Strawberry clover (Trifolium fragiferum): current status and future role in Australian agriculture
R. W. Smith A * , B. Penrose
A * , B. Penrose  B , A. D. Langworthy
B , A. D. Langworthy  C , A. W. Humphries D , C. A. Harris E , M. E. Rogers
C , A. W. Humphries D , C. A. Harris E , M. E. Rogers  F , P. G. H. Nichols G and R. C. Hayes
F , P. G. H. Nichols G and R. C. Hayes  H
H
A Tasmanian Institute of Agriculture, University of Tasmania, Private Bag 1375, Prospect, Tas. 7250, Australia.
B Tasmanian Institute of Agriculture, University of Tasmania, Private Bag 98, Sandy Bay, Tas. 7001, Australia.
C Tasmanian Institute of Agriculture, University of Tasmania, Private Bag 3523, Burnie, Tas. 7320, Australia.
D South Australian Research and Development Institute, Waite Campus, 2b Hartley Grove, Urrbrae, SA 5064, Australia.
E New South Wales Department of Primary Industries, 444 Strathbogie Road, Glen Innes, NSW 2370, Australia.
F Agriculture Victoria, Ferguson Road, Tatura, Vic. 3616, Australia.
G School of Agriculture and Environment and Institute of Agriculture, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
H New South Wales Department of Primary Industries, Wagga Wagga Agricultural Institute, Pine Gully Road, Wagga Wagga, NSW 2650, Australia.
Crop & Pasture Science - https://doi.org/10.1071/CP22301
Submitted: 5 September 2022 Accepted: 23 January 2023 Published online: 7 March 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing
Abstract
Strawberry clover (Trifolium fragiferum L.) is periodically raised as an alternative perennial pasture legume for temperate regions of Australia. Its tolerance of waterlogging is widely known, yet its ability to persist through periods of soil moisture deficit is often understated. Other desirable characteristics include its stoloniferous growth habit and tolerance of mildly saline conditions. Only four strawberry clover cultivars have been registered in Australia, and the most popular, cv. Palestine, is a direct introduction, released in 1938 and first certified in 1951. Furthermore, strawberry clover’s distribution has largely been confined to niche environments, particularly waterlogged and saline areas. This paper reviews the taxonomy and breeding system, morphology, distribution and ecology, and subsequent transfer of strawberry clover to Australia. It reviews and maps the suitability of strawberry clover for perennial pasture systems in the medium–-high rainfall and irrigated temperate zones of Australia, with reference to future climates. The paper also highlights the breeding focus, commercialisation and marketing required to supersede cv. Palestine and lists the germplasm available in the Australian Pastures Genebank, with origins. We conclude that, although strawberry clover is unlikely to become a dominant perennial pasture legume species in Australia, it could be used in a wider range of environments than just those affected by salinity and/or waterlogging stress.
Keywords: cultivars, germplasm, legumes, pastures, perennial, review, stress tolerance, suitability, waterlogging.
Introduction
The benefits of including legumes in mixed pasture swards are well understood. Most notably, legumes increase groundcover, improve nutritional quality of grazed forage, and provide nitrogen for companion pasture species and future crops grown in rotation (Howieson et al. 2008; Nichols et al. 2012). Relative to annual plants, perennial legumes can reduce the detrimental impacts of dryland salinity from rising water tables by utilising soil water from greater depths and for longer periods (Dunin et al. 1999; Fillery and Poulter 2006; Ward and Micin 2006). Despite these advantages, only three temperate perennial legumes, lucerne (Medicago sativa L.), white clover (Trifolium repens L.) and, to a lesser extent, red clover (T. pratense L.), are widely sown in Australia (Dear et al. 2003). Oram (1993) outlined the reasons for expanding the number and area of alternative legumes as: (1) the need to fill diverse ecological niches within paddocks; (2) better buffering against pests and diseases; and (3) improving sustainable soil management. In addition, recent research has shown that alternative legumes may have a role in reducing methane emissions from livestock (Badgery et al. 2023) and reducing incidence of metabolic disorders in livestock associated with issues such as bloat (Marshall et al. 1979) and mineral imbalance (Refshauge et al. 2022).
Identification and adoption of perennial legume species that will prosper in climates and soil types where the traditional species fail to persist remains an opportunity for pastoral systems. This will be particularly important for adaptation of pastoral systems to the drier climate and increased frequency of extreme climatic events predicted for much of southern Australia, which are likely to reduce annual pasture production (Harrison et al. 2016; Harrison et al. 2017). Dear et al. (2003) and Li et al. (2008) suggested strawberry clover (T. fragiferum L.) as one of only a few alternative perennial legumes that offer greatest immediate potential for southern Australia, along with sainfoin (Onobrychis viciifolia Scop.), sulla (Hedysarum coronarium L.) and several Lotus species; more recently, Real et al. (2014) also suggested tedera (Bituminaria bituminosa C.H. Stirton var. albomarginata) as an alternative. As observed by Morley (1963):
Strawberry clover has long been identified as a candidate perennial pasture legume, which responds to summer rains, and grows well, and produces nitrogen in other seasons in regions rather too dry for white clover.
Strawberry clover is an interesting species with unfulfilled potential, remaining a niche perennial legume species in pasture systems worldwide. Likely reasons for limited adoption is that it lacks the dry matter (DM) production of white clover (Kemp et al. 2002; Gerard et al. 2022), the persistence of lucerne in dry environments (Li et al. 2008), or the vigour of red clover (Dear et al. 2003). However, strawberry clover has waterlogging and salinity tolerance superior to any of these species (Nichols et al. 2008). It also can persist through dry summers and spread rapidly via above-ground stolons when soil moisture returns to non-limiting levels. It is perceived to be sensitive to soil acidity (Pires et al. 1992; Wheeler and Dodd 1995; McVittie et al. 2012; Moir et al. 2016; Hayes et al. 2023), an issue examined in some detail in this review owing to the extent of soil acidity across much of southern Australia.
Only two cultivars of strawberry clover (Palestine and O’Connors) are currently grown for certified seed in Australia, with cv. Palestine comprising >94% of seed production over the past 5 years (Australian Seeds Authority 2021a, 2021b). Two New Zealand cultivars (Grasslands Onward and Grasslands Upward) were developed in the 1980s (Rumball et al. 1991a, 1991b), but have had little impact in Australia. Several authors, including Craig (1994), McDonald et al. (2005), Rogers et al. (2005), Li et al. (2008), and Nichols et al. (2008), have suggested opportunities for developing improved strawberry clover cultivars for Australian conditions. This review examines the use of strawberry clover in pastures, considers its strengths and weaknesses, and presents a case for further research and breeding efforts to enable greater use in future farming systems.
Taxonomy and morphological description
Strawberry clover was included as the species Trifolium fragiferum by Linnaeus (1753) in his botanical work ‘Species Plantarum’. In their taxonomic revision of the genus Trifolium, Zohary and Heller (1984) categorised strawberry clover as follows:
Ellison et al. (2006), in a phylogenetic study of the Trifolium genus, suggested a new classification, with T. fragiferum placed in section Vesicastrum within subgenus Trifolium of the genus Trifolium.
An extensive botanical description of strawberry clover can be found in Zohary and Heller (1984). Strawberry clover is generally prostrate in growth (although genotypes vary), originating as a tap-rooted seedling (Frame 2005), before it develops secondary root branching and spreads vegetatively via creeping stems (stolons) that can root at the nodes (Hollowell 1939; Tiver 1954; Forde et al. 1981) (Fig. 1a–d). It has some similarities to white clover, but can be distinguished from that species in the field by having leaflets more oval in shape and distinctly different flower heads that are more rounded and pink in colour, and a composite fruit containing dried petals, resembling the structure of a strawberry (Hollowell 1939; Tiver 1954) (Fig. 1b, e). Leaf markings in strawberry clover cultivars tend to be less prominent than those of white clover, typically ranging from no marking (Fig. 1b) to a broad, pale green chevron. Seeds are light brown (Frame 2005) (Fig. 1f), with 660 000–770 000 seeds/kg (Tiver 1954; Forde et al. 1981; Frame 2005). Zohary and Heller (1984) further describe five botanical varieties of T. fragiferum: var. fragiferum, var. majus, var. modestum, var. pulchellum and var. orthodon. These varieties differ slightly in morphology and natural distribution. Variety fragiferum has more botanical specimens and a broader distribution, with the other varieties being much rarer (Zohary and Heller 1984). On this basis, it appears most likely that cv. Palestine belongs to var. fragiferum. It is not known to which variety other germplasm in Australian collections belong.
The breeding system of strawberry clover has been debated by several authors. Early work on strawberry clover claimed that the species was self-fertile (Williams 1931, in Davies 1971), although research by Morley (1963) and Wright (1964) found that it was outcrossing and predominantly self-sterile. Davies (1971) points out that 11 of 63 clones in the crossing data from Wright (1964) set an average of 46% seed autogamously, suggesting a breeding system with variable self-compatibility. Davies and Young (1966) found strawberry clover largely self-sterile, following examination of accessions collected from Europe and in material derived from the accessions, but they also found a small percentage of plants that were self-fertile. By contrast, some populations were largely self-fertile in northern Europe with ability to be maintained under selfing conditions without loss of vigour or self-fertility (Davies 1971). One hypothesis for this might be a lack of suitable pollinators in more northerly climates. Regardless, strawberry clover flowers are cross-pollinated by honey bees (Frame 2005) (Fig. 1e).
