Production losses caused by red leather leaf in hay and milling oats
Rama Harinath Reddy Dadu A * , Mark S. McLean B and Grant J. Hollaway C
A * , Mark S. McLean B and Grant J. Hollaway C
A
B
C
Abstract
Red leather leaf (RLL), caused by Neospermospora avenae, is a common foliar disease of oats in south-eastern Australia but its impact on hay and grain yield and quality is unknown.
We aimed to determine the effect of RLL on grain yield and quality of commercial milling oat varieties, and biomass and nutrition of hay oat varieties, with a range of host plant resistance responses by comparing fungicide and disease treatments.
A total of eight field experiments were conducted in 2019 and 2020; four experiments each investigated the effects of RLL on hay or milling oats. In each experiment, six or seven oat varieties were sown with a disease or fungicide imposed. RLL severity was estimated visually for each experiment. Biomass yield and quality traits at early milk development stage were measured for hay oat experiments; and grain yield and quality for the milling oat experiments.
RLL severity (% leaf area affected: 0–44% in hay oats and 2–61% in milling oats) varied with the season, varietal resistance, and treatments. RLL caused significant losses in most experiments, with reductions in biomass of up to 3.5 t/ha (22%) in hay oats and grain yield of up to 1.1 t/ha (21%) in milling oats.
RLL can be a severe foliar disease of oats in south-eastern Australia and cause significant losses in both hay and milling oat crops.
Growing varieties with resistance can effectively reduce potential loss due to RLL and growers are required to adapt fungicide strategies reflecting seasonal conditions and product type (grain or hay).
Keywords: foliar disease, host plant resistance, management, oat, production loss, red leather leaf, stubble-borne, yield.
Introduction
Red leather leaf (RLL) is a foliar disease of oats (Avena sativa) caused by Neospermospora avenae (previously known as Pseudodiscosia avenae (Sprague and Johnson 1936) and Spermospora avenae (Sprague 1951)). RLL was first identified on oats in 1932 in North America (Sprague and Johnson 1936) and has since been identified in Turkey (1943), New Zealand (1966), Germany (1969), Australia (1978), and Ireland (1988) (Bremer 1943; Latch 1966; Schlösser 1969; Pascoe and Woodcock 1981; Cunningham 1990; Murray 2007). To date, RLL has been considered a minor issue for oat production globally and only few studies have been done on its epidemiology, associated crop losses, and management (Clifford 1995; Murray 2007). The demand for oat grains and fodder has increased significantly during the past decade with production, and exports of oat grain and hay from Australia increasing by more than 59%, from 0.92 Mt in 1994 to 2.3 Mt in 2017 (FAOSTAT 2021). More information is required on the impacts of RLL on grain and biomass yield, and the quality of oat in south-eastern Australia, where the crop is common.
RLL is known to cause damage to oat crops where environmental conditions are conducive. Severe infections in Europe cause stunting, leaf discolouration and some yield losses (Cunningham 1990). Although these losses have not been quantified, greater RLL severity has been observed in regions with greater rainfall (Sprague 1939) and during the wetter winter months (Murray 2007). In Australia, RLL is known to be present in south-eastern Australia and common in the medium to high rainfall zones (~410–615 mm average annual rainfall) (Collis 2020). These observations were consistent with findings of a phylogenetic study in which N. avenae was genetically similar to Rynchsporium commune, which causes scald of barley (Hordeum vulgare) (Crous et al. 2021). Scald is also found to be severe in medium and high rainfall regions and is favoured by cool (5–16°C) temperatures and wet conditions (Davis and Fitt 1994).
RLL is spread by conidia, most likely from infected stubble and seed (Crous et al. 2021). Primary infection of an oat crop occurs when wet and cool conditions are present, with lesions appearing as water soaked or blue green areas surrounded by a broad chlorotic zone. Lesions grow in size, become necrotic, reddish brown and leathery textured. Lesions sometimes disintegrate to form holes in the centre and leaf margins and tips may die prematurely (Murray 2007). Conidia are produced within necrotic lesions and serve as secondary inoculum, causing infection in the upper canopy (Chambers and Thomas 2020).
Host-plant resistance is an essential component of integrated disease management of cereal foliar diseases, reducing the risk of production losses and reliance on fungicides for control of many foliar pathogens in cereal crops. Effective host plant resistance has been documented in oat varieties toward rusts and septoria leaf blotch (caused by Phaeosphaeria avenaria) (Barr 1994) but little is known about the host plant resistance to RLL in oats, although it is present in some varieties. Host plant resistance to RLL in oats was first reported by Sprague (1939) where less severe RLL was observed in the variety Grey Winter. Cunningham (1990) also observed that some varieties grown in Ireland had less severe RLL, and Clifford (1995) noted that some varieties were affected but others were not. In Australia, oat cultivars are rated for their disease expression in the presence of RLL on a nine-point scale; from resistant (R) through to very susceptible (VS) with differences among commercial cultivars (Hollaway and McLean 2022). The underlying resistance mechanism within these varieties remains unknown. But the limited field testing conducted in Australia through national varietal trials (NVT) (Hollaway and McLean 2022) revealed that most of these varieties appear to have partial resistance, with few potentially harbouring major sources of host-plant resistance. The actual losses associated with different reaction responses, however, have not been studied.
Fungicides are used in oat crops for control of foliar diseases such as septoria leaf blotch, stem rust (caused by Puccinia graminis f. sp. avenae) and crown rust (caused by Puccinia coronata). Foliar applied fungicides can provide effective suppression of these diseases and associated grain yield increases (Clark et al. 1975; Barr 1994). However, information is limited on the effectiveness of fungicides for control of RLL. One study reported that fungicides applied as a single application or mixtures of fenpropidin, fenpropimorph and propiconazole suppressed RLL (Cunningham 1990). Another study reported that triazoles suppressed sporulation (Clifford 1995). No published information is available on efficacy of other fungicide chemistries.
RLL has the potential to cause significant production losses in oats and affect the export industry under conditions favourable to disease development. Information is required on the potential grain and hay yield, and quality losses to determine the risk of production loss, and the appropriate management strategies needed for control. This study aimed to determine the effect of RLL on grain yield and quality of current commercial milling oats, and biomass and nutritional losses in hay oats varying in host plant resistance by comparing fungicide and disease treatments.
