many studies ensued, and we now know that gaseous Hg undergoes photo-chemical reactions involving halogens.[5] Despite more than a decade of effort to measure and understand these Atmospheric Mercury Depletion Events (AMDEs), a recent comprehensive review of this process[6] makes but a very tenuous link between AMDE-deposited Hg and the Hg observed in Arctic aquatic biota. Here, we propose that any connection between AMDEs and Hg exposure in top predators is presently masked by a poor understanding of the transfers and transformations of Hg in aquatic systems, and these latter need more research.
Mercury is a global contaminant that cycles between air, soil and water.[7] The assessment of the risks from contaminant Hg in the Arctic is fraught with difficulty. There is a natural background cycle, difficulty in measuring ultra-trace concentrations, and the capacity of Hg to change chemical form, with monomethyl mercury (MeHg) exhibiting the greatest toxicity.[8,9] Based on Sunderland and Mason’s[10] pre- and post-industrial Hg budgets we have produced a global contaminant Hg budget (Fig. 1). This budget implies: (1) terrestrial soils contain the largest reservoir of contaminant Hg; (2) as a result, fluxes of contaminant Hg to estuaries and continental shelves are important; (3) fluxes between atmosphere and ocean are large with the net direction uncertain; and (4) deep (>1500 m) ocean contains as much contaminant Hg as upper ocean. This last point is important as it shows that Hg flux to the deep ocean has likely reduced Hg exposure in the euphotic zone. The residence time of the upper ocean (~70 years) and the atmosphere (~0.7 years) are both long enough to permit transport of Hg from industrial source regions to the Arctic, a conclusion supported by a recent mass balance for Hg.[11] Furthermore, the long residence time of the ocean implies that this part of the system is likely no longer in steady-state.
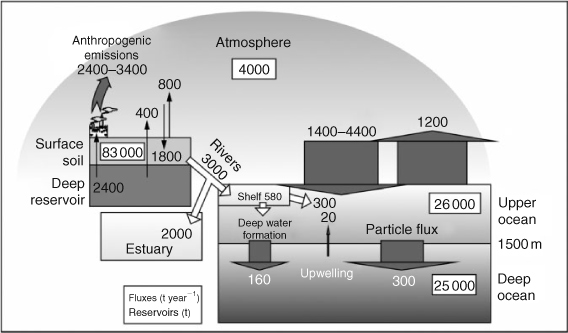
|
With respect to the Hg cycle, the Arctic Ocean has several key characteristics. It receives large inputs of sediment, freshwater and carbon from land,[12,13] shelves comprise 50% of the ocean’s area,[13] which is large by global standards, and the interior ocean (beyond the shelf) exhibits low productivity under the polar pack ice, which leads to a very low particle flux into the deep ocean.[13] The ice cover, acting as a seasonally-variable barrier between atmosphere and ocean, implies that deposition of atmospherically transported contaminants differs from open oceans. The Outridge et al.[11] mass balance implies that atmospheric Hg loadings to the Arctic Ocean cannot explain the increasing Hg trends in higher trophic level biota.
Here we propose several post-depositional Hg processes that are crucial to understand before we can link Hg deposition in the Arctic to Hg burdens in high trophic biota in the ocean.
The importance of post-depositional processes within the Arctic
Elemental gaseous mercury (Hg0) prevails in the atmosphere (greater than 90%), but this form of Hg partitions weakly into water, does not absorb strongly onto particles and is relatively low in toxicity. To deposit Hg0 and hold it within aquatic systems requires conversion to HgII (Fig. 2). More important is the conversion to the biomagnifying form, MeHg, which dominates higher levels of the food chain (80–99%). Fig. 2 clearly implies that transformations and transfers between Hg0, HgII and MeHg must be understood before connections can be made between Hg0 transported by the atmosphere and exposures to MeHg in apex aquatic animals. Not only need we worry about transformations, which can occur in both directions, but we also need to understand what controls the efficiency of such transformations and transfers.
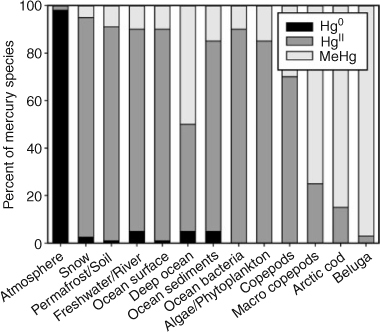
|
Further emphasising the importance of transformations and transfer efficiencies is the apparent lack of coherence between trends of Hg emissions[14] or Hg in physical media,[5,15,16] and biota.[17,18] The complexity of the Hg cycle in the Arctic (Fig. 3) together with recent climate change in the cryosphere offer several opportunities for climate variability to influence trends in final receptors.[19,20] Outridge et al.[11] proposed that the large inventory of HgII in the upper ocean could provide the means by which variable methylation alone could produce observed MeHg trends in biota. We identify four components of the Arctic Ocean’s Hg cycle that are presently poorly understood, and yet have a large potential to alter Hg exposure in top predators (Fig. 3).
