Detection of Fusarium graminearum in wheat grains in Western Australia
D. G. Wright A D , G. J. Thomas A , R. Loughman A , J. Fuso-Nyarko B and S. Bullock CA Department of Agriculture and Food, Western Australia, Locked Bag 4, Bentley Delivery Centre, Perth, WA 6983, Australia.
B WA State Agricultural Biotechnology Centre, School of Biological Sciences and Biotechnology, Murdoch University, Perth, WA 6150, Australia.
C Plant Disease Diagnostic Unit, Royal Botanical Gardens, Sydney, NSW, Australia.
D Corresponding author. Email: dominie.wright@agric.wa.gov.au
Australasian Plant Disease Notes 5(1) 82-84 https://doi.org/10.1071/DN10029
Submitted: 9 July 2010 Accepted: 16 July 2010 Published: 28 July 2010
Abstract
Shrivelled, bleached wheat grain detected by bulk grain handlers during quality checks was submitted to AGWEST Plant Laboratories, Department of Agriculture and Food, Western Australia. Fusarium graminearum was detected. Further testing of the wheat grain delivered to bulk silos found other Fusarium spp. associated with the grain samples. This is the first record of F. graminearum in wheat grains in Western Australia.
Fusarium head blight (FHB) is an important disease in cereal crops causing major economic losses of 20–100% (McMullen et al. 1997; Manning et al. 2000) in both yield reduction and grain quality. FHB can be caused by a complex of Fusarium spp. or by an individual Fusarium sp. The most important species involved is F. graminearum Schwabe. Other Fusarium spp. often associated with the disease include: F. avenaceum (Fr.:Fr.) Sacc; F. culmorum (Wm. G. Sm.) Sacc; F. poae (Peck) Wollenweb; F. pseudograminearum O’Donnell & T. Aoki; F. sporotrichiodes Sherb; and Microdochium nivale (Fr.) Samuels & I.C. Hallett (Dill-Macky 1997, 2010).
The dominant species associated with the disease is dependent upon climatic conditions. F. graminearum is widely distributed and common in tropical, subtropical and warm temperate regions of the world, including USA, Canada, southern and central Europe, China and Japan (Nicholson et. al. 1998; Stack 2000; CABI 2007). However, it tends to be uncommon in cool-temperate regions. In northern Europe, where climatic conditions are cooler, F. avenaceum, F. culmorum and F. poae are the prevalent species (Stack 2000).
In Australia, F. graminearum is the major cause of FHB in Queensland (Qld) and New South Wales (NSW) (Manning et al. 2000); however, F. pseudograminearum has also been associated with FHB in these regions. F. graminearum was first detected in Western Australia (WA) in 1959 on sorghum causing a stalk rot (Shivas 1989). Fusarium spp. including F. graminearum have been reported at low concentrations in weather-stained barley seed in WA (Young and Loughman 2001); however, it had not been previously detected in wheat crops in WA.
Wheat seeds showing unusual discoloration were submitted to the diagnostic service (AGWEST Plant Laboratories) of the Department of Agriculture and Food (DAFWA) in 2004. The seed had been found by a commercial seed handler during standard grain quality checks of grain delivered from the 2003/04 harvest. The grain was shrivelled and quite bleached in colour. In some cases pink staining could be seen on the seed coat (Fig. 1). The seed was tested for the presence of Fusarium spp. using the International Seed Testing Association (ISTA) freeze blotter method for Fusarium (Mathur and Kongsdal 2003). Fusarium graminearum was detected in the sample submitted. The identification of the pathogen was confirmed by the Plant Disease Diagnostics Unit, Royal Botanical Gardens in Sydney, NSW. Cultures have been lodged with DAFWA Culture Collection (WAC 11354).