Origin and distribution
Native distribution and habitat
On the basis of reported botanical specimens, Zohary and Heller (1984) claim strawberry clover to be native to all European countries (including the British Isles), all other Mediterranean countries, and West and Central Asia (including western Siberia, the Caucasus and Turkestan regions, Iraq, Iran, Afghanistan and Pakistan), and it has also been recorded in Ethiopia. This distribution is supported by seed collection site information, referred to as ‘passport data’, consisting of latitude, longitude, altitude, temperature and rainfall parameters, and soil pH and texture for 480 geo-referenced wild populations, referred to as ‘accessions’. These are held in genebanks worldwide, with a total of 1095 collected accessions (Genesys 2022; Fig. 2).
|
|
Strawberry clover is relatively tolerant of flooding and of moderately saline soils (up to 20 mM NaCl), being commonly found in low-lying swampy, or occasionally saline, environments (Townsend 1985). It has been collected across a wide range of latitudes (15.4–55.6°N) and elevations (−20 to 2278 m a.m.s.l.). Cv. Palestine was collected as an ecotype in Israel close to the Dead Sea (Barnard 1972a). A comparison of the passport data for cv. Palestine with those for accessions collected from extremes in its native distribution for temperature, latitude, elevation and rainfall is shown in Table 1. Accessions have been collected in environments with mean temperatures in the warmest quarter >30°C and maximum temperatures in the warmest month of 40°C; at the other extreme, accessions have been collected in environments with average minimum temperatures in the coldest month as low as −25°C. Strawberry clover has also been collected in environments with average annual rainfall from 98 to 1718 mm. However, the lower rainfall limit per se may be misleading, because strawberry clover is often found in the most favoured niches within localities such as irrigation ditches or watercourses. Genesys data (Genesys 2022) were used, in combination with satellite maps in Google Earth, to identify accessions growing in low-rainfall environments with minimal supplementary water. The presence of wild strawberry clover populations in very hot and dry environments suggests potential to improve the heat and drought tolerance of cultivars.

|
Subsequent transfer to Australia
Outside its native range, strawberry clover has spread to Australia, New Zealand and western USA (Townsend 1985; Frame 2005). It is unclear how and when it first came to Australia. According to Whittet (1964a), the introduction of strawberry clover to Australia was by design for the purposes of pasture improvement. However, it is likely that the first strawberry clovers were introduced accidentally into Australia as seed contaminants of other agricultural seeds, fodder, wool fleeces or packing containers imported in the late 18th and 19th centuries, in a manner similar to other exotic pasture legumes (Gladstones 1966; Nichols et al. 2012). Naturalised strawberry clover populations are known to have become established in the south-east of South Australia and in the Hunter region of New South Wales (NSW) prior to the 1890s, when they were first commercialised, later becoming known as cvv. O’Connors and Shearmans, respectively) (Barnard 1972a, 1972b). However, cv. Palestine, the most widely used strawberry clover in Australia, was a deliberate introduction in 1929 from Zimbabwe (formerly Rhodesia), having been originally collected as an ecotype from Israel (Barnard 1972c).
Breeding and cultivar development
Existing cultivars: strains, origins and utilisation
Early development of strawberry clover in Australia was founded largely on locally adapted strains. More than 20 strains from Australia, New Zealand and the USA were characterised by Tiver (1954). O’Connors was the name given to a strain that developed over more than 30 years in the Penola district in south-eastern South Australia and was known for its value on poorly drained soils (Tiver 1954). It is a more prostrate strain than cv. Palestine, with smaller leaves and finer runners, making it more tolerant of close grazing, although less productive, particularly in winter (Tiver 1954).
Similar to O’Connors, Shearmans was a locally adapted biotype that was directly multiplied for sale (Donald 1970) but was considered a distinct variety of strawberry clover and named by Breakwell (1923). It was first recognised in 1897 and subsequently cultivated by a dairy producer on marshy saline soils near Newcastle, NSW (Breakwell 1923). Early observations under such conditions suggested that Shearmans was faster spreading and grew more herbage than the known strawberry clover strain at the time, but produced little seed (Breakwell 1923); the last of these observations was later contradicted in South Australia according to Tiver (1954). Other early Australian strains included Hamilton, Parklands and Swan Hill, which were characterised as having medium leaf size, being finer and more prostrate and generally of poor winter production (Tiver 1954). Later, Prinsep Park (sometimes spelt Princep Park; Nichols et al. 2012) was selected out of Palestine in the Bunbury region of Western Australia (WA) with comparable persistence and yield (McDonald 2006).
Cultivar Palestine was the first certified in Australia in 1951 by the South Australian Department of Agriculture (Donald and Trumble 1940; Barnard 1972a; Australian Pastures Genebank 2022). The ecotype from which it is derived was found in Israel near the Dead Sea (Barnard 1972a; Nichols et al. 2012) and introduced to Australia in 1929 by HC Trumble; cv. Palestine was commercially available from 1938 (Tiver 1954). It was described by Tiver (1954) as a vigorous and erect strain with coarse runners and large leaflets, providing good winter production and flowering earlier than cv. O’Connors. Donald (1970) suggested that it had proven to be ‘outstanding value on 400 000 ha of winter flooded rendzina soils in south-east South Australia’ but had not been extensively adopted elsewhere in Australia. It remains the most widely cultivated variety (Australian Seeds Authority 2021a, 2021b). Phenotypic variation within and among Australian populations of strawberry clover cv. Palestine has been studied, using seed collected from five different environments in WA (McDonald et al. 2005). The results showed that, although considerable within-population variation exists, the five populations exhibit significantly different traits including leaf markers, growth habit, flowering time, plant width and height, seed production and seed size. The authors concluded that large variation existed within strawberry clover cv. Palestine in Australia, with the potential to express different phenotypes as it evolves to adapt to different environments.
Salina is one of the two main cultivars developed in the USA by selection within cv. Palestine (Townsend 1985; Frame 2005). It was developed for Californian conditions (Forde et al. 1981) and released in 1962 by the Californian Agricultural Experiment Station (Oregon State University 2022). Cv. Fresa was developed by New Mexico State University and was released in 1982 for turf purposes from material collected in Turkey (Frame 2005; Oregon State University 2022). It was actively selected for its low dense habit and found to be less than half as productive as cvv. Palestine, O’Connors and Salina (Baltensperger et al. 1982; Baltensperger and Gaussoin 1984).
Grasslands Onward was developed in New Zealand by W Rumball of AgResearch at Palmerston North in the 1980s (IP Australia 2022), released in New Zealand in 1991 (Rumball et al. 1991a), and granted Plant Breeder’s Rights (PBR) registration in Australia in 1997 (IP Australia 2022). The parent material originates from a range of germplasm including naturalised New Zealand germplasm, wild accessions collected in Europe, and commercial seed lines including O’Connors and Palestine (Rumball et al. 1991a). Cv. Grasslands Onward is most similar to O’Connors, being small-leaved, compact and prostrate, yet is more productive and best suited to sheep grazing (Rumball et al. 1991a). Grasslands Upward was developed in conjunction with Grasslands Onward from the same broad range of germplasm, but plants were selected for a more erect, open type suitable for dairy pastures (Rumball et al. 1991a, 1991b). The cultivar is thus more similar to cv. Palestine (Rumball et al. 1991b). Field evaluations in New Zealand showed Grasslands Onward and Grasslands Upward to have performance superior to cvv. O’Connors and Palestine, respectively (Rumball et al. 1991a, 1991b). However, they have had limited commercial success in Australia, with PBR registration of Grasslands Onward being terminated in 2007 (IP Australia 2022). In 2005, the turf-type germplasm ‘GAO153’ was released by AgResearch Grasslands in New Zealand for the purpose of low maintenance, dense groundcover, similar to cv. Fresa (Rumball and Claydon 2005).
Commercial seed production
Cultivars O’Connors and Palestine, both of which are maintained by the South Australian Research and Development Institute (SARDI), are now the only eligible varieties for certification in Australia (Australian Seeds Authority 2021c). In the 1952–53 season, certified seed of O’Connors reached 20 t, whereas that of Palestine was 50 t (Gorman 1953). By comparison, in the 5 years to 2021, mean certified seed production (under OECD, AOSCA and Australian Certification Schemes) of O’Connors was 4 t and cv. Palestine 48 t (Australian Seeds Authority 2021b). According to Lucas (2020), there was no seed production of proprietary strawberry clover cultivars between 2014 and 2018 in Australia.
Agronomy
Productivity
Strawberry clover is commonly considered less productive than white and red clovers. This appears to be an issue of lower production potential rather than a density-dependent relationship, from observations in both spaced-plant and plot experiments (Kemp et al. 2002; Gerard et al. 2022). On the Central Tablelands of NSW, Kemp et al. (2002) observed inconsistent growth of strawberry clover, producing comparable aboveground DM yields to white clover only under favourable conditions, and in drier years producing only 20% of the aboveground DM of subterranean clover. Li et al. (2008) found that strawberry clover was more productive and persistent than white clover at two sites in WA and one site in NSW, but was less productive than lucerne.
We can only speculate as to the apparent disparity of results reported by Kemp et al. (2002) and Li et al. (2008). In the former study, strawberry clover performance was inconsistent and inferior to white clover, compared with the latter, where strawberry clover performance surpassed white clover and approached that of lucerne. Although site descriptions for both studies are brief, it is probable that the more fertile topsoil was substantially deeper at the sites reported by Li et al. (2008) but quite shallow at most of the sites reported by Kemp et al. (2002). It is also likely that summer temperatures were cooler at the NSW Central Tablelands sites (Kemp et al. 2002) than in the cropping environments reported by Li et al. (2008). Neither study reported prolonged periods of inundation at any site. White clover certainly favours cooler summer temperatures and is known to tolerate moderate soil acidity and infertility (Lane et al. 2000). Both lucerne (Norton et al. 2021) and strawberry clover (Hofmann et al. 2007) tolerate moisture stress better than white clover, helping to explain their superior persistence in the drier environments described by Li et al. (2008) compared with white clover.