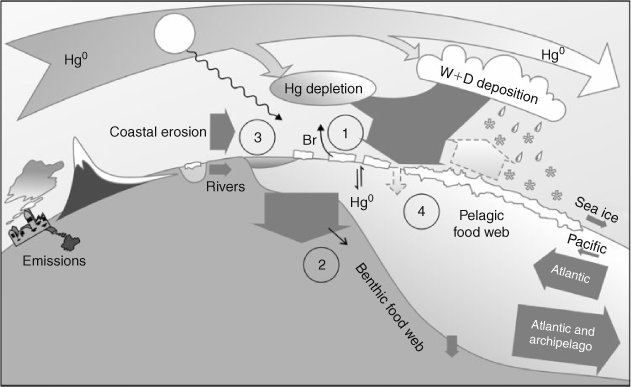
|
Mercury deposition onto and entry into the upper ocean
Mercury deposits on ice and snow through wet and dry deposition and AMDEs potentially leading to high concentrations in snow.[21,22] Once air temperatures rise above the melting point of water, ice and snow can begin to thaw and some portion – maybe a large portion – of the deposited HgII is reduced and re-emitted to the atmosphere.[23–25] How efficient is the transfer of deposited Hg from the snow and ice surface into the water and thence into biota (Fig. 4)? Some mechanisms that have escaped direct measurement might be very important. For example, blowing snow contaminated with Hg deposited during AMDEs might enter polynyas thence to enter biota during spring production. Microbes are also available in this environment to reduce HgII to Hg0 and therefore promote evasion back to the atmosphere.[26] Presently we have no quantitative estimates of the efficiencies of these processes such that we could predict how much of the deposited HgII actually winds up as MeHg in the food web, but we do know that surface water in the Arctic is generally supersaturated with Hg0.[27] In freshwater systems, Loseto et al.[28] found snowmelt water to be the most significant source of MeHg to Arctic lake systems, and even larger than wetlands where methylation occurs in soils.
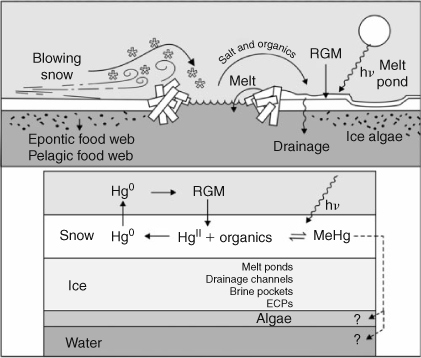
|
The methylation of Hg in sediments
Microbial communities in sediments that reduce sulfate also produce MeHg (Fig. 5).[29,30] The Arctic Ocean contains large estuaries and the greatest percentage of shelf area among all world oceans. Clearly, production of MeHg in sediments could be especially important as a source to bottom water and biota, but given the large range in organic carbon supply over Arctic shelves[31] we expect there to be, likewise, a large range in the activity of methylation. There are no measurements with which to assess the rate of production of MeHg in sediments, the factors that control its production, and the importance of sediment sources for MeHg in benthic and pelagic biota.
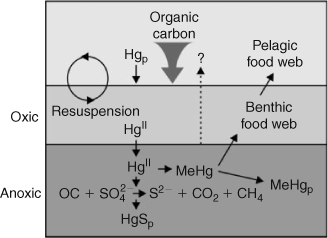
|
Transformation and delivery of Hg from terrestrial and freshwater systems
Large inflows to the Arctic Ocean from rivers brings organic carbon and various forms of Hg including particulate, dissolved and MeHg (Fig. 6). We know that the Mackenzie River delivers ~2.2 t of Hg and 15 kg of MeHg to its estuary.[32] Likewise, the Churchill and Nelson Rivers deliver ~13 kg year–1 of MeHg to Hudson Bay, a number that is far greater than that delivered by direct snow melt.[33,34] The Mackenzie River is particle rich and may, therefore, differ from other rivers in how it transports Hg and in how accessible the Hg is to biota. The connections between terrestrial and marine systems offer many opportunities for change in the movement of Hg and in its transformations, especially given projections of permafrost melt-out and the subsequent alteration of drainage basin hydrology and organic productivity.[35] Where permafrost melts, soils may dry out or inundate. The latter process leads to the release of Hg (e.g. see Klaminder et al.[36]) as well as the creation of MeHg (St. Louis et al.[37]). In particular, recently deposited Hg may be more susceptible to release and uptake by freshwater fish.[38] We presently have very few measurements for these systems in the Arctic.
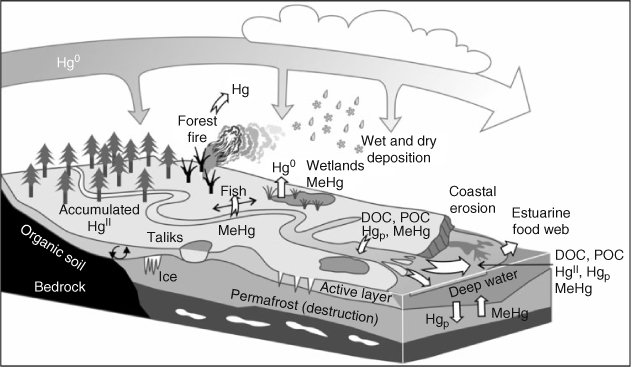
|
Transformations with the Arctic Ocean water column