Methods
Site details
In both 2019 and 2020, field experiments were conducted at two sites. At each site in both years, one hay and one milling oat experiment was conducted for a total of eight experiments. Experiments were at Inverleigh (E144°05.657′, S38°17.369′) in the high rainfall zone (the arable areas where annual rainfall is between 500 and 900 mm in south-eastern Australia) in 2019 and 2020 and in the medium rainfall zone (the arable areas where annual rainfall is between 300 and 500 mm), Longerenong (E142°29.494′, S36°67.180′) in 2019 and Vectis (E142°11.712′, S36°73.947′) in 2020. All the sites were characterised by grey vertisol soil. Average annual rainfall and temperature differed between sites. Long-term average (1960–2020) growing season rainfall (GSR) (April–November) was 406 mm at Inverleigh, 345 mm at Vectis and 313 mm at Longerenong. Average long-term growing season maximum temperatures (GST) were 11.6–19.7°C at Inverleigh, 13.6–26.1°C at Vectis and 13.2–24.8°C at Longerenong.
Oat varieties
For all experiments, the selected varieties were grown commercially in south-eastern Australia with different RLL resistance, phenology, and maturity (Table 1). For the hay oat experiments, six varieties were grown in all experiments and an additional variety, Tungoo, was included in the Vectis and Longerenong experiments as it is adapted only to the medium rainfall zone. For the milling oat experiments, six varieties were sown. Williams and Yallara are dual purpose varieties and were included in both hay and milling experiments.
Experiment design and establishment
All experiments had two treatments applied. (1) Disease, which had ~500 g of oat stubble infected with RLL applied to each plot evenly. (2) Fungicide, which had multiple foliar fungicide applications during the season to supress RLL. The RLL infected stubble was sourced from disease blocks of a single susceptible variety (Mitika), naturally infected with N. avenae grown in the preceding year at Vectis was harvested, used to spread across all the trials at Vectis, Longerenong and Inverleigh so that the resistance expression within the varieties across the locations and years is independent of phenotypic and/or genotypic changes within the pathogen. At each site, the infected stubble was spread around 3 weeks from sowing. In the fungicide treatment, foliar applications of 500 mL/ha of propiconazole (625 g of active ingredient/L) were made using a tractor mounted boom sprayer at Z25 (mid tillering), Z31 (early stem elongation) and Z39 (flag leaf emergence) crop growth stages (Zadoks et al. 1974). There were no fungicides registered in Australia to control RLL at the time of experiments. Hence, propiconazole, which was commonly applied to control other diseases in oats such as rusts and septoria leaf blotch, was used in these experiments to evaluate its efficacy against RLL. Varieties and treatments were arranged in complete randomised block designs where all variety × treatment combinations were replicated once in each of the six blocks. At Vectis and Longerenong, plots were 8 m × 1.8 m with six rows at 30 cm row spacing. At Inverleigh, plots were 10 m long and 2 m wide with eight rows at 22.5 cm row spacing. At all sites, wheat plots of the same dimensions as the oat plots were sown (Table 2) in parallel to minimise disease spread between two oat plots. Hay and milling experiments were sown at 150 and 190 seeds per m2 respectively. At Vectis and Longerenong, 50 kg/ha of urea (N: 46%) and 70 kg/ha of Mono-Ammonium Phosphate (MAP; N: 10%, P: 22%, S: 1.5%, Ca: 1.6%) fertiliser was applied at sowing. At Inverleigh, 130 kg/ha Urea and 100 kg/ha MAP fertiliser were applied at sowing. Herbicides were applied according to recommendations for the local area to control grass and broad leaf weeds. Prior to sowing, the seed of all varieties was treated with tebuconazole (25 g a.i./L) at 100 mL/100 kg of seed to control smut disease.
| Medium rainfall zone | High rainfall zone | ||||
|---|---|---|---|---|---|
| Vectis | Longerenong | Inverleigh | Inverleigh | ||
| 2019 | 2020 | 2019 | 2020 | ||
| Milling oat experiments | |||||
| Sowing date | 14 May 2019 | 28 April 2020 | 6 May 2020 | 29 April 2020 | |
| Harvest date | 10 December 2019 | 15 December 2020 | 10 December 2019 | 14 December 2020 | |
| Hay oat experiments | |||||
| Sowing date | 14 May 2019 | 28 April 2020 | 6 May 2019 | 5 May 2020 | |
| Biomass cut date | 1 November 2019 A | 14 October 2020 | 21 October 2019 and 4 November 2019 B | 27 October 2020 and 17 November 2020 | |
Disease severity, biomass and grain yield and quality
For all experiments, RLL severity was assessed by visually estimating the percentage leaf area affected (%LAA) by disease on three to five occasions during the growing season. Assessments targeted the stem elongation (Z30-2), flag leaf emergence (Z37-9), ear emergence (Z51-5) and flowering stages (Z61-9) of crop development. From early stem elongation to head emergence stages, RLL severity of whole plots (%LAA) was measured on the centre three or four rows for each plot. After head emergence and until grain fill stages, %LAA of the top four leaves (flag to flag ‒3) from 10 randomly sampled main stems in each plot were estimated. Leaves were not assessed if senesced. For hay oat experiments at Vectis, a single assessment was done for each plot at flowering to estimate total plant area affected by RLL. Minor incidences of bacterial blight disease were present in all the trials at Inverleigh in both the seasons and in both the milling and hay oat trials at Longerenong during 2020. However, the disease levels were low and did not confound with grain or hay yield estimates.
For hay oat experiments, plant height was measured at maturity for three plants within each plot by measuring from soil surface to the base of the ear. Hay cuts were made at two locations in each plot and for each variety (except Forester at Vectis and Longerenong) at the start of milk-dough development for hay yield and quality analysis (Table 2). Forester at Vectis (2019) and Longerenong (2020) was cut at late flowering (Z65). For each cut, two internal rows, 1 m in length starting 15 cm above the ground were cut using hand-held shears. The samples were dried for ~3 days at 70°C and dry weight measured. Stem thickness and plant height were measured at the Vectis and Longerenong sites, but not Inverleigh. Stem thickness was assessed differently across the two seasons. In 2020, 10 dried stems from each plot were flattened between the first and second nodes and diameter measured with digital callipers. In 2019, stems were not flattened prior to diameter being measured with digital callipers. Near infrared spectroscopy (NIR) analysis of hay protein and digestibility were done at the South Australia Research and Development Institute (SARDI), Waite Campus, South Australia. Traits included digestibility, dry matter percent (DM), acid detergent fibre (ADF), neutral detergent fibre (NDF), lignin content, potassium percent, crude protein (CP) and water-soluble carbohydrates (WSC).