Abiotic stress tolerance
Strawberry clover is generally purported to have better tolerance of abiotic stresses such as drought, salinity, waterlogging and extremes of temperature than many other temperate perennial forage legumes, particularly white clover (Tiver 1954; Dūmiņš et al. 2021). Salinity continues to be a major environmental problem in parts of Australia, as a consequence of the clearing of perennial vegetation causing rising water tables (National Land and Water Resources Audit 2001). This land may also be subject to waterlogging due to shallow water tables, or due to decreased infiltration of surface water or irrigation and poor drainage (Barrett-Lennard 2003). The productivity of conventional pastures in these saline and waterlogged areas is very low and has prompted efforts to develop improved fodder species for saline and waterlogged conditions (Rogers et al. 2005; Nichols et al. 2008). Less is known about the tolerance of strawberry clover to soil acidity.
Response to soil acidity and fertility
Strawberry clover is grown on soils in western USA that are wet, saline or alkaline, and it is generally advantaged by continuous grazing in mixed pastures (Van Keuren and Hoveland 1985). One of the primary limitations to the broader adaptation of strawberry clover in environments across southern Australian is its sensitivity to soil acidity and associated mineral toxicities. In hydroponic solution culture experiments, McVittie et al. (2012) compared species for seedling root and shoot reductions in response to elevated concentrations of aluminium (Al) and manganese (Mn), respectively, and reported that the response of strawberry clover cv. Palestine to both elements was similar to that of lucerne cv. SARDI 10. These results were broadly consistent with an earlier screening in hydroponic solution involving Al alone (Wheeler and Dodd 1995). Similarly, in a glasshouse experiment, Moir et al. (2016) demonstrated that strawberry clover had a response to lime and optimal pH similar to that of lucerne when grown in an acidic soil over a 10-month period. In a separate study, strawberry clover seedlings were shown to be highly sensitive to soil acidity and grew only when lime was applied (Pires et al. 1992). Together, these studies affirm the fundamental importance of ameliorating soil acidity for the successful establishment of strawberry clover seedlings.
Less is known about the relative tolerance to soil acidity of mature strawberry clover plants. Seedling response to soil acidity does not necessarily reflect mature plant performance in field environments. This is illustrated by the perennial grass phalaris (Phalaris aquatica L.); its seedlings are highly sensitive to Al toxicity, which prompted a breeding initiative to develop more tolerant cultivars (Culvenor et al. 1986, 2011). Yet, this species has been endemic across acidic soil environments of south-eastern Australia for many decades, and in the Millennial Drought, mature plants of phalaris persisted in acutely acidic soils better than some species regarded as more tolerant of acidity (Hayes et al. 2015). Key factors impacting strawberry clover performance in field environments include the severity of soil acidity, the extent to which low pH is associated with Al and Mn toxicities, and the depth of any acidic soil layer (i.e. the proportion of the root system impacted by low pH). Recruitment of seedlings is also likely to be reduced, owing to the seedling sensitivity of strawberry clover.
Soil acidity in a field environment can often be difficult to disentangle from other soil fertility properties because many acidic soils also have low fertility (Hayes et al. 2019). Low fertility in acidic soils can be caused directly or indirectly by soil acidity (Hayes et al. 2022), or can exist simply as an association due to factors such as the parent material from which the soil was derived. Most previous studies that have examined strawberry clover response to soil fertility issues other than pH have focused on phosphorus (P). In a glasshouse experiment using a P-deficient soil, Moir et al. (2016) observed a similar response to P fertiliser in strawberry clover and white clover. Both species achieved ‘biologically optimum yields’ at lower P-fertiliser rates than lucerne, which is known to have very high P requirements (Sandral et al. 2019). Caradus (1980) demonstrated in a pot experiment that the total root length of strawberry clover was only 30% of that of white clover, suggesting a relatively limited capacity of strawberry clover to explore the soil volume for nutrients compared with other legumes. Taken together, these studies suggest a vulnerability in strawberry clover on acidic soils, due not only to sensitivity to toxicities but also to low fertility conditions that are often associated with acidity. In practice, this suggests that strawberry clover is probably more suited to soil types with pH and fertility levels considered suitable for lucerne, or where lucerne might otherwise be suited but for a high risk of waterlogging. In a recent evaluation of perennial legume persistence in tableland environments of NSW, Hayes et al. (2022a) demonstrated that strawberry clover generally performed well at only a subset of sites, where lucerne also performed well.
A study conducted on the Central Tablelands of NSW showed the DM production of strawberry clover to be lower and more variable than either white or subterranean clover (Kemp et al. 2002). Although an extensive characterisation of soil type was not undertaken, those authors reported initial soil tests from the surface 10 cm as being generally acidic (pHCa ≤5.1), with low levels of Al (≤6% of cations) and variable P fertility (Bray P, 8–32 mg/L).
Waterlogging and salinity tolerance
In southern Australia, strawberry clover is often found in dryland areas that are poorly drained, sodic, waterlogged and/or saline, or where the soil pH is too alkaline for white clover (pHwater 8–9) (Craig 1994). Similarly, in irrigated regions, strawberry clover may be included in pastures if the irrigation water is too saline for white clover (Craig 1994).
Strawberry clover has been highlighted numerous times in multi-species evaluations for waterlogging and/or salinity tolerant forage legumes (Masters et al. 2001; Dear et al. 2003; Rogers et al. 2005; Masters et al. 2007; Dear and Ewing 2008; Striker and Colmer 2017). However, much of the research has also been conducted under controlled-climate conditions, making it challenging to extrapolate findings accurately to the field.
Strawberry clover’s salinity tolerance was first documented ~80 years ago (Ahi and Powers 1938; Gauch and Magistad 1943), with the first Australian reports published by Tiver (1954) and Rogers and Bailey (1963). Most of this, and subsequent, research (Russell 1976; Mehanni and Repsys 1986; Craig 1994; Rogers et al. 1997a; Nichols et al. 2008; Rogers et al. 2009; Galloway et al. 2010) concluded that strawberry clover had moderate salinity tolerance, especially compared with other forage legumes. A conservative salinity threshold of 1–2 dS/m (~20 mM NaCl) has been identified as the maximum salinity level before strawberry clover herbage growth is depressed (Gauch and Magistad 1943; Rogers et al. 1997b). Strawberry clover’s tolerance of saline conditions has been associated with the exclusion of Na+ and Cl− from the shoots and the maintenance of a reasonable internal K+/Na+ ratio (<2.0) (Rogers and West 1993; Munns 2005; Rogers et al. 2009; Dūmiņš et al. 2021).
Recent research by Dūmiņš et al. (2021), in a non-agricultural environment, confirmed strawberry clover’s superior salinity tolerance compared with white clover and its adaptation to coastal saline sites. At 21 dS/m (~350 mM NaCl), strawberry clover’s DM yield was depressed by 50% (Can et al. 2013). An extensive review of the salinity tolerance of a wide range of forages identified strawberry clover as one of 53 forage legumes with a potential role in ‘salt-affected’ Australian areas (Rogers et al. 2005). Based on this review, the salinity tolerances of 38 annual and perennial Trifolium species, including six strawberry clover genotypes, were evaluated under glasshouse conditions (Rogers et al. 2009). Again, strawberry clover proved to be one of the more salt-tolerant clover species, with significant intraspecific variation in the salinity tolerance of evaluated genotypes. At 160 mM NaCl (~9.5 dS/m), herbage growth of strawberry clover cv. Palestine was significantly more depressed than that of the other strawberry clover accessions evaluated (54% vs 73% of growth achieved under non-saline conditions), highlighting an opportunity for further cultivar development.
Strawberry clover is well known to have waterlogging tolerance (root-zone hypoxia or even anoxia) superior to that of many other perennial forage legumes (Rogers et al. 2009; Stevenson et al. 2017; Striker and Colmer 2017). A review by Striker and Colmer (2017) of the published literature found that moderate waterlogging induced growth reductions of <25% for strawberry clover, 35% for white clover, and >50% for both lucerne and red clover. Tiver (1954) reported intraspecific variation in the winter waterlogging tolerance of strawberry clover, attributing the greater tolerance of cv. Palestine to its superior winter growth potential. More recent studies have shown the development of aerenchyma and both adventitious and lateral roots underpinning strawberry clover’s ability to avoid the oxygen deficit incurred by waterlogged conditions (Rogers and West 1993; Rogers et al. 2009). Such root morphological responses have also been observed in strawberry clover in response to the co-occurrence of salinity and waterlogging stress (Rogers and West 1993; Fig. 3). Under the combined stresses, root porosity (aerenchyma) of strawberry clover has been shown to be higher than under waterlogging stress in isolation (11.5% vs 5.8%; Rogers and West 1993). This greater root porosity may be a response to the increased oxygen demand of energy-dependent transport processes responsible for the exclusion of Na+ and Cl− from the shoot (Teakle et al. 2007).
|
|
Genetic diversity for salinity tolerance has been found within the species at various growth stages including germination, early seedling growth and mature vegetative growth (Schachtman and Kelman 1991; Rumbaugh et al. 1993; Rogers et al. 2009). Similarly, Stevenson et al. (2017) and Andersone-Ozola et al. (2021) identified strawberry clover genotypes with greater waterlogging tolerance than the current commercial cv. Palestine. Although the abovementioned studies suggest potential to develop strawberry clover cultivars that are more salinity and waterlogging tolerant than Palestine, other studies have reported contrasting findings (Craig 1994), and selection of plants with superior salinity tolerance has proved difficult (Gauch and Magistad 1943; Rumbaugh et al. 1993). Despite this, interest remains in selection for strawberry clover genotypes with increased salinity and waterlogging tolerance (McDonald et al. 2005; Nichols et al. 2008), so that better adapted and more productive genotypes are available for saline land. Such genotypes are critically needed, owing to the current absence of perennial forage legumes suitable for highly saline and waterlogged soils (winter, 0.8–5.0 dS/m; summer, 30.8–36.2 dS/m) (Nichols et al. 2008). Breeding programs also need to ensure that selected genotypes not only survive in these environments but also produce sufficient herbage to justify their sowing and establishment costs (Rogers et al. 1997b; Boschma et al. 2008; Nichols et al. 2008).