Variability in MeHg concentration has been observed in Arctic Ocean plankton,[24] but there are no published data on MeHg distributions within the water column from which to infer source. Recent findings in the Mediterranean Sea and Pacific Ocean show that methylation can occur in the water column as part of the detrital remineralisation process (Fig. 7).[39,40] Most of the MeHg is produced during the regeneration process rather than transported with the detritus.[39] The western Arctic Ocean contains a pervasive cold, nutrient-rich halocline. This region has the potential to harbour MeHg which has either been imported from the Pacific Ocean, or produced within the Arctic as part of the vigorous production and regeneration cycle over the Chukchi Shelf.[31] There is a large inventory of HgII in the upper ocean of the Arctic that would supply ample HgII to feed the methylation engine.[11] We presently have no measurements from which we could assess this pathway to produce MeHg.
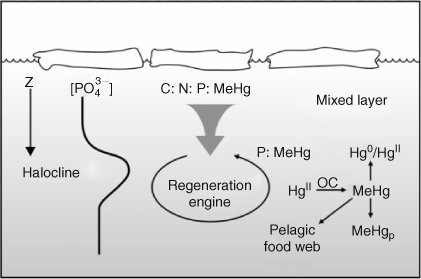
|
Open questions for future research
The question we have posed here is how do processes operating in within the Arctic (terrestrial and marine) define the vulnerability of the Arctic Ocean to mercury? This contrasts the overwhelming recent interest in atmospheric deposition chemistry as the major driver of marine food web exposure to mercury.
We describe four potential processes, about which we know practically nothing, that lead to conversions between Hg0, HgII and the organic bioaccumulative neurotoxic form, MeHg. Lack of knowledge of these processes severely limits our understanding of what has been driving recent trends of Hg in biota and predictions of future trends. In particular, future work needs to resolve whether or not Hg recently deposited from the atmosphere is especially prone to methylation and biological uptake in marine systems as seems to be the case in freshwater systems.[38]
Accordingly, future research should focus on:
-
Processes at the ice–ocean interface that permit the entry of deposited Hg into food webs
-
Methylation in sediments and subsequent entry into benthic or pelagic food webs
-
The importance of mercury and methylmercury inputs from rivers and coastal erosion to marine food webs
-
Production of MeHg within the Arctic Ocean’s water column.
-
The removal of Hg from the surface of the interior Arctic Ocean by vertical flux
Research along these lines is essential before realistic modelling of Hg speciation, especially MeHg, and trophic level transfers can be conducted for the Arctic.
Our lack of understanding of the mercury cycle as it impacts food webs is now compounded by an Arctic undergoing rapid change.[20] This change presents a new set of challenges to understanding mercury trends in biota and the underlying cause of mercury exposure in high trophic levels. Accordingly, a second set of questions is clearly relevant to future studies of mercury in the Arctic, and we can propose several promising lines of research. For example, how does the loss of ice affect air-sea interaction, the balance between ice and pelagic food webs, the connectivity of such food webs with benthos, the intensity of primary production and regeneration, and how do hydrological interactions in surface soil horizons affect the production of MeHg? Where higher trophic level biota are involved, we could ask another set of questions such as how do foraging strategy and plasticity in diet affect exposure?[41,42] Most of these questions involve, directly or indirectly, an interaction between the mercury cycle and the carbon cycle.
Is the Arctic especially vulnerable to global Hg emissions? We conclude that the sensitivity of the Arctic Ocean’s ecosystem to Hg lies not so much in the Arctic’s position in the global deposition hierarchy but more in the methylation processes within the Arctic and their vulnerability to climate change.
Acknowledgements
This article is based on a keynote presentation given at the 2009 Goldschmidt conference. We thank A. Green for encouraging us to focus our ideas in this paper, and three anonymous reviewers for providing constructive comments on an earlier version. Both authors appreciate financial support from the Northern Contaminants Program (NCP), ArcticNet and NSERC for a Postdoctoral Fellowship to L. Loseto. The perspectives presented here reflect the influence of a long association with colleagues who have re-written Arctic contaminant science.
[1]
[2]
S. Booth ,
D. Zeller ,
Mercury, food webs, and marine mammals: implications of diet and climate change for human health.
Environ. Health Perspect. 2005
, 113, 521.
|
CAS |
PubMed |