For milling oat experiments, grain was harvested at maturity from each plot with a plot harvester and each plot weighed individually at 10% moisture content to determine grain yield (Table 2). Subsamples were retained and tested for grain quality measurements of screenings (percentage of grain less than 2.2 mm in width), retention (percentage of grain greater than 2.5 mm in width) and 1000 grain weight.
Statistical analysis
RLL severity, biomass yield, grain yield and quality of each experiment were estimated using the following linear mixed model (LMM):
where y is the vector of observations such as disease severity, biomass yield, grain yield or quality, τ is the vector of treatment by variety fixed effects with associated design matrix X. u is the vector of random block and plot effects with design matrix Z (Gilmour et al. 1997) and e is the residual error. The residual error was assumed to be normally distributed and independent from random effects (Norman et al. 2017). All models were diagnostically tested to ensure that the assumptions of LMM, normality and homoscedasticity are satisfied. Logit transformation was applied where the assumptions were violated. Fisher’s protected least significance of difference (l.s.d.) test at 5% significance level was used to perform possible pairwise comparisons between means. All analysis were performed in GENSTAT (18th Edition; VSN International, Hemel Hempstead, UK).
Multi-site analysis of milling oat experiments to determine the global statistical differences between four experiments were estimated using the below LMM:
where y is the vector of observations such as disease severity or grain yield of all experiments together, β is the vector of fixed effects including experiment, treatment, and variety main and interaction effects with associated design matrix X. α is the vector of random experimental blocking structure (replicate, range and row) effects with associated design matrix Z. e is the residual error partitioned by experiments, where experiments are a combination of site and year. Residual errors were assumed to be normally distributed with a mean zero and independent among trials, with a separate trial error variance and separable first-order autoregressive process (AR1 ⊗ AR1) in both the Row and Column directions. This aimed to account for spatial correlation among the neighbouring plots.
The models were diagnostically tested to ensure that the assumptions of LMM, normality and homoscedasticity are satisfied, and where appropriate, were refitted with traits transformed. Conditional Wald tests were used to infer the significance of the fixed terms. The model was fitted with ASReml-R package ver. 4.3.0 (Butler et al. 2017) in the R statistical computing environment (R Core Team 2023). Multi-site analysis of hay oats was not performed because of different varieties used at Inverleigh, Vectis and Longerenong.
Results
Hay oat experiments
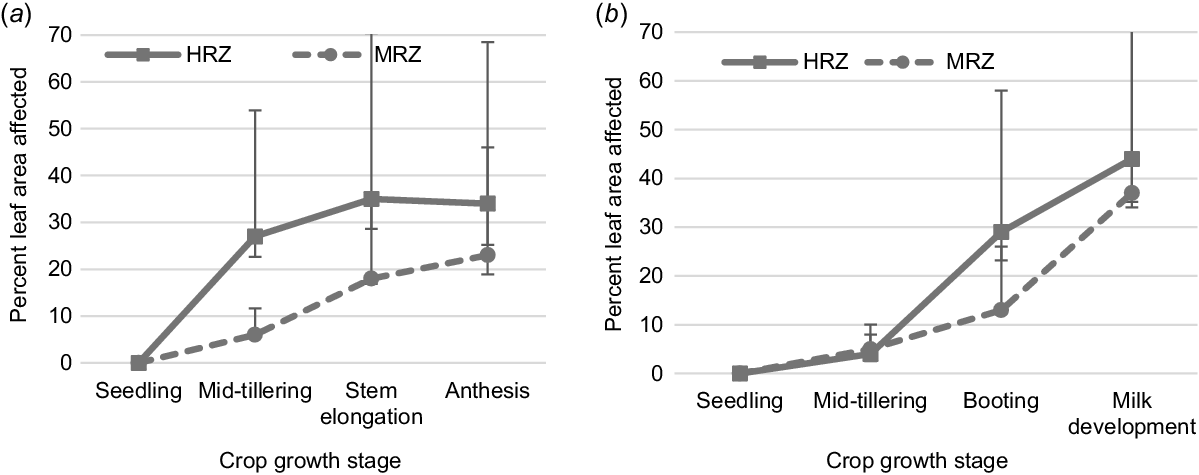
RLL severity varied between sites, varieties, and treatments, which was related to rainfall and temperature (Tables 3, 4 and Supplementary Table S1). RLL severity was lower at Vectis than Inverleigh during both seasons (Fig. 1), which was associated with warmer temperatures and less rainfall at Vectis (Table 3). In general, RLL severity was related to varietal resistance ratings at both sites and years, except for Wintaroo (MRMS) which had equal or higher RLL severity than other susceptible rated varieties at three of the four sites. RLL severity was found less for the fungicide treatment compared to the disease treatments in most cases, except for Tungoo (MRMS) in 2019 (Vectis), 2020 (Longerenong), Yallara (S) in 2019 at Vectis, and Forester (MRMS) in both seasons at Inverleigh. Suppression of RLL by fungicide treatment was greater during 2020 at both sites compared to 2019, with average reductions of up to 19% in RLL severity in 2020 compared to 10% during 2019 when measured between flowering and milk development growth stages.