Heat (supraoptimal temperature) tolerance
No published research has quantified the heat tolerance of strawberry clover. However, it can be presumed to tolerate supraoptimal ambient temperatures (i.e. ≥30°C) based on its prevalence in environments where ambient temperatures often exceed 40°C (Frame 2004). Further research is required to evaluate whether strawberry clover has greater heat tolerance than other perennial legume species. The need for such research is further validated by the fact that, whereas red clover occurs in such environments, it is relatively sensitive to supraoptimal temperatures compared with other common temperate perennial pasture species (Langworthy et al. 2018). Claims of intraspecific variation in the temperature sensitivity of strawberry clover require such research to evaluate a range of strawberry clover genotypes (Duke 2012). Because many of the Australian regions where strawberry clover is cultivated for its salinity and waterlogging tolerance are characterised by hot summers, identification of genotypes with superior heat tolerance will be beneficial to the grazing industries in these regions.
Drought tolerance
Strawberry clover is a more drought-tolerant perennial Trifolium species than white clover (Dear et al. 2003; Milne 2011). During short periods of soil moisture deficit stress (≤54 days), studies under glasshouse and field conditions have shown strawberry clover’s superior capacity to maintain leaf production and functionality relative to red and white clovers (Dodd and Orr 1995; Hofmann et al. 2007). Although the glasshouse study of Hofmann et al. (2007) failed to confirm the mechanisms underlying this tolerance, strawberry clover was noted to maintain higher leaf water potential, net photosynthesis and transpiration rate than white clover during a drying cycle. Over longer periods of soil moisture deficit (84 days), the three species (strawberry, red and white clovers) exhibited almost complete above-ground tissue senescence with very limited capacity to resume herbage production when irrigation was applied to alleviate stress conditions (Dodd and Orr 1995). On the basis of these studies, further investigation is warranted of strawberry clover for inclusion in temperate perennial pastures subject to intermittent periods of soil moisture deficit or in areas where irrigation is limited by water restrictions. Strawberry clover’s advantage will most likely be targeted at environments where other constraints (notably waterlogging) impede the use of more drought-tolerant temperate perennial legumes such as lucerne (Dodd and Orr 1995; Bouton 2012).
Sowing rate and cost of seed
Relatively few management guidelines are available that are specific to strawberry clover, with management often presumed to be the same as for white clover. Sowing rates recommended for strawberry clover are 0.5–1.0 kg bare seed/ha when sown as a component in a mixture, 1–2 kg bare seed/ha when sown alone under dryland conditions, or 2–4 kg bare seed/ha under irrigation (Lattimore and McCormick 2012). Owing to differences in seed size, a sowing rate of 4 kg/ha strawberry clover was calculated by Hayes et al. (2022a) to deliver an approximately equivalent seeding density of white clover at 2 kg bare seed/ha, red clover at 5 kg bare seed/ha, or lucerne at 8 kg bare seed/ha, unless there was a factory coating on seed, which diminishes seeding density substantially. This puts the seed cost of strawberry clover into some context. A survey by the authors of three seed retailers across southern Australia showed that seed costs of strawberry clover were almost double those of white clover. If strawberry clover is to be sown at double the rate of white clover to achieve equivalent plant density, the farmer could be paying around four times the cost for seed to include strawberry clover rather than white clover. Morley (1963) suggested that one limitation to the broader utilisation of strawberry clover was seed cost: ‘There seems no good reason why seed yields cannot be as high as those of white clover, and costs about the same, if care is taken to provide sufficient honey bees at flowering time’. It would seem that little progress has been made on this front in the last 60 years, and it is likely to be a contributing reason for lower areas of strawberry clover sowings.
Grazing management and forage conservation
Owing to its prostrate growth habit, strawberry clover is rarely used in forage conservation (Forde et al. 1981). There are several reports of it tolerating continuous, close grazing (Raguse et al. 1971; Forde et al. 1981), but few studies have been conducted to test the optimal grazing regime for this species. Raguse et al. (1971) included strawberry clover and white clover, along with cocksfoot and perennial ryegrass, in a grazing study in California where mixed pastures were grazed either continuously or in rotation by steers. The authors concluded that strawberry clover was the only species to respond positively to the continuous grazing regime. However, their study was unable to separate the confounding effects of competition from the different grazing regimes. The authors postulated that the reduced shading of strawberry clover by companion species in the continuous grazing treatment may have aided its performance. Further work is needed to understand the response of pure strawberry clover swards to different grazing regimes.
Strawberry clover persists largely through stolons, although recruitment from seed is also possible. In white clover, frequent defoliation of plants in pots depleted stores of non-structural carbohydrates, threatening plant survival (Singh and Sale 1997a), and effects were more extreme under low soil P fertility (Singh and Sale 1997b). If strawberry clover responds similarly on account of its stoloniferous growth habit, this species may also benefit from rotational grazing that allows periods for recovery of carbohydrate reserves and maximises stolon development. Continuous close grazing, especially by sheep, may reduce strawberry clover persistence in the long term by reducing stolon development. It may also reduce seedling recruitment, whereby flowers are continually grazed by livestock before they have a chance to set seed. Further work is needed to improve understanding of the factors affecting persistence and recovery of strawberry clover under different grazing regimes.
Forage quality
Crude protein (CP) concentrations in strawberry clover range between 13% and 26%, depending on maturity and environmental conditions. Kenny and Reed (1984), Norman et al. (2021) and Stutz et al. (2023) found that protein content decreased substantially with maturity and floral development, presumably because of the decline in the leaf:stem ratio with maturity (Minson 1980). In the comparative experiment of Norman et al. (2021), strawberry clover had higher CP content at the vegetative stage than 29 other legume species/accessions, and had CP content at the flowering stage similar to or higher than white and red clovers, although lower than lucerne. At the mature stage, the CP content in strawberry clover herbage was considerably lower than in all of the lucerne and red clover cultivars, as well as white clover cv. Storm (Norman et al. 2021). By contrast, Stutz et al. (2023) showed that strawberry clover had a similar protein content to white clover across a range of vegetative stages.
Norman et al. (2021) found that the acid detergent fibre content of strawberry clover at the vegetative stage was not significantly different from that of white clover (cv. Storm), sulla (Hedysarum coronarium L.), birdsfoot trefoil (Lotus corniculatus L.) and sainfoin (Onobrychis viciifolia Scop.) and was lower than that of lucerne. Stutz et al. (2023) also found strawberry clover to be similar to white clover cultivars with respect to neutral detergent fibre content.
There is a paucity of published literature regarding the metabolisable energy (ME) content of strawberry clover. Norman et al. (2021) measured the cumulative energy production (GJ ME/ha) for 30 accessions of perennial legumes over a series of successive cuts over a 12-month period. Strawberry clover had greater cumulative energy production than red clover (cvv. Rubitas and Tuscan), but lower than all of the white clover, sulla and lucerne cultivars. Norman et al. (2021) suggest that the cumulative energy of strawberry clover may become more similar to other commonly used legumes after the end of the second autumn; therefore, we recommend more long-term comparative studies that measure metabolisable energy over an extended period of time.
Andrew and Robins (1969) found strawberry clover had greater concentrations (% of DM) of chloride (0.43–2.50%), potassium (K) (0.54–3.93%), nitrogen (4.26–0.51%) and sodium (0.37–0.82%), and lower concentrations of magnesium (0.20–0.23%) and P (0.35–0.51%), than white clover and lucerne under several K fertiliser regimes. Micronutrient concentrations in strawberry clover were similar to the ranges in red clover and lucerne collated by Kao et al. (2020), except for boron concentration, which was found to be higher in strawberry clover than in both of the other species. Iron concentration was higher, and zinc lower, in strawberry clover than in lucerne. We recommend that collection of more data regarding the micronutrient profile of strawberry clover should be a research priority, so that producers and consultants can understand better the nutritional effects of using strawberry clover in pastures.
Overall, these data suggest that in terms of protein, acid and neutral detergent fibre, and mineral contents, strawberry clover can provide an alternative nutritionally similar to white clover. However, it is important to note that all data were from cv. Palestine, and all but one of the studies were conducted in Australia. Nutritional composition is known to be affected by environmental factors (Givens et al. 2000) and can vary widely between cultivars (e.g. Norman et al. 2021). Therefore, measuring nutritional composition of strawberry clover grown in other environments and from a variety of cultivars is a research priority for achieving better understanding of the nutritional composition of strawberry clover.
Anti-nutritional factors
As with many salt-tolerant species, strawberry clover has an enhanced ability to take up contaminant metals (Lutts and Lefèvre 2015). Cadmium (Cd) is a contaminant found in many P fertilisers, and thus has accumulated in many pastures that have had recurrent P fertiliser application (Langlands et al. 1988). When Cd is accumulated in the liver and kidneys of sheep and in offal at concentrations that exceed guideline limits, the organs must be disposed of, reducing the production value of the carcass (Langlands et al. 1988). Strawberry clover has been found to take up Cd to a greater extent than red clover, crimson clover (T. incarnatum L.) and white clover, but to a lesser extent than lucerne (Stafford et al. 2016).