[3]
J. Van Oostdam ,
S. G. Donaldson ,
M. Feely ,
D. Arnold ,
P. Ayotte ,
G. Bondy ,
L. Chan ,
E. Dewaily ,
et al. Human health implications of environmental contaminants in Arctic Canada: a review.
Sci. Total Environ. 2005
, 351–352, 165.
| Crossref | GoogleScholarGoogle Scholar |

[4]
W. H. Schroeder ,
K. G. Anlauf ,
L. A. Barrie ,
J. Y. Lu ,
A. Steffen ,
D. R. Schneeberger ,
T. Berg ,
Arctic springtime depletion of mercury.
Nature 1998
, 394, 331.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[5]
S. E. Lindberg ,
S. Brooks ,
C.-J. Lin ,
K. J. Scott ,
M. S. Landis ,
R. K. Stevens ,
M. Goodsite ,
A. Richter ,
Dynamic oxidation of gaseous mercury in the arctic troposphere at polar sunrise.
Environ. Sci. Technol. 2002
, 36, 1245.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[6]
A. Steffen ,
T. Douglas ,
M. Amyot ,
P. A. Ariya ,
K. Aspmo ,
T. Bergm ,
J. Bottenheim ,
S. Brooks ,
et al. A synthesis of atmospheric mercury depletion event chemistry linking atmosphere, snow and water.
Atmos. Chem. Phys. 2008
, 8, 1445.
|
CAS |