| Year | Location | Growing season (April–November) | Red leather leaf severity (% leaf area affected) of Mulgara (S) treated with infected stubble during anthesis (Z61) | ||||
|---|---|---|---|---|---|---|---|
| Total rainfall (mm) | Spring (September– November) rainfall (mm) | Mean number of rain days/monthA | Mean maximum daily temperature (°C) | ||||
| 2019 | Vectis (MRZ) | 254.0 | 57.7 | 13 | 18.6 | 23 | |
| Inverleigh (HRZ) | 442.6 | 126.2 | 18 | 15.5 | 34 | ||
| 2020 | Longerenong (MRZ) | 320.4 | 155.0 | 13 | 18.3 | 37 | |
| Inverleigh (HRZ) | 479.2 | 223.6 | 13 | 15.4 | 44 | ||
| Variety | Mean disease severity (percent leaf area affected) logit transformation values in parenthesisA | |||||
|---|---|---|---|---|---|---|
| RatingB | Medium rainfall zone | High rainfall zone | ||||
| Vectis 2019 | Longerenong 2020 | Inverleigh 2019 | Inverleigh 2020 | |||
| 11 October, Z61 | 15 October, Z75 | 18 October, Z61 | 16 October, Z71 | |||
| Disease treatment | ||||||
| Forester | MRMS | –C | 8.7c (−2.4) | 1.6a (−4.3) | 0a (−1.1) | |
| Tungoo | MRMS | 6.1a (−2.7) | 3.3ab (−3.4) | –D | – | |
| Williams | MRMS | 23.0ef (−1.2) | 20.5d (−1.4) | 23.0e (−1.2) | 19.8d (−1.4) | |
| Brusher | MS | 18.8de (−1.5) | 29.3f (−0.9) | 20.5de (−1.4) | 13.2c (−2.0) | |
| Mulgara | S | 23.0ef (−1.2) | 36.7g (−0.5) | 34.5f (−0.7) | 43.7f (−0.2) | |
| Yallara | S | 21.8ef (−1.3) | 24.8e (−1.1) | 40.4g (−0.4) | 35.7e (−0.6) | |
| Wintaroo | S | 26.7f (−1.0) | 30.2f (−0.8) | 16.7cd (−1.6) | 20.9d (−1.3) | |
| Fungicide treatment | ||||||
| Forester | MRMS | – | 5.7b (−2.8) | 2.3a (−4.2) | 0a (−3.8) | |
| Tungoo | MRMS | 8.8ab (−2.4) | 2.7a (−3.6) | – | – | |
| Williams | MRMS | 11.5bc (−2.0) | 3.7ab (−3.3) | 17.1cd (−1.6) | 2.9ab (−3.2) | |
| Brusher | MS | 13.3bc (−1.9) | 10.0c (−2.2) | 8.7b (−2.4) | 0.6a (−4.0) | |
| Mulgara | S | 16.1cd (−1.7) | 2.2a (−3.8) | 14.8c (−1.8) | 6.8b (−2.8) | |
| Yallara | S | 18.5de (−1.5) | 4.0ab (−3.2) | 19.1cde (−1.5) | 1.3a (−4.7) | |
| Wintaroo | S | 9.0ab (−2.4) | 3.3ab (−3.4) | 4.1ab (−3.2) | 1.2a (−4.8) | |
| Variety | ||||||
| P | <0.001 | <0.001 | <0.001 | <0.001 | ||
| l.s.d. (0.05) | 0.26 | 0.20 | 0.36 | 0.50 | ||
| Treatment | ||||||
| P | <0.001 | <0.001 | <0.001 | <0.001 | ||
| l.s.d. (0.05) | 0.14 | 0.11 | 0.21 | 0.29 | ||
| Variety × Treatment | ||||||
| P | <0.001 | <0.001 | <0.001 | <0.001 | ||
| l.s.d. (0.05) | 0.37 | 0.29 | 0.51 | 0.71 | ||
Variety and treatment choice had impacts on plant biomass (dry weight) in all experiments but the interaction effect of variety and treatment was only significant in two of the four experiments (Table 5). RLL impacted biomass in both the experiments conducted at Longerenong (P = 0.004) and Inverleigh in 2020 (P = 0.045), but not in 2019. Reductions varied between the two experiments and varieties. On average, losses were greatest at the Inverleigh site with reductions of up to 3.2 t/ha (22%) in Yallara (S) (Table 6). Reductions were also found to be related to variety susceptiblity rating at Inverleigh but were not at Longerenong. Two MRMS rated varieties Forester and Williams had biomass loss of 0.9 t/ha each and two susceptible varieties Brusher (MS) and Yallara (S) did not have losses due to RLL at Longerenong. Only Wintaroo (S) and Mulgara (S) had losses of up to 3.1 and 0.8–2.5 t/ha respectively, at both the sites.
| Variety | Biomass yield (t/ha)A | |||||
|---|---|---|---|---|---|---|
| RatingB | Medium rainfall zone | High rainfall zone | ||||
| Vectis 2019 | Longerenong 2020 | Inverleigh 2019 | Inverleigh 2020 | |||
| Disease treatment | ||||||
| ForesterC | MRMS | 6.9 | 5.9a | 16.2 | 13.9efgh | |
| Tungoo | MRMS | 7.3 | 7.1cde | –D | – | |
| Williams | MRMS | 6.9 | 6.5abc | 15.0 | 13.0cde | |
| Brusher | MS | 7.2 | 7.0cde | 16.7 | 12.3bcd | |
| Mulgara | S | 7.9 | 7.0cde | 13.8 | 10.9a | |
| Yallara | S | 7.4 | 7.2def | 14.1 | 11.5ab | |
| Wintaroo | S | 6.7 | 6.4abc | 14.7 | 11.9abc | |
| Fungicide treatment | ||||||
| Forester | MRMS | 7.5 | 6.8bcde | 16.0 | 14.9gh | |
| Tungoo | MRMS | 7.3 | 6.7bcde | – | – | |
| Williams | MRMS | 7.0 | 7.4ef | 15.4 | 13.7defg | |
| Brusher | MS | 7.5 | 7.3ef | 16.8 | 14.6fgh | |
| Mulgara | S | 7.9 | 7.8f | 15.7 | 13.4def | |
| Yallara | S | 7.8 | 7.1cdef | 15.3 | 14.7fgh | |
| Wintaroo | S | 7.4 | 7.2def | 16.8 | 15.0h | |
| Variety | ||||||
| P | <0.001 | 0.002 | 0.001 | <0.001 | ||
| l.s.d. (0.05) | 0.41 | 0.48 | 1.4 | 0.96 | ||
| Treatment | ||||||
| P | 0.006 | 0.009 | 0.003 | <0.001 | ||
| l.s.d. (0.05) | 0.22 | 0.26 | 0.6 | 0.56 | ||
| Variety × Treatment | ||||||
| P | 0.501 | 0.004 | 0.127 | 0.045 | ||
| l.s.d. (0.05) | n.s. | 0.69 | n.s. | 1.36 | ||
| Variety | Rating | Medium rainfall zone | High rainfall zone | |||
|---|---|---|---|---|---|---|
| Vectis 2019 | Longerenong 2020 | Inverleigh 2019 | Inverleigh 2020 | |||
| Biomass difference between treatments (% Loss)A | ||||||
| Forester | MRMS | 0.7 (9)B | 0.9 (13) | −0.2 (−1)B | 1 (7)B | |
| Tungoo | MRMS | 0.0 (0)B | −0.4 (−6)B | – | – | |
| Williams | MRMS | 0.1 (1)B | 0.9 (12) | 0.4 (3)B | 0.7 (5)B | |
| Brusher | MS | 0.3 (4)B | 0.3 (4)B | 0.1 (1)B | 2.3 (16) | |
| Mulgara | S | 0.0 (0)B | 0.8 (10) | 1.9 (12)B | 2.5 (9) | |
| Yallara | S | 0.3 (4)B | −0.1 (−1)B | 1.2 (8)B | 3.2 (22) | |
| Wintaroo | S | 0.7 (10)B | 0.8 (11) | 1.9 (13)B | 3.1 (21) | |
Stem thickness was not affected by RLL at either Vectis or Longerenong but plant height was affected by RLL at Vectis during 2019 (P = 0.024) (Table 7). Plant height was reduced in three varieties, Williams (MRMS), Mulgara (S) and Yallara (S). This was not related to variety susceptibility rating.