Bloat (ruminant tympany) is a common disorder of livestock, particularly cattle grazing lush, legume-dominant pastures (Brightling 1994). It is reported to be a particular risk in pastures dominated by white or red clover, the foliage of which is low in condensed tannins that bind to soluble proteins and prevent the formation of rumen foam (Lane et al. 2000). Strawberry clover is rarely flagged as a high-risk forage for bloat (Frame 2005), although this may reflect a lower incidence of pastures with high strawberry clover content. The bloat potential and occurrence of condensed tannins in strawberry clover has been little studied. Redgut (intestinal torsion) is another common disorder particularly in sheep, caused by grazing highly digestible, legume-dominant pastures (Gumbrell 1997). Few instances have been reported in the literature of redgut caused by strawberry clover; however, this may also reflect a lower incidence of strawberry clover dominant pastures. Further studies are needed to establish both the bloat and redgut potential of strawberry clover.
The herbage of many common legume species contains phytoestrogens, which can lower the fertility of the grazing flock or herd (Reed 2016), yet the risk posed by strawberry clover is uncertain. Surprisingly, in a screening of the herbage of 100 Trifolium species for oestrogenic isoflavones, Francis et al. (1967) did not include T. fragiferum. Herbage of T. neglectum was included in that study and is a species sometimes considered a synonym of T. fragiferum (Forde et al. 1981); it was not reported to contain high oestrogenic isoflavone concentrations (Francis et al. 1967). Further research is required to determine the levels of phytoestrogens in strawberry clover. Notwithstanding, most of the risks posed by anti-nutritional compounds in legumes can be managed by growing the species in mixtures to provide diversity in the diet of grazing ruminants.
Rhizobia and nitrogen fixation
Seeds of strawberry clover are recommended to be inoculated with Rhizobium leguminosarum bv. trifolii strain TA1 (Group B) (Drew et al. 2012). In studies by Rigg et al. (2021), strawberry clover cv. Palestine formed effective nodules with both TA1 and WSM1325 (Group C) rhizobial strains, but formed ineffective symbioses with rhizobial strains used for other perennial legumes. The cross-host compatibility observed in strawberry clover cv. Palestine may be an advantage in many environments of south-eastern Australia where older Group C rhizobia strains are endemic owing to a long history of subterranean clover use (Rigg et al. 2021). There are no data available to inform the cross-host rhizobial compatibility of other strawberry clover cultivars, and further studies are needed.
Use in mixtures
The lower productive potential of strawberry clover than of other legumes suggests that it is unlikely to become a dominant species encroaching in a significant way on the mainstream perennial legume species white clover, red clover and lucerne. However, justification exists for the inclusion of strawberry clover as a component of pasture mixtures. First, it is a perennial legume. Agriculture in southern Australia relies heavily on self-regenerating annual legumes (Nichols et al. 2012), but they often struggle to compete for light and water with perennial pasture species, especially beyond the year of sowing, resulting in reduced persistence (Dear et al. 1998). The perennial habit of strawberry clover does not require annually set seed and regeneration of seedlings for persistence, and this provides a competitive advantage over self-regenerating legume species. Second, perennial legumes such as strawberry clover can provide more early autumn feed than annual legumes because they already have extensive root systems and above-ground branches when annual species are still establishing. Third, perennial species such as strawberry clover provide increased options to establish a companion legume in spring-sown, perennial-based pastures. As demonstrated by Li et al. (2014), perennial pastures sown in autumn can include subterranean clover, whereas those sown in spring should be sown only to perennial species, owing to the inability of spring-sown subterranean clover to set seed and regenerate in subsequent years.
The superior heat and moisture stress tolerance of strawberry clover compared with red and white clovers is another advantage in mixtures. Vigorous perennial companion species such as phalaris or lucerne are known to exacerbate the apparent moisture deficit experienced by companion species (Dear and Cocks 1997; Dear et al. 2010), and this might be expected to diminish the persistence of profligate water users such as white clover (Norton et al. 2021). The lower productivity of strawberry clover may be an advantage in this scenario, by reducing the amount of tissue to be maintained under drought and reducing the competitive effects on other sward components that can drive overall pasture productivity. The extent to which strawberry clover can endure this competition requires further testing across a diversity of environments. The prostrate habit of strawberry clover also offers advantages in increasing groundcover, which is sub-optimal in lucerne pastures (Hayes et al. 2018). Such increased groundcover is likely to increase water infiltration over summer and reduce soil temperatures, both of which are likely to enhance lucerne persistence and productivity (Hayes et al. 2021). The case for including strawberry clover in mixtures with lucerne offers additional advantages where lucerne density is reduced through periodic waterlogging (Dear et al. 2010).
Other uses
Strawberry clover has a flowering window quite similar to white clover, which can last from mid-spring to autumn under favourable conditions (Dodd and Orr 1995; Somerville 2019). Honey bees have been noted to forage on flowers of this species (Forde et al. 1981), but its value for honey production remains in question. It is noted as a species of only moderate value to apiarists across south-eastern Australia (Somerville 2019), which may simply reflect the fact that it has not traditionally been grown widely. There are several reports in the literature that the flowers of strawberry clover are ‘extremely attractive to honey bees for both nectar and pollen’ (McGregor 1976), but data quantifying this to provide comparisons with other, more studied species are scarce. As a largely cross-pollinating species in which seed production has been suggested to increase almost 3-fold where honey bees are actively foraging (Morley 1963), there is little doubt that strawberry clover flowers offer rewards for foraging honey bees, and are assumed to be of similar quality to white clover flowers (Somerville 2019). However, the timing, quantity and quality of nectar and pollen from this species has not been quantified.
Strawberry clover provides excellent groundcover and is a useful component of lawn or turf mixtures. In fact, cv. Fresa was selected for its low, dense habit and was developed as a low-maintenance groundcover for use in lawns (Baltensperger et al. 1982; Baltensperger and Gaussoin 1984). It may also be useful in vineyards and orchards where low biomass production is an advantage, or on sporting fields, particularly where drainage or irrigation systems are imperfect, because it can tolerate extremes of too much or too little water better than many other species. Its year-round growth habit also makes it a useful addition in sporting fields; it is less affected by frost than kikuyu (Cenchrus clandestinus Hochst. ex. Chiov. syn. Pennisetum clandestinum) or other C4 grass species often used, thereby maintaining an aesthetically pleasing green appearance throughout the year. Strawberry clover was also advocated by Whittet (1964b) for planting along irrigation channel banks with kikuyu to control weeds and limit erosion caused by livestock, highlighting the value of this species in a diversity of settings.
Pest and disease issues
Pasture pests and diseases can contribute to the poor persistence of legumes within a mixed pasture (Allen 1989; Irwin 1989). However, there are few published reports concerning the economic impact of pests and diseases on temperate perennial legume species other than white clover, red clover and lucerne (Allen 1989; Irwin 1989; Jones 2013).
Major pests considered across southern Australia that can negatively impact strawberry clover production and persistence are likely to be similar to those affecting white clover (Allen 1989). These include redlegged earth mite (Halotydeus destructor); blue oat mite (Penthaleus major); lucerne flea (Sminthurus viridis); root feeding scarabs (Sericesthis spp.); corbies, pasture web worms and related caterpillars (Oncopera spp.); pasture cockchafers (Aphodius spp.); cutworms (Agrotis spp.); native budworm (Helicoverpa punctigera); and bluegreen aphid (Acyrthosiphon kondoi) (Roberts et al. 1979; Allen 1989).
There have been limited screenings of disease-causing pathogens that impact strawberry clover in Australia (Buchen-Osmond et al. 1988; Jones 1996, 2013). The only record of a virus disorder occurring in strawberry clover in Australia is of White clover mosaic virus in Western Australia (Buchen-Osmond et al. 1988). Several fungal diseases have been identified as occurring in strawberry clover, including Fusarium spp. (Kellock et al. 1978), and pepper spot caused by the fungi Leptosphaerulina trifolii, Colletotrichum trifolii, Pleospora herbarum and Pseudopeziza trifolii (Shipton 1967).
Soil nematodes may significantly reduce perennial legume performance by reducing root growth and nitrogen fixation, and by stunting both leaf and stolon growth. Strawberry clover has been reported to be susceptible to root-knot nematodes (Meloidogyne spp.) (Shipton 1967; Quesenberry et al. 1986) and Pratylenchus thornei (Grandison and Wallace 1974).
The use of resistant cultivars to combat both pasture pests and disease-causing pathogens is likely to be the best long-term, low-cost option. However, because little published information exists for strawberry clover, there is an initial need to determine the relevant benchmarks for pasture pest and disease incidences, and yield losses caused by the most prevalent pests and diseases affecting the species. This will inform the selection of pests and diseases for which to conduct resistance screening programs among current cultivars and diverse germplasm, and the need for future breeding programs for pest and disease resistance.