[7]
J. O. Nriagu ,
J. M. Pacyna ,
Quantitative assessment of worldwide contamination of air, water and soils by trace metals.
Nature 1988
, 333, 134.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[8]
W. F. Fitzgerald ,
C. H. Lamborg ,
C. R. Hammerschmidt ,
Marine biogeochemical cycling of mercury.
Chem. Rev. 2007
, 107, 641.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[9]
D. Mergler ,
H. A. Anderson ,
L. H. M. Chan ,
K. R. Mahaffey ,
M. Murray ,
M. Sakamoto ,
A. H. Stern ,
Methylmercury exposure and health effects in humans: a worldwide concern.
Ambio 2007
, 36, 3.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[10]
E. M. Sunderland ,
R. P. Mason ,
Human impacts on open ocean mercury concentrations.
Global Biogeochem. Cycles 2007
, 21, GB4022.
| Crossref | GoogleScholarGoogle Scholar |

[11]
P. M. Outridge ,
R. W. Macdonald ,
F. Wang ,
G. A. Stern ,
A. P. Dastoor ,
A mass balance inventory of mercury in the Arctic Ocean.
Environ. Chem. 2008
, 5, 89.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[12]
M. C. Serreze ,
A. P. Barrett ,
A. G. Slater ,
R. A. Woodgate ,
K. Aagaard ,
R. B. Lammers ,
M. Steele ,
R. Moritz ,
et al. The large-scale freshwater cycle of the Arctic.
J. Geophys. Res. 2006
, 111, C11010.
| Crossref | GoogleScholarGoogle Scholar |

[13]
[14]
E. B. Swain ,
P. M. Jakus ,
G. Rice ,
F. Lupi ,
P. A. Maxson ,
J. M. Pacyna ,
A. Penn ,
S. J. Spiegel ,
et al. Socioeconomic consequences of mercury use and pollution.
Ambio 2007
, 36, 45.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[15]
C. Li ,
J. Cornett ,
S. Willie ,
J. Lam ,
Mercury in Arctic air: the long-term trend.
Sci. Total Environ. 2009
, 407, 2756.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[16]
A. Steffen ,
W. Schroeder ,
R. W. Macdonald ,
L. Poissant ,
A. Konoplev ,
Mercury in the arctic atmosphere: an analysis of eight years of measurements of GEM at Alert (Canada) and a comparison with observations at Amderma (Russia) and Kuujjuarapik (Canada).
Sci. Total Environ. 2005
, 342, 185.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[17]
W. L. Lockhart ,
G. A. Stern ,
R. Wagemann ,
R. V. Hunt ,
D. A. Metner ,
J. DeLaronde ,
B. Dunn ,
R. E. A. Stewart ,
et al. Concentrations and trends of mercury in tissues of beluga whales (Delphinapterus leucas) from the Canadian Arctic from 1981 to 2002.
Sci. Total Environ. 2005
, 351–352, 391.
| Crossref | GoogleScholarGoogle Scholar |