| Variety | RatingA | Stem thickness (mm)B , C | Plant height (cm) | |||
|---|---|---|---|---|---|---|
| Vectis 2019 | Longerenong 2020 | Vectis 2019 | Longerenong 2020 | |||
| Disease treatment | ||||||
| Forester | MRMS | 3.7 | 1.1 | 82.8cdef | 116.8 | |
| Tungoo | MRMS | 3.7 | 1.1 | 80.5bcd | 90.6 | |
| Williams | MRMS | 4.3 | 1.4 | 71.9a | 94.4 | |
| Brusher | MS | 3.8 | 1.1 | 84.0def | 93.8 | |
| Mulgara | S | 4.1 | 1.3 | 93.8h | 102.6 | |
| Yallara | S | 3.7 | 1.2 | 79.5bc | 80.8 | |
| Wintaroo | S | 4.1 | 1.0 | 84.8efg | 98.4 | |
| Fungicide treatment | ||||||
| Forester | MRMS | 4.0 | 1.1 | 81.0bcd | 116.8 | |
| Tungoo | MRMS | 3.9 | 1.1 | 81.5bcde | 89.4 | |
| Williams | MRMS | 4.7 | 1.4 | 78.7b | 74.1 | |
| Brusher | MS | 3.8 | 1.2 | 85.3fg | 94.0 | |
| Mulgara | S | 4.3 | 1.4 | 99.1i | 102.3 | |
| Yallara | S | 4.2 | 1.2 | 84.0defg | 79.6 | |
| Wintaroo | S | 4.2 | 1.1 | 87.7g | 74.1 | |
| Variety | ||||||
| P | <0.001 | <0.001 | <0.001 | <0.001 | ||
| l.s.d. (0.05) | 0.19 | 0.09 | 2.55 | 2.57 | ||
| Treatment | ||||||
| P | <0.001 | 0.08 | <0.001 | 0.167 | ||
| l.s.d. (0.05) | 0.1 | n.s. | 1.36 | n.s. | ||
| Variety × Treatment | ||||||
| P | 0.221 | 0.63 | 0.024 | 0.461 | ||
| l.s.d. (0.05) | n.s. | n.s. | 3.61 | n.s. | ||
There were no significant differences in hay quality measurements of digestibility, dry matter percent (DM), acid detergent fibre (ADF), neutral detergent fibre (NDF), lignin content, potassium percent, crude protein (CP) and water-soluble carbohydrates (WSC) when the disease and fungicide treatments were compared for any variety in any experiment (data not shown).
Milling oat experiments
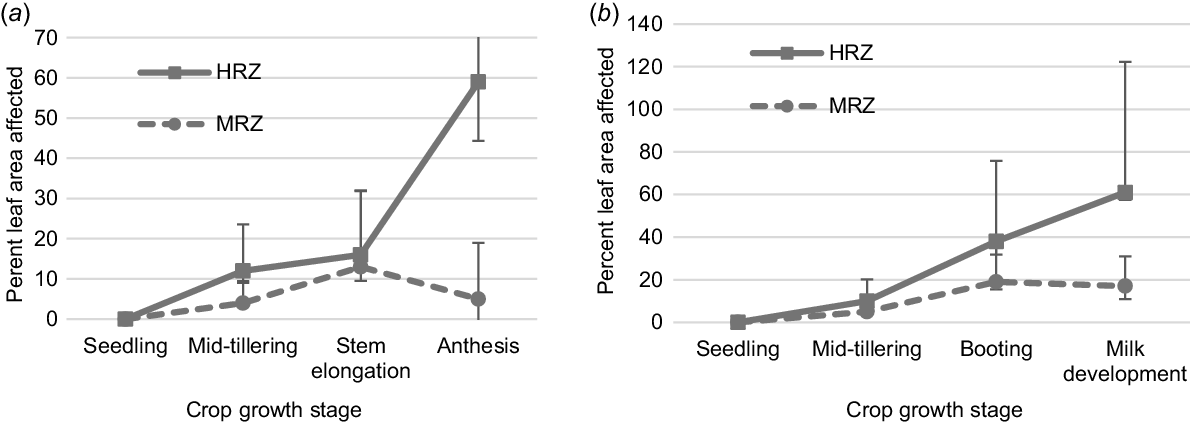
RLL severity varied between experiments, varieties and treatments (Tables 8, S2 and S3). There was no significant effect of RLL at Vectis (P = 0.175). RLL was more severe at the Inverleigh sites compared to the Vectis and Longerenong sites (Fig. 2). At Inverleigh, RLL severity was similar for disease treatments in both seasons. RLL severity was greater in susceptible varieties Mitika and Kowari in both the seasons and Yallara in 2019. Williams (MRMS) had less disease. RLL severity of Bannister (MS) and Bilby (S) did not correspond to their ratings. At Longerenong, RLL severity was not related to varietal resistance and Bannister (MS) had a significantly higher disease compared to other varieties.