Climate and soil recommendations
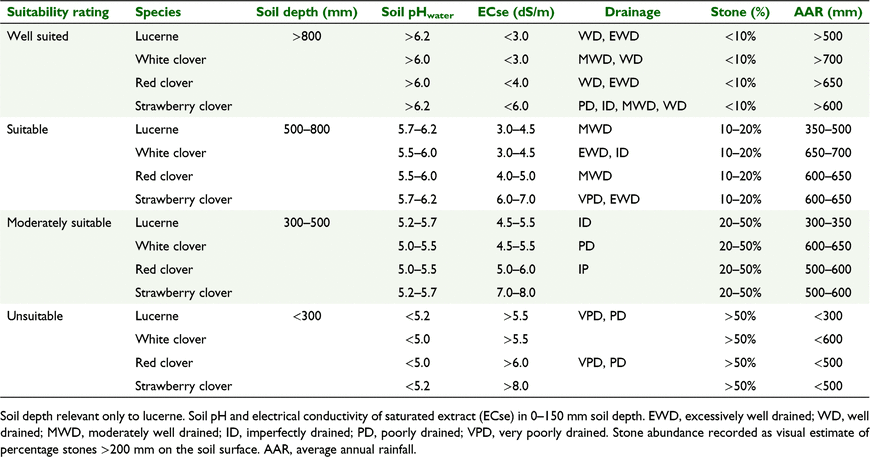
Using the information compiled in this review, we have suggested growth suitability rules for strawberry clover (Table 2) for the purpose of mapping its suitability in Tasmania, Australia (Fig. 4). These rules include both soil and rainfall parameters developed for land suitability mapping (Kidd et al. 2015). To determine the niche areas where strawberry clover might have superior adaptation over other perennial legumes, growth suitability rules have also been suggested for lucerne, white clover and red clover (Table 2). These niche areas for Tasmania are highlighted in Fig. 5. Although strawberry clover has broad adaptation, the other perennial species will likely be more productive. Nonetheless, the niche areas where strawberry clover may be more suitable than the other species is not insignificant. Similar growth suitability maps for strawberry clover could be drawn for the other states of Australia and, indeed, for other parts of the globe.
|
|
|
|
|
Future research and conclusions
Strawberry clover has a clear advantage over other temperate perennial legume species in environments prone to waterlogging or to moderate salinity. Outside this niche, its potential zone of adaptation has hitherto been poorly defined. Strawberry clover seems unlikely to enjoy widespread adoption in areas with shallow and acidic soils, or in environments where white clover or subterranean clover are more productive (Kemp et al. 2002). Strawberry clover may be successful in high-rainfall ‘cropping zone’ environments such as those described by Li et al. (2008), although more evidence and research is required on drought tolerance. However, we acknowledge that strawberry clover is unlikely to match the productivity of lucerne in most environments. Therefore, the case for replacing lucerne with strawberry clover is not proposed unless for areas subject to waterlogging. Rather, we propose that a possible fit for strawberry clover in future pasture systems could be as a companion species with lucerne in semi-permanent pastures for grazing, for the following reasons:
-
Both species are broadly suited to more fertile soils with mild levels of acidity.
-
The lower yield potential of strawberry clover may make it a less competitive neighbour with lucerne than alternative legume species.
-
The prostrate habit of strawberry clover promises many advantages for lucerne swards that are commonly plagued by low levels of groundcover (Hayes et al. 2018).
-
The perennial habit of strawberry clover promises advantages over self-regenerating annual legume species that struggle to co-exist with lucerne in the long term (Dear et al. 2000; Dear et al. 2007).
-
More productive cultivars of strawberry clover have yet to be developed; a less risky approach is to target it as a companion species to be grown in mixtures.
To our knowledge, no research has examined strawberry clover in mixtures with lucerne. One major question surrounding adding strawberry clover with lucerne is whether it can persist in the low soil moisture content environments that develop when lucerne dries the soil profile during drought conditions. Use of alternative spatial sowing arrangements may reduce this competition, although it may increase intraspecific competition if sowing rates are not reduced (Hayes et al. 2021). This would seem to be a high priority for future research to increase the utilisation of this important species and add value to contemporary production systems. In addition to the suggested improvement of waterlogging and salinity tolerance (Nichols et al. 2008, 2012), development of tolerance to acidic soils should be continued, but coupled with increased productivity. Further, the drought tolerance and recovery through vegetative stolon spread following periods of moisture deficit where white clover fails to persist requires examination. Strawberry clover remains an underutilised species, although new research and demonstration is required for this species to be adopted across a wider range of environments and grazing systems.
Data availability
Data sharing is not applicable on the basis that this is a review article.
Conflicts of interest
The authors declare no conflicts of interest
Declaration of funding
The Tasmanian Institute of Agriculture received matching funding from the Meat & Livestock Australia (MLA) Donor Company for a project titled ‘Growing red meat productivity through the selection and establishment of perennial legumes (P.PSH.2052)’. This project was part of the Livestock Productivity Partnership, a collaborative research and development partnership involving the MLA Donor Company, NSW Department of Primary Industries, University of New England, CSIRO, the Tasmanian Institute of Agriculture and the University of Melbourne.
Acknowledgements
The authors acknowledge the contributions of numerous technical staff that over many years that have improved our understanding of strawberry clover as a species though field and pot evaluation studies. The authors also acknowledge the key role of the Australian Pastures Genebank in securing the future of pasture breeding and research through the regeneration, characterisation and storage of germplasm and by providing information for this paper. Further, the authors acknowledge the contribution of Mathew Webb and Darren Kidd from Natural Values Science and Services Branch, Natural Resources and Environment Tasmania for developing the maps of strawberry clover suitability.
References
Ahi SM, Powers WL (1938) Salt tolerance of plants at various temperatures. Plant Physiology 13, 767–789.| Salt tolerance of plants at various temperatures.Crossref | GoogleScholarGoogle Scholar |
Allen PG (1989) Arthropod pests and the persistence of pasture legumes in Australia. In ‘Persistence of forage legumes. Proceedings of trilateral workshop on legume persistence (Australia/New Zealand/USA), Hawaii’. (Eds GC Marten, AG Matches, RF Barnes, RW Broughton, RJ Clements, GW Sheath) pp. 419–439. (American Society of Agronomy: Madison, WI, USA)
Andersone-Ozola U, Jēkabsone A, Purmale L, Romanovs M, Ievinsh G (2021) Abiotic stress tolerance of coastal accessions of a promising forage species, Trifolium fragiferum. Plants 10, 1552
| Abiotic stress tolerance of coastal accessions of a promising forage species, Trifolium fragiferum.Crossref | GoogleScholarGoogle Scholar |
Andrew CS, Robins MF (1969) The effect of potassium on the growth and chemical composition of some tropical and temperate pasture legumes. I. Growth and critical percentages of potassuim. Australian Journal of Agricultural Research 20, 999–1007.
| The effect of potassium on the growth and chemical composition of some tropical and temperate pasture legumes. I. Growth and critical percentages of potassuim.Crossref | GoogleScholarGoogle Scholar |
Australian Pastures Genebank (2022) APG 383. Trifolium fragiferum L. ‘PALESTINE’. PIRSA/SARDI, Government of South Australia. Available at https://apg.pir.sa.gov.au/gringlobal/accessiondetail.aspx?id=40569
Australian Seeds Authority (2021a) Areas registered in Australia for certification under OECD, AOSCA and Australian Seed Certififcation Schemes – 2017/19 to 2021/22. Australian Seeds Authority Ltd, Melbourne, Vic., Australia.
Australian Seeds Authority (2021b) Certified seed produced in Australia under OECD, AOSCA and Australian Seed Certification Schemes in the 12 months to 30 September 2021. Australian Seeds Authority Ltd, Melbourne, Vic., Australia.
Australian Seeds Authority (2021c) National list of plant varieties eligible for seed certification in Australia. Australian Seeds Authority Ltd, Melbourne, Vic., Australia. Available at https://aseeds.com.au/wp-content/uploads/asgarosforum/23/ASA-NATIONAL-LIST-OF-VARIETIES-10Sept2021.pdf [Accessed 3 February 2022]
Badgery W, Li G, Cowie A, Smith R, Humphries A, Peck D, Simmons A, Eckard R, Vercoe P, Wood J, Durmic Z, Hutton P (2023) Reducing enteric methane of ruminants in Australian grazing systems – a review of the role for temperate legumes and herbs. Crop & Pasture Science
| Reducing enteric methane of ruminants in Australian grazing systems – a review of the role for temperate legumes and herbs.Crossref | GoogleScholarGoogle Scholar |
Baltensperger AA, Gaussoin R (1984) ‘Fresa’ strawberry clover: a new ground cover variety. Bulletin 708. New Mexico State University Library, Las Cruces, NM, USA. Available at https://nmsu.contentdm.oclc.org/digital/collection/AgCircs/id/28143
Baltensperger AA, Watson CE, Smith MA, Mclean SD, Gaussoin RE (1982) Registration of Fresa strawberry clover1 (Reg. No. 38). Crop Science 22, 1260–1260.
| Registration of Fresa strawberry clover1 (Reg. No. 38).Crossref | GoogleScholarGoogle Scholar |
Barnard C (1972a) Trifolium fragiferum L. (strawberry clover) cv. Palestine. In ‘Register of Australian herbage plant cultivars.’ (Division of Plant Industry, Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia)
Barnard C (1972b) Trifolium fragiferum L. (strawberry clover) cv. O’Connors. In ‘Register of Australian herbage plant cultivars.’ (Division of Plant Industry, Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia)
Barnard C (1972c) Trifolium fragiferum L. (strawberry clover) cv. Shearmans. In ‘Register of Australian herbage plant cultivars.’ (Division of Plant Industry, Commonwealth Scientific and Industrial Research Organization: Melbourne, Australia)
Barrett-Lennard EG (2003) The interaction between waterlogging and salinity in higher plants: causes, consequences and implications. Plant and Soil 253, 35–54.
| The interaction between waterlogging and salinity in higher plants: causes, consequences and implications.Crossref | GoogleScholarGoogle Scholar |
Boschma SP, Lodge GM, Harden S (2008) Herbage mass and persistence of pasture legumes and grasses at two potentially different saline and waterlogging sites in northern New South Wales. Australian Journal of Experimental Agriculture 48, 553–567.
| Herbage mass and persistence of pasture legumes and grasses at two potentially different saline and waterlogging sites in northern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Bouton JH (2012) An overview of the role of lucerne (Medicago sativa L.) in pastoral agriculture. Crop & Pasture Science 63, 734–738.
| An overview of the role of lucerne (Medicago sativa L.) in pastoral agriculture.Crossref | GoogleScholarGoogle Scholar |
Breakwell E (1923) Clovers. In ‘The grasses and fodder plants of New South Wales’. pp. 290–324. (Afred James Kent, Government Printer: Sydney, NSW, Australia)
Brightling A (1994) ‘Stock diseases. Diseases of beef cattle, sheep, goats and farm dogs in temperate Australia.’ (Butterworth-Heinemann: Sydney, NSW, Australia)
Buchen-Osmond C, Crabtree K, Gibbs A, McLean G (1988) ‘Viruses of plants in Australia.’ (Australian National University Printing Service: Canberra, ACT, Australia)
Can E, Arslan M, Sener O, Daghan H (2013) Response of strawberry clover (Trifolium fragiferum L.) to salinity stress. Research on Crops 14, 576–584.