[18]
B. M. Braune ,
Temporal trends of organochlorines and mercury in seabird eggs from the Canadian Arctic, 1975–2003.
Environ. Pollut. 2007
, 148, 599.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[19]
P. M. Outridge ,
H. Sanei ,
G. A. Stern ,
P. B. Hamilton ,
F. Goodarzi ,
Evidence for control of mercury accumulation rates in Canadian high arctic lake sediments by variations in aquatic primary productivity.
Environ. Sci. Technol. 2007
, 41, 5259.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[20]
R. W. Macdonald ,
T. Harner ,
J. Fyfe ,
Recent climate change in the Canadian Arctic and its impact on contaminant pathways and interpretation of temporal trend data.
Sci. Total Environ. 2005
, 342, 5.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[21]
J. Y. Lu ,
W. H. Schroeder ,
L. A. Barrie ,
A. Steffen ,
H. E. Welch ,
K. Martin ,
L. Lockhart ,
R. V. Hunt ,
et al. Magnification of atmospheric mercury deposition to polar regions in springtime: the link to tropospheric ozone depletion chemistry.
Geophys. Res. Lett. 2001
, 28, 3219.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[22]
T. A. Douglas ,
M. Sturm ,
W. R. Simpson ,
J. D. Blum ,
L. Alvarez-Aviles ,
J. J. Keeler ,
D. K. Perovich ,
A. Biswas ,
et al. Influence of snow and ice crystal formation and accumulation on mercury deposition to the Arctic.
Environ. Sci. Technol. 2008
, 42, 1542.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[23]
J. D. Lalonde ,
A. J. Poulain ,
M. Amyot ,
The role of mercury redox reactions in snow on snow to air mercury transfer.
Environ. Sci. Technol. 2002
, 36, 174.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[24]
G. A. Stern ,
R. W. Macdonald ,
Biogeographic provinces of total and methyl mercury in zooplankton and fish from the Beaufort and Chukchi Seas: results from the SHEBA drift.
Environ. Sci. Technol. 2005
, 39, 4707.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[25]
J. L. Kirk ,
V. L. St. Louis ,
M. J. Sharp ,
Rapid reduction and reemission of mercury deposited into snowpacks during atmospheric mercury depletion events at Churchill, Manitoba, Canada.
Environ. Sci. Technol. 2006
, 40, 7590.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[26]
A. J. Poulain ,
S. M. Ní Chadhain ,
P. A. Ariya ,
M. Amyot ,
E. Garcia ,
P. G. C. Campbell ,
G. J. Zylstra ,
T. Barkay ,
Potential for mercury reduction by microbes in the High Arctic.
Appl. Environ. Microbiol. 2007
, 73, 2230.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[27]
M. E. Andersson ,
J. Sommar ,
K. Gardfeldt ,
O. Lindqvist ,
Enhanced concentrations of dissolved gaseous mercury in the surface waters of the Arctic Ocean.
Mar. Chem. 2008
, 110, 190.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[28]
L. L. Loseto ,
D. R. S. Lean ,
S. D. Siciliano ,
Snowmelt sources of mercury in High Arctic ecosystems.
Environ. Sci. Technol. 2004
, 38, 3004.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[29]
R. P. Mason ,
E.-H. Kim ,
J. Cornwell ,
D. Heyes ,
An examination of the factors influencing the flux of mercury, methylmercury and other constituents from estuarine sediment.
Mar. Chem. 2006
, 102, 96.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[30]
N. Ogrinc ,
M. Monperrus ,
J. Kotnik ,
V. Fajon ,
K. Vidimova ,
D. Amouroux ,
D. Kocman ,
E. Tessier ,
et al. Distribution of mercury and methylmercury in deep-sea surficial sediments of the Mediterranean Sea.
Mar. Chem. 2007
, 107, 31.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[31]
A. D. McGuire ,
L. G. Anderson ,
T. R. Christensen ,
S. Dallimore ,
L. Guo ,
D. J. Hayes ,
M. Heimann ,
T. D. Lorenson ,
et al. Sensitivity of the carbon cycle in the Arctic to climate change.
Ecol. Monogr. 2009
, 79, 523.
| Crossref | GoogleScholarGoogle Scholar |