| Mean disease severity (percent leaf area affected) logit transformation values in parenthesisA | ||||||
|---|---|---|---|---|---|---|
| Variety | RatingB | Medium rainfall zone | High rainfall zone | |||
| Vectis 2019 | Longerenong 2020 | Inverleigh 2019 | Inverleigh 2020 | |||
| 14th October, Z65 | 29th September, Z69 | 16th October, Z71 | 15th October, Z73 | |||
| Disease treatment | ||||||
| Williams | MRMS | 2.3 | 11.2de (−2.1) | 21.7b | 24.7bc | |
| Bannister | MS | 4.2 | 18.6f (−1.5) | 39.0cd | 42.8d | |
| Bilby | S | 6.0 | 9.5cd (−2.4) | 19.0ab | 29.9c | |
| Yallara | S | 5.6 | 13.8de (−1.9) | 48.9de | 32.6c | |
| Mitika | S | 6.8 | 11.1de (−2.2) | 56.4e | 53.5e | |
| Kowari | S | 5.0 | 14.0e (−1.9) | 58.9e | 61.3e | |
| Fungicide treatment | ||||||
| Williams | MRMS | 1.8 | 1.8a (−4.0) | 21.9b | 6.9a | |
| Bannister | MS | 2.1 | 2.5ab (−3.7) | 10.4a | 15.6ab | |
| Bilby | S | 2.7 | 2.5ab (−3.7) | 9.8a | 8.8a | |
| Yallara | S | 3.3 | 3.8ab (−3.3) | 18.9ab | 10.6a | |
| Mitika | S | 3.4 | 6.3bc (−2.7) | 32.7c | 15.4a | |
| Kowari | S | 4.4 | 5.0abc (−2.9) | 32.2c | 25.6c | |
| Variety | ||||||
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| l.s.d. (0.05) | 1.43 | 0.35 | 7.23 | 6.57 | ||
| Treatment | ||||||
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| l.s.d. (0.05) | 0.82 | 0.20 | 4.17 | 3.79 | ||
| Variety × Treatment | ||||||
| P-value | 0.175 | <0.001 | <0.001 | 0.013 | ||
| l.s.d. (0.05) | n.s. | 0.50 | 10.22 | 9.3 | ||
Grain yield varied between experiments (Table S4). RLL caused grain yield losses in both the experiments conducted in 2020 (Longerenong, P < 0.001; Inverleigh, P = 0.03) but not in 2019 (Table 9). RLL severity was comparatively similar and higher at Inverleigh in 2019 than at Inverleigh and Longerenong during 2020, however, reductions in grain yield were not measured (Vectis, P = 0.148; Inverleigh, P = 0.162). During 2020, losses varied between varieties and sites and all six oat varieties measured grain yield loss. Some losses were related to RLL resistance rating, with Williams (MRMS) having the lowest loss of 0.3 t/ha at Longerenong and second lowest loss of 0.6 t/ha at Inverleigh (Table 10). At Longerenong, Bannister (MS) and Bilby (S) had the greatest losses of 1.0 t/ha, whereas susceptible varieties, Mitika, Kowari and Yallara had losses of 0.7–0.9 t/ha. At Inverleigh, Bilby (S) had the lowest loss of 0.5 t/ha, and Kowari (S) had the greatest loss of 1.1 t/ha. Yallara (S), Bannister (MS) and Mitika (S) had losses of 0.7–0.9 t/ha.
| Variety | Grain yield (t/ha)A | |||||
|---|---|---|---|---|---|---|
| RatingB | Medium rainfall zone | High rainfall zone | ||||
| Vectis 2019 | Longerenong 2020 | Inverleigh 2019 | Inverleigh 2020 | |||
| Disease treatment | ||||||
| Williams | MRMS | 3.8 | 5.5f (−2.9) | 5.5 | 7.6g | |
| Bannister | MS | 3.9 | 5.1d (−2.9) | 4.9 | 7.3g | |
| Bilby | S | 3.5 | 3.8a (−3.2) | 3.6 | 4.9a | |
| Yallara | S | 3.3 | 3.7a (−3.2) | 3.8 | 5.3b | |
| Mitika | S | 4.0 | 4.6c (−3.0) | 3.7 | 5.4b | |
| Kowari | S | 4.2 | 4.4b (−3.1) | 4.6 | 5.7c | |
| Fungicide treatment | ||||||
| Williams | MRMS | 4.2 | 5.8g (−2.8) | 5.5 | 8.2h | |
| Bannister | MS | 4.2 | 6.1h (−2.7) | 5.4 | 8.1h | |
| Bilby | S | 3.9 | 4.8c (−3.0) | 4.1 | 5.4b | |
| Yallara | S | 3.8 | 4.6c (−3.0) | 4.2 | 6.0d | |
| Mitika | S | 4.4 | 5.3e (−2.9) | 4.4 | 6.3e | |
| Kowari | S | 4.2 | 5.2de (−2.9) | 5.1 | 6.8f | |
| Variety | ||||||
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| l.s.d. (0.05) | 0.22 | 0.02 | 0.27 | 0.18 | ||
| Treatment | ||||||
| P-value | <0.001 | <0.001 | <0.001 | <0.01 | ||
| l.s.d. (0.05) | 0.12 | 0.01 | 0.15 | 0.11 | ||
| Variety × Treatment | ||||||
| P-value | 0.148 | <0.001 | 0.162 | 0.03 | ||
| l.s.d. (0.05) | n.s. | 0.03 | n.s. | 0.26 | ||
| Variety | Rating | Medium rainfall zone | High rainfall zone | |||
|---|---|---|---|---|---|---|
| Vectis 2019 | Longerenong 2020 | Inverleigh 2019 | Inverleigh 2020 | |||
| Grain yield difference between treatments (% Loss)A | ||||||
| Williams | MRMS | 0.4 (9)B | 0.3 (5) | 0.0 (0)B | 0.6 (7) | |
| Bannister | MS | 0.2 (5)B | 1.0 (16) | 0.5 (7)B | 0.8 (10) | |
| Bilby | S | 0.2 (5)B | 1.0 (21) | 0.3 (7)B | 0.5 (9) | |
| Yallara | S | 0.5 (13)B | 0.9 (20) | 0.2 (5)B | 0.7 (12) | |
| Mitika | S | 0.4 (6)B | 0.7 (15) | 0.7 (16)B | 0.9 (10) | |
| Kowari | S | 0.0 (0)B | 0.8 (15) | 0.5 (10)B | 1.1 (16) | |
Grain quality was unaffected by RLL for all varieties in both locations and seasons when the disease and fungicide treatments were compared (data not shown).