Caradus JR (1980) Distinguishing between grass and legume species for efficiency of phosphorus use. New Zealand Journal of Agricultural Research 23, 75–81.
| Distinguishing between grass and legume species for efficiency of phosphorus use.Crossref | GoogleScholarGoogle Scholar |
Craig AD (1994) The strawberry clover (Trifolium fragiferum L.) scene in Australia. Department of Primary Industries, South Australia, Adelaide, SA, Australia.
Culvenor RA, Oram RN, Fazekas dSGC (1986) Variation in tolerance in Phalaris aquatica L. and a related species to aluminium in nutrient solution and soil. Australian Journal of Agricultural Research 37, 383–395.
| Variation in tolerance in Phalaris aquatica L. and a related species to aluminium in nutrient solution and soil.Crossref | GoogleScholarGoogle Scholar |
Culvenor RA, McDonald SE, Veness PE, Watson D, Dempsey W (2011) The effect of improved aluminium tolerance on establishment of the perennial grass, phalaris, on strongly acid soils in the field and its relation to seasonal rainfall. Crop & Pasture Science 62, 413–426.
| The effect of improved aluminium tolerance on establishment of the perennial grass, phalaris, on strongly acid soils in the field and its relation to seasonal rainfall.Crossref | GoogleScholarGoogle Scholar |
Davies WE (1971) Host/pollinator relationships in the evolution of herbage legumes in Britain. Science Progress 59, 573–589.
| Host/pollinator relationships in the evolution of herbage legumes in Britain.Crossref | GoogleScholarGoogle Scholar |
Davies WE, Young NR (1966) Self-fertility in Trifolium fragiferum. Heredity 21, 615–624.
| Self-fertility in Trifolium fragiferum.Crossref | GoogleScholarGoogle Scholar |
Dear BS, Cocks PS (1997) Effect of perennial pasture species on surface soil moisture and early growth and survival of subterranean clover (Trifolium subterraneum L.) seedlings. Australian Journal of Agricultural Research 48, 683–694.
| Effect of perennial pasture species on surface soil moisture and early growth and survival of subterranean clover (Trifolium subterraneum L.) seedlings.Crossref | GoogleScholarGoogle Scholar |
Dear BS, Ewing MA (2008) The search for new pasture plants to achieve more sustainable production systems in southern Australia. Australian Journal of Experimental Agriculture 48, 387–396.
| The search for new pasture plants to achieve more sustainable production systems in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Dear BS, Cocks PS, Collins DP, Wolfe EC (1998) Established perennial grasses reduce the growth of emerging subterranean clover seedlings through competition for water, light, and nutrients. Australian Journal of Agricultural Research 49, 41–52.
| Established perennial grasses reduce the growth of emerging subterranean clover seedlings through competition for water, light, and nutrients.Crossref | GoogleScholarGoogle Scholar |
Dear BS, Cocks PS, Swan AD, Wolfe EC, Ayre LM (2000) Effect of phalaris (Phalaris aquatica L.) and lucerne (Medicago sativa L.) density on seed yield and regeneration of subterranean clover (Trifolium subterraneum L.). Australian Journal of Agricultural Research 51, 267–278.
| Effect of phalaris (Phalaris aquatica L.) and lucerne (Medicago sativa L.) density on seed yield and regeneration of subterranean clover (Trifolium subterraneum L.).Crossref | GoogleScholarGoogle Scholar |
Dear BS, Moore GA, Hughes SJ (2003) Adaptation and potential contribution of temperate perennial legumes to the southern Australian wheatbelt: a review. Australian Journal of Experimental Agriculture 43, 1–18.
| Adaptation and potential contribution of temperate perennial legumes to the southern Australian wheatbelt: a review.Crossref | GoogleScholarGoogle Scholar |
Dear BS, Sandral GA, Virgona JM, Swan AD, Orchard BA, Cocks PS (2007) Lucerne, phalaris, and wallaby grass in short-term pasture phases in two eastern Australian wheatbelt environments. 2. Effect of perennial density and species on subterranean clover populations and the relative success of 3 clover cultivars of different maturity. Australian Journal of Agricultural Research 58, 123–135.
| Lucerne, phalaris, and wallaby grass in short-term pasture phases in two eastern Australian wheatbelt environments. 2. Effect of perennial density and species on subterranean clover populations and the relative success of 3 clover cultivars of different maturity.Crossref | GoogleScholarGoogle Scholar |
Dear BS, Peoples MB, Hayes RC, Swan AD, Chan KY, Oates AA, Morris SG, Orchard BA (2010) Effect of gypsum on establishment, persistence and productivity of lucerne and annual pasture legumes on two grey Vertosols in southern New South Wales. Crop & Pasture Science 61, 435–449.
| Effect of gypsum on establishment, persistence and productivity of lucerne and annual pasture legumes on two grey Vertosols in southern New South Wales.Crossref | GoogleScholarGoogle Scholar |
Dodd MB, Orr SJ (1995) Seasonal growth, phosphate response, and drought tolerance of 11 perennial legume species grown in a hill-country soil. New Zealand Journal of Agricultural Research 38, 7–20.
| Seasonal growth, phosphate response, and drought tolerance of 11 perennial legume species grown in a hill-country soil.Crossref | GoogleScholarGoogle Scholar |
Donald CM (1970) Temperate pasture species. In ‘Australian grasslands’. (Ed. RM Moore) pp. 303–320. (Australian National University Press: Canberra, ACT, Australia)
Donald CM, Trumble HC (1940) Palestine strawberry clover (Trifolium fragiferum L.). Journal of the Australian Institute of Agricultural Science 7, 124–125.
Drew E, Herridge D, Ballard R, O’Hara G, Deaker R, Denton M, Yates R, Gemell G, Hartley E, Phillips L, Seymour N, Howieson J, Ballard N (2012) Inoculating legumes: a practical guide. Grains Research and Development Corporation, Kingston, ACT, Australia.
Duke J (2012) ‘Handbook of legumes of world economic importance.’ (Springer Science & Business Media: New York, NY, USA)
Dunin FX, Williams J, Verburg K, Keating BA (1999) Can agricultural management emulate natural ecosystems in recharge control in south eastern Australia? Agroforestry Systems 45, 343–364.
| Can agricultural management emulate natural ecosystems in recharge control in south eastern Australia?Crossref | GoogleScholarGoogle Scholar |
Dūmiņš K, Andersone-Ozola U, Samsone I, Elferts D, Ievinsh G (2021) Growth and physiological performance of a coastal species Trifolium fragiferum as affected by a coexistence with Trifolium repens, NaCl treatment and inoculation with rhizobia. Plants 10, 2196
| Growth and physiological performance of a coastal species Trifolium fragiferum as affected by a coexistence with Trifolium repens, NaCl treatment and inoculation with rhizobia.Crossref | GoogleScholarGoogle Scholar |
Ellison NW, Liston A, Steiner JJ, Williams WM, Taylor NL (2006) Molecular phylogenetics of the clover genus (Trifolium—Leguminosae). Molecular Phylogenetics and Evolution 39, 688–705.
| Molecular phylogenetics of the clover genus (Trifolium—Leguminosae).Crossref | GoogleScholarGoogle Scholar |
Fillery IRP, Poulter RE (2006) Use of long-season annual legumes and herbaceous perennials in pastures to manage deep drainage in acidic sandy soils in Western Australia. Australian Journal of Agricultural Research 57, 297–308.
| Use of long-season annual legumes and herbaceous perennials in pastures to manage deep drainage in acidic sandy soils in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Forde MB, Duke JA, Gibson P, Reed CF, Smith RR (1981) Trifolium fragiferum L. (strawberry clover). In ‘Handbook of legumes of world economic importance’. (Ed. JA Duke) pp. 238–241. (Plenum Press: New York, NY, USA)
Frame J (2004) ‘Forage legumes for temperate grasslands.’ (Science Publishers: Enfield, NH, USA)
Frame J (2005) Trifolium fragiferum L. In ‘Forage legumes for temperate grasslands’. (Ed. J Frame) pp. 168–173. (Food and Agriculture Organization of the United Nations: Rome, Italy)
Francis CM, Millington AJ, Bailey ET (1967) The distribution of oestrogenic isoflavones in the genus Trifolium. Australian Journal of Agricultural Research 18, 47–54.