|
Trace-back identified the sample had originated from a single farm in the south coast region of WA. After the initial detection, further samples of wheat and barley that had originated from that farm were submitted from bulk handlers. The sample size varied from 500 g to 1 kg. Grain and stubble samples were collected from wheat and barley crops from the farm where the grain samples originated. Grain was collected from the on-farm silos and spilled grain lying in the paddock.
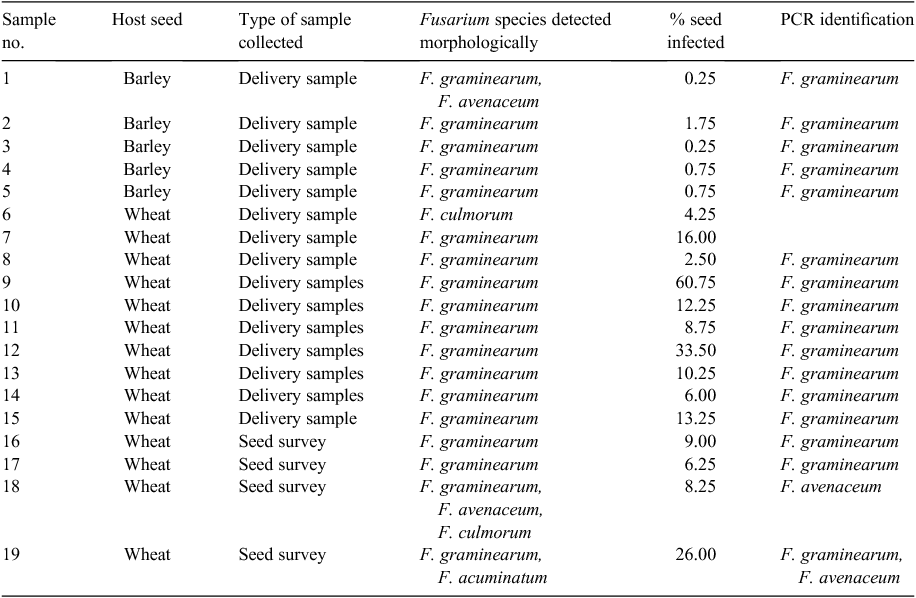
Seed samples were tested using the ISTA freeze blotter method for Fusarium. A 400-seed sample test was done and the number of seed infected with Fusarium was recorded. If Fusarium was detected on the seed, single spore cultures were taken and plated on carnation leaf agar (CLA) and potato dextrose agar (PDA) for morphological identification according to Burgess et al. (1994). Polymerase chain reaction (PCR), using species-specific primers was used where possible to verify the morphological results. The primer sets used were: (a) F.g 16NF and F.g 16NR for F. graminearum (Nicholson et al. 1998); (b) FPG-F, FPG-R (Williams et al. 2002) and Fp1–1 and Fp1–2 (Aoki and O’Donnell 1999) for F. pseudograminearum; and (c) FA-ITSF (20 MER) and FA-ITSR (20 MER) for F. avenaceum (Schilling et al. 1996). F. graminearum was detected in 14/14 wheat and 5/9 barley seed samples collected (Table 1) from the affected area. Levels of infection in seed samples ranged from 0.25% to 60.75%. The variation in the infection level reflects the type of samples collected and submitted for testing. F. avanaceum, F. acuminatum and F. culmorum were also present in four wheat and barley samples tested. The number of white shrivelled seed present in the sample did not correlate with the level of infection detected in the seed sample. In most cases the results from the PCR testing confirmed the species identification of the Fusarium determined morphologically.
|
The stubble collected was examined visually for the presence or absence of perithecia. In these samples, no perithecia were present; however, sporodochia were present. Single-spore cultures were then grown on CLA and on PDA for 4 weeks. Cultures were identified morphologically according to Burgess et al. (1994) and where possible assayed by PCR using the species-specific primers listed above to verify results. F. avenaceum was found to be colonising both wheat and barley heads and barley stubble collected from the ground in the paddock. F. graminearum was not detected on wheat or barley stubble and no other Fusarium spp. were detected from stubble samples collected from the farm.
This is the first report of F. graminearum associated with grain of wheat in WA. F. graminearum and F. pseudograminearum have been previously associated with causing FHB in wheat (Burgess et al. 1987; Akinsanmi et al. 2006) and also causing discoloured grain of wheat in NSW (Klein 1987). The grain symptoms observed in the WA grain sample are consistent with those caused by FHB as described in the literature (Burgess et al. 1987; Dill-Macky 2010). Grain was shrivelled and in some instances very bleached and white in colour. Pink colouration could be seen on some grains, and after testing the grain, F. graminearum was detected. Other Fusarium spp. detected on wheat seed included F. culmorum, F. avenaceum, and F. acuminatum. The level of these detected in the seed samples were at a lower level than F. graminearum detected. F. pseudograminearum was not detected in these seed and stubble samples. Both F. graminearum and F. avenaceum were confirmed on barley seed, although at lower levels than in wheat.
FHB is favoured by rainfall or high humidity during the flowering to grainfill period of crop growth (Stack 2000; Dill-Macky 2010). Weather records for the 2003 season from the affected area indicate that heavy rainfall (68 mm) fell over a 3-day period coinciding with flowering/grainfill of wheat crops, providing conditions conducive for Fusarium infection to occur in the field (Dill-Macky 2010). This infection was then detected in the harvested grain. This finding indicates that Fusarium contamination of wheat and barley grain occurs in WA when conditions are conducive to infection.
Acknowledgements
We would like to thank all regional and metro-based staff of DAFWA involved in the collection of samples during the survey. We would also like to thank all technical staff involved in the processing of samples and the laborious work involved in the identification of the cultures.
Akinsanmi OA,
Backhouse D,
Simpendorfer S, Chakraborty S
(2006) Genetic diversity of Australian Fusarium graminearum and Fusarium pseudograminearum. Plant Pathology 55, 494–504.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Aoki T, O’Donnell K
(1999) Morphological and molecular characterisation of Fusarium pseudograminearum sp. nov., formerly recognised as the Group 1 population of F. graminearum. Mycologia 91, 597–609.
| Crossref | GoogleScholarGoogle Scholar |

Burgess LW,
Klein TA,
Bryden WL, Tobin NF
(1987) Head blight of wheat caused by Fusarium graminearum Group 1 in New South Wales in 1983. Australasian Plant Pathology 16, 72–78.
| Crossref | GoogleScholarGoogle Scholar |

Klein TA
(1987) Fungi associated with discoloured wheat grain in northern New South Wales. Australasian Plant Pathology 16, 69–71.
| Crossref |

McMullen M,
Jones R, Gallenberg D
(1997) Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Disease 81, 1340–1348.
| Crossref | GoogleScholarGoogle Scholar |

Nicholson P,
Simpson DR,
Weston G,
Rezanoor HN,
Lees AK,
Parry DW, Joyce D
(1998) Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiological and Molecular Plant Pathology 53, 17–37.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Schilling A,
Möller EM, Geiger HH
(1996) Molecular differentiation and diagnosis of the cereal pathogens Fusarium culmorum and F. graminearum. Sydowia 48, 71–82.

Shivas R
(1989) Fungal and bacterial diseases of plants in Western Australia. Journal of the Royal Society of Western Australia 72, 1–62.

Williams KJ,
Dennis JI,
Smyl C, Hallwork H
(2002) The application of species-specific assays based on the polymerase chain reaction to analyse Fusarium crown rot of durum wheat. Australasian Plant Pathology 31, 119–127.
| Crossref | GoogleScholarGoogle Scholar |