Discussion
RLL can be a severe foliar disease of oats in south-eastern Australia and cause significant losses to hay and milling oat crops. This study demonstrated that RLL can cause reductions in biomass and grain yield of up to 3.5 t/ha (22%) and 1.1 t/ha (21%), respectively. This is the first study of production losses caused by RLL infection in oats, demonstrating that previous assumptions that RLL was a minor issue were inaccurate. These losses were very similar to the grain yield losses caused by other diseases of oats such as stem rust (Martens 1985) and powdery mildew (Clifford 1995), crown rust (Lovatto et al. 2021) and septoria leaf blotch (Clark et al. 1975). Grain quality was generally unaffected by RLL, with small and infrequent reductions observed. This was likely due to disease progress of RLL occurring during the cool, wet winter and early spring months, prior to grain development stages of crop development.
The reductions in biomass and grain yield demonstrated in this study were likely an underestimate of actual losses, given that fungicide treatments did not provide complete control of RLL. Fungicide treated plots did not have any infected stubble applied, however RLL symptoms developed rapidly early in the growing season in disease free treatments in all experiments. This may have been due to infection from airborne spores from neighbouring plots but was most likely from seed-borne infection, in which case RLL could develop rapidly and cause significant damage, even where oats have not previously been grown. In addition to biomass and grain yield losses, this study demonstrated that plant morphology could be affected by RLL. In hay oats, RLL caused reductions to plant height, which may likely influence biomass. Similar effects were observed in oat plant morphology and biomass losses due to RLL infection (Cunningham 1990). Plant morphology was also previously observed to be affected by other diseases. Oat plants infected with barley yellow dwarf virus (BYDV) were significantly shorter and tillers were generally stunted (Bisnieks et al. 2005). Crown rust infection in susceptible hay oat varieties reduced stem thickness and biomass yield in Western Australia (K. Chambers, unpubl. data). Previous studies measured reductions in biomass of up to 66% due to other diseases including septoria leaf blotch (Bisnieks et al. 2005; Malik et al. 2011). The losses reported in this and other studies demonstrate that foliar diseases need to be managed in oat crops to maximise production.
RLL severity and associated losses were influenced by seasonal conditions, particularly wet weather during spring months (September–November). The greatest severity and losses occurred during the 2020 season, which had 54% and 31% higher spring rainfall at Longerenong and Inverleigh respectively, compared to 2019. Days with minimum temperatures as low as 5–7°C and maximum temperatures between 16 and 20°C likely favoured disease development. Spring rainfall likely accelerated disease progression on to flag leaves through rain splash of spores during post anthesis and early milk development stages and caused reductions in biomass and grain yield. In contrast, temperatures greater than 20°C during the spring months (September–November) appeared to slow disease progression and also increase crop growth and green leaf area, especially in later maturing hay varieties such as Williams and Forester. Drier conditions in spring meant no impact of RLL on biomass or grain yield in any variety at Vectis and Inverleigh during 2019. There was also an interaction between biomass and the fungicide treatment in some cases. For example, the moderately resistant to moderately susceptible (MRMS) rated variety Forester had low RLL infection (~9%) at Longerenong in 2020 but had a 13% (0.9 t/ha) reduction in biomass. The reason for the reduction is unclear but may have been due to fungicides causing a greening effect that prolonged green leaf area and improved biomass yield (Dietz et al. 2019).
This study demonstrated the benefits of growing oat varieties with host-plant resistance to RLL as varieties with greater resistance had less RLL infection and associated reductions in production. In milling oat experiments, variety Williams (MRMS) had the least reduction in grain yield, 6% on average in all experiments. In hay oat experiments, Tungoo (MRMS) did not have any losses and Williams (MRMS) had 0.9 t/ha (12%) of biomass loss only at Longerenong but not at other sites. Biomass reductions at Longerenong, unlike at Inverleigh in 2020, were inconsistent and did not relate to varietal resistance rating. These findings suggest likely presence of different pathotypes with varied virulence in both the regions. Continuous monitoring of pathogen populations is required to detect virulence changes and improve disease management strategies for RLL accordingly. Currently, virulence patterns of Puccinia coronata (Henningsen et al. 2024) and Phaeosphaeria avenaria (Garrard and Atieno 2023) in oats are monitored annually in Australia to inform industry about new strains and corresponding resistance changes in the commercial varieties.
Significant RLL severity and associated yield reductions in moderately susceptible (MS) and worse-rated varieties demonstrate the need for additional control strategies where such varieties are commonly grown, and conducive conditions occur. As one of the control strategy methods, fungicides can potentially be used to suppress RLL and avoid production losses. Previously propiconazole provided suppression (Cunningham 1990) and this study confirmed that multiple foliar applications of propiconazole at tillering, early stem elongation and inflorescence emergence generally provided significant reductions in RLL. The efficacy of fungicide varied between seasons, with greater control achieved during 2020 than 2019 at both sites. The reason for this is unclear but could be due to fungicide application coinciding with disease development when fungicides are usually found effective (Poole and Arnaudin 2014). The three-stage application treatment used in this study is not practical for oat growers as this may potentially increase the cost of production and limit economic returns, hence it is not a recommended strategy. Further investigation is required to identify one or two fungicide application strategies similar to that identified for other foliar diseases of cereals such as the spot form of net blotch and scald in barley (McLean and Hollaway 2018; McLean et al. 2022)
In conclusion, this study identified that RLL causes reductions in biomass and grain yield in hay and milling oats in the medium and high rainfall zones of south-eastern Australia. Losses will be greatest and most frequent where infected seeds are used, oats are grown in close rotation and seasonal conditions are favourable. Most of current commercial varieties are rated as susceptible and are at risk of loss as there are few control options available. Fungicides are not registered to control RLL in Australia, but propiconazole, which is registered for controlling other diseases of oats, showed promise to manage RLL. However, further research is required to develop integrated disease management strategies for economic control.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
We are grateful to Grains Research and Development Corporation (Project code: DJP1907-001RTX), AgriFutures (Project code: PRJ-011029) and Agriculture Victoria for investment and support.