| The distribution of oestrogenic isoflavones in the genus Trifolium.Crossref | GoogleScholarGoogle Scholar |
Galloway A, Cables J, Parsons D, Lane P, Hall E (2010) Growth and development of Lotus and Trifolium species under saline and waterlogging conditions. In ‘Food security from sustainable agriculture. Proceedings of 15th agronomy conference’. Lincoln, New Zealand, 15–18 November 2010. (Eds EH Dove, RA Culvenor). (The Regional Institute Online Publishing)
Gauch HG, Magistad OC (1943) Growth of strawberry clover varieties and of alfalfa and ladino clover as affected by salt. Agronomy Journal 35, 871–880.
| Growth of strawberry clover varieties and of alfalfa and ladino clover as affected by salt.Crossref | GoogleScholarGoogle Scholar |
Genesys (2022) Online Database - Available at https://www.genesys-pgr.org/a/overview [Accessed 2022]
Gerard PJ, Aalders LT, Hardwick S, Wilson DJ (2022) Investigation into the contrasting production of eight perennial clover cultivars in the first two years at field sites in in Waikato and Canterbury. New Zealand Journal of Agricultural Research 65, 271–289.
| Investigation into the contrasting production of eight perennial clover cultivars in the first two years at field sites in in Waikato and Canterbury.Crossref | GoogleScholarGoogle Scholar |
Givens DI, Owen E, Axford RFE, Omed HM (2000) ‘Forage evaluation in ruminant nutrition.’ (CAB International: Wallingford, UK)
Gladstones JS (1966) Naturalized subterranean clover (Trifolium subterraneum L.) in Western Australia: the strains, their distributions, characteristics, and possible origins. Australian Journal of Botany 14, 329–354.
| Naturalized subterranean clover (Trifolium subterraneum L.) in Western Australia: the strains, their distributions, characteristics, and possible origins.Crossref | GoogleScholarGoogle Scholar |
Gorman LW (1953) Strains of strawberry clover. Journal of New Zealand Grasslands 15, 151–156.
| Strains of strawberry clover.Crossref | GoogleScholarGoogle Scholar |
Grandison GS, Wallace HR (1974) The distribution and abundance of Pratylenchus thornei in fields of strawberry clover (Trifolium fragiferum). Nematologica 20, 283–290.
| The distribution and abundance of Pratylenchus thornei in fields of strawberry clover (Trifolium fragiferum).Crossref | GoogleScholarGoogle Scholar |
Gumbrell RC (1997) Redgut in sheep: a disease with a twist. New Zealand Veterinary Journal 45, 217–221.
| Redgut in sheep: a disease with a twist.Crossref | GoogleScholarGoogle Scholar |
Harrison MT, Cullen BR, Rawnsley RP (2016) Modelling the sensitivity of agricultural systems to climate change and extreme climatic events. Agricultural Systems 148, 135–148.
| Modelling the sensitivity of agricultural systems to climate change and extreme climatic events.Crossref | GoogleScholarGoogle Scholar |
Harrison MT, Cullen BR, Armstrong D (2017) Management options for dairy farms under climate change: Effects of intensification, adaptation and simplification on pastures, milk production and profitability. Agricultural Systems 155, 19–32.
| Management options for dairy farms under climate change: Effects of intensification, adaptation and simplification on pastures, milk production and profitability.Crossref | GoogleScholarGoogle Scholar |
Hayes RC, Li GD, Culvenor RA (2015) Changed recommendations for the use of phalaris on acid soils. In ‘Building productive, diverse and sustainable landscapes. Proceedings of the 17th Australian society of agronomy conference’. Hobart, Tas., Australia. 20–24 September 2015. (Eds T Acuña, C Moeller, D Parsons, M Harrison). (Australian Society of Agronomy)
Hayes RC, Li GD, Norton MR, Culvenor RA (2018) Effects of contrasting seasonal growth patterns on composition and persistence of mixed grass-legume pastures over 5 years in a semi-arid Australian cropping environment. Journal of Agronomy and Crop Science 204, 228–242.
| Effects of contrasting seasonal growth patterns on composition and persistence of mixed grass-legume pastures over 5 years in a semi-arid Australian cropping environment.Crossref | GoogleScholarGoogle Scholar |
Hayes RC, Ara I, Badgery WB, Culvenor RA, Haling RE, Harris CA, Li GD, Norton MR, Orgill SE, Penrose B, Smith RW (2019) Prospects for improving perennial legume persistence in mixed grazed pastures of south-eastern Australia, with particular reference to white clover. Crop & Pasture Science 70, 1141–1162.
| Prospects for improving perennial legume persistence in mixed grazed pastures of south-eastern Australia, with particular reference to white clover.Crossref | GoogleScholarGoogle Scholar |
Hayes RC, Newell MT, Pembleton KG, Peoples MB, Li GD (2021) Sowing configuration affects competition and persistence of lucerne (Medicago sativa) in mixed pasture swards. Crop & Pasture Science 72, 707–722.
| Sowing configuration affects competition and persistence of lucerne (Medicago sativa) in mixed pasture swards.Crossref | GoogleScholarGoogle Scholar |
Hayes RC, Condon JR, Li GD (2022) The role of liming in improving soil health. In ‘Improving soil health’. (Ed. W Horwath) pp. 403–441. (Burleigh Dodds Publishing: Cambridge, UK)
Hayes RC, Newell MT, Li GD, Haling RE, Harris CA, Culvenor RA, Badgery WB, Munday N, Price A, Stutz RS, Simpson RJ (2023) Legume persistence for grasslands in tableland environments of south-eastern Australia. Crop & Pasture Science 74,
| Legume persistence for grasslands in tableland environments of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Hofmann RW, Lin W, Stilwell SA, Lucas RJ (2007) Comparison of drought resistance in strawberry clover and white clover. In ‘Proceedings of the New Zealand Grassland Association’. Vol. 69. pp. 219–222. (New Zealand Grassland Association: Mosgiel, New Zealand)
Hollowell EA (1939) Strawberry clover. Leaflet No. 176. U.S. Department of Agriculture, Government Printing Office.
Howieson JG, Yates RJ, Foster KJ, Real D, Besier RB (2008) Prospects for the future use of legumes. In ‘Nitrogen-fixing leguminous symbioses.’ (Eds MJ Dilworth, EK James, JI Sprent, WE Newton) pp. 363–393. (Springer: Berlin, Germany)
IP Australia (2022) Plant breeders rights registration – strawberry clover (Trifolium fragiferum) variety: grasslands onward. IP Australia, Australian Government, Canberra, ACT.
Irwin JAG (1989) Diseases of pasture legumes in Australia. In ‘Persistence of forage legumes. Proceedings of trilateral workshop on legume persistence (Australia/New Zealand/USA). Hawaii’. (Eds GC Marten, AG Matches, RF Barnes, RW Broughton, RJ Clements, GW Sheath) (American Society of Agronomy: Madison, WI, USA)
Jones RAC (1996) Virus diseases of Australian pastures. In ‘Pasture and forage crop pathology’. (Eds S Chackraborty, KT Leath, RA Skipp, GA Pederson, RA Bray, GCM Latch, FW Nutter) pp. 288–307. (American Society of Agronomy: Madison, WI, USA)
Jones RAC (2013) Virus diseases of perennial pasture legumes in Australia: incidences, losses, epidemiology, and management. Crop & Pasture Science 64, 199–215.
| Virus diseases of perennial pasture legumes in Australia: incidences, losses, epidemiology, and management.Crossref | GoogleScholarGoogle Scholar |
Kao P-T, Darch T, McGrath SP, Kendall NR, Buss HL, Warren H, Lee MRF (2020) Chapter Four – Factors influencing elemental micronutrient supply from pasture systems for grazing ruminants. In ‘Advances in agronomy. Vol. 164’. (Ed. DL Sparks) pp. 161–229. (Academic Press: Cambridge, MA, USA)
Kellock AW, Stubbs LL, Parbery DG (1978) Seed-borne Fusarium species on subterranean clover and other pasture legumes. Australian Journal of Agricultural Research 29, 975–982.
| Seed-borne Fusarium species on subterranean clover and other pasture legumes.Crossref | GoogleScholarGoogle Scholar |
Kemp DR, Michalk DL, Goodacre M (2002) Productivity of pasture legumes and chicory in central New South Wales. Australian Journal of Experimental Agriculture 42, 15–25.
| Productivity of pasture legumes and chicory in central New South Wales.Crossref | GoogleScholarGoogle Scholar |
Kenny PT, Reed KFM (1984) Effects of pasture type on the growth and wool production of weaner sheep during summer and autumn. Australian Journal of Experimental Agriculture 24, 322–331.
| Effects of pasture type on the growth and wool production of weaner sheep during summer and autumn.Crossref | GoogleScholarGoogle Scholar |
Kidd D, Webb M, Malone B, Minasny B, McBratney A (2015) Digital soil assessment of agricultural suitability, versatility and capital in Tasmania, Australia. Geoderma Regional 6, 7–21.
| Digital soil assessment of agricultural suitability, versatility and capital in Tasmania, Australia.Crossref | GoogleScholarGoogle Scholar |
Lane LA, Ayres JF, Lovett JV (2000) The pastoral significance, adaptive characteristics, and grazing value of white clover (Trifolium repens L.) in dryland environments in Australia: a review. Australian Journal of Experimental Agriculture 40, 1033–1046.
| The pastoral significance, adaptive characteristics, and grazing value of white clover (Trifolium repens L.) in dryland environments in Australia: a review.Crossref | GoogleScholarGoogle Scholar |
Langlands JP, Donald GE, Bowles