[32]
D. R. Leitch ,
J. Carrie ,
D. Lean ,
R. W. Macdonald ,
G. A. Stern ,
F. Wang ,
The delivery of mercury to the Beaufort Sea of the Arctic Ocean by the Mackenzie River.
Sci. Total Environ. 2007
, 373, 178.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[33]
J. L. Kirk ,
V. L. St. Louis ,
Multiyear total and methyl mercury exports from two major sub-Arctic rivers draining into Hudson Bay.
Environ. Sci. Technol. 2009
, 43, 2254.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[34]
A. Hare ,
G. A. Stern ,
R. W. Macdonald ,
Z. A. Kuzyk ,
F. Wang ,
Contemporary and pre-industrial mass budgets of mercury in the Hudson Bay marine system: the role of lateral sediment recycling transport.
Sci. Total Environ. 2008
, 406, 190.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[35]
F. J. Wrona ,
T. D. Prowse ,
J. D. Reist ,
J. E. Hobbie ,
L. M. J. Levesque ,
R. W. Macdonald ,
W. F. Vincent ,
Effects of ultraviolet radiation and contaminant-related stressors on Arctic freshwater ecosystems.
Ambio 2006
, 35, 388.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[36]
J. Klaminder ,
K. Yoo ,
J. Rydberg ,
R. Giesler ,
An explorative study of mercury export from a thawing palsa mire.
J. Geophys. Res. 2008
, 113, G04034.
| Crossref | GoogleScholarGoogle Scholar |

[37]
V. L. St. Louis ,
J. W. M. Rudd ,
C. A. Kelly ,
R. A. Bodaly ,
M. J. Paterson ,
K. G. Beaty ,
R. H. Hessleiin ,
A. Heyes ,
et al. The rise and fall of mercury methylation in an experimental reservoir.
Environ. Sci. Technol. 2004
, 38, 1348.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[38]
S. R. Harris ,
J. W. M. Rudd ,
M. Amyot ,
C. L. Babiarz ,
K. G. Beaty ,
P. J. Blanchfield ,
R. A. Bodaly ,
B. A. Branfireun ,
et al. Whole-ecosystem study shows rapid fish-mercury response to changes in mercury deposition.
Proc. Natl. Acad. Sci. USA 2007
, 104, 16586.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

[39]
D. Cossa ,
B. Aveerty ,
N. Pirrone ,
The origin of methylmercury in open Mediterranean waters.
Limnol. Oceanogr. 2009
, 54, 837.
|
CAS |

[40]
E. M. Sunderland ,
D. P. Krabbenhoft ,
J. W. Moreau ,
S. A. Strode ,
W. M. Landing ,
Mercury sources, distribution and bioavailability in the North Pacific Ocean: insights from data and models.
Global Biogeochem. Cycles 2009
, 23, GB2010.
| Crossref | GoogleScholarGoogle Scholar |

[41]
L. L. Loseto ,
G. A. Stern ,
D. Deibel ,
T. L. Connelly ,
A. Prokopowicz ,
L. Fortier ,
D. R. S. Lean ,
S. H. Ferguson ,
Linking mercury exposure to habitat and feeding behaviour in Beaufort Sea beluga whales.
J. Mar. Syst. 2008
, 74, 1012.
| Crossref | GoogleScholarGoogle Scholar |

[42]
L. L. Loseto ,
G. A. Stern ,
S. H. Ferguson ,
Size and biomagnification: how habitat selection explains beluga mercury levels.
Environ. Sci. Technol. 2008
, 42, 3982.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[43]
L. Poissant ,
H. H. Zhang ,
J. Canário ,
P. Constant ,
Critical review of mercury fates and contamination in the arctic tundra ecosystem.
Sci. Total Environ. 2008
, 400, 173.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

[44]
C. J. Watras ,
N. S. Bloom ,
Mercury and methylmercury in individual zooplankton: implications for bioaccumulation.
Limnol. Oceanogr. 1992
, 37, 1313.
| Crossref |