Acknowledgements
The authors thank the field crops pathology team for scientific input and experiments at Vectis and Longerenong and Field Applied Research (FAR) Australia for experiments at Inverleigh, Geoff Thomas and Kylie Chambers at DPIRD, WA for project collaborations, and Beata Sznajder and Julian Taylor at University of Adelaide, SA for assistance with statistical analysis.
References
Bisnieks M, Persson T, Eckersten H, Sigvald R (2005) The effects on yield and components of yield in oats infected with BYDV-PAV at different growth stages. Journal of Plant Diseases and Protection 112, 521-528.
| Crossref | Google Scholar |
Bremer H (1943) An American oat disease found in western Anatolia. Phytopathotogy 33, 165-167.
| Google Scholar |
Clark RV, Gourley CO, Johnston HW, Piening LJ, Pelletier G, Santerre J, Genereux H (1975) Oat yield losses from Septoria leaf blotch at four locations in eastern Canada. Canadian Plant Disease Survey 55, 36-43.
| Google Scholar |
Crous PW, Braun U, McDonald BA, Lennox CL, Edwards J, Mann RC, Zaveri A, Linde CC, Dyer PS, Groenewald JZ (2021) Redefining genera of cereal pathogens: Oculimacula, Rhynchosporium and Spermospora. Fungal Systematics and Evolution 7, 67-98.
| Crossref | Google Scholar | PubMed |
Cunningham PC (1990) A serious attack by Spermospora avenae on oats – a disease new to western Europe. Plant Pathology 39, 191-196.
| Crossref | Google Scholar |
Davis H, Fitt BDL (1994) Effects of temperature and leaf weness on the latent period of Rhynchosporium secalis (leaf blotch) on leaves of winter barley. Journal of Phytopathology 140(3), 269-279.
| Crossref | Google Scholar |
Dietz JI, Schierenbeck M, Simón MR (2019) Impact of foliar diseases and its interaction with nitrogen fertilization and fungicides mixtures on green leaf area dynamics and yield in oat genotypes with different resistance. Crop Protection 121, 80-88.
| Crossref | Google Scholar |
FAOSTAT (2021) Food and Agriculture Organization of the United Nations. FAOSTAT statistical database. Available at https://www.fao.org/faostat/en/#data/QCL/visualize [accessed 1 June 2021]
Gilmour AR, Cullis BR, Verbyla AP (1997) Accounting for natural and extraneous variation in the analysis of field experiments. Journal of Agricultural, Biological, and Environmental Statistics 2(3), 269-293.
| Crossref | Google Scholar |
Henningsen EC, Lewis D, Nguyen DT, Sperschneider J, Kianian SF, Stone E, Dodds PN, Figueroa M (2024) Virulence patterns of oat crown rust in Australia – season 2022. Plant Disease 108, 1959-1963.
| Crossref | Google Scholar | PubMed |
Latch G (1966) Fungous diseases of ryegrasses in New Zealand: I. Foliage diseases. New Zealand Journal of Agricultural Research 9, 394-409.
| Crossref | Google Scholar |
Lovatto M, Silva GBPd, Coelho FK, Martinelli JA, Pacheco MT, Federizzi LC, Delatorre CA (2021) Crown rust on oat genotypes with different levels of resistance: damages and losses. Ciência Rural 51(3), e20200298.
| Crossref | Google Scholar |
Malik R, Paynter B, Webster C, McLarty A (2011) Growing oats in Western Australia for hay and grain. Department of Primary Industries and Regional Development, WA, Perth. Bulletin, 4798. Available at https://library.dpird.wa.gov.au/bulletins/116
Martens J (1985) Oat stem rust. In ‘The Cereal Rusts, Vol. 2: Diseases, Distribution, Epidemiology, and Control’. (Eds AP Roelfs, WR Bushnell) pp. 103–129. (Academic Press) 10.1016/B978-0-12-148402-6.50012-2
McLean MS, Hollaway GJ (2018) Suppression of scald and improvements in grain yield and quality of barley in response to fungicides and host-plant resistance. Australasian Plant Pathology 47, 13-21.
| Crossref | Google Scholar |
McLean MS, Poole N, Santa IM, Hollaway GJ (2022) Efficacy of spot form of net blotch suppression in barley from seed, fertiliser and foliar applied fungicides. Crop Protection 153, 105865.
| Crossref | Google Scholar |
Norman A, Taylor J, Tanaka E, Telfer P, Edwards J, Martinant J-P, Kuchel H (2017) Increased genomic prediction accuracy in wheat breeding using a large Australian panel. Theoretical and Applied Genetics 130, 2543-2555.
| Crossref | Google Scholar | PubMed |
Pascoe I, Woodcock T (1981) Plant pathogenic fungi from Victoria, I. Spermospora avenae on oats. Australasian Plant Pathology 10, 60-61.
| Crossref | Google Scholar |
Poole NF, Arnaudin ME (2014) The role of fungicides for effective disease management in cereal crops. Canadian Journal of Plant Pathology 36, 1-11.
| Crossref | Google Scholar |
Schlösser UG (1969) Nachweis des Gramineenparasiten Spermospora subulata (Sprague) Sprague in Deutschland. Nachrichlenblatt des Deutschen Pflanzenschutzdienste (Braunschweig) 21(11), 166-168.
| Google Scholar |
Sprague R, Johnson AG (1936) A new Pseudodiscosia. Mycologia 28, 181-185.
| Crossref | Google Scholar |
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Research 14, 415-421.
| Crossref | Google Scholar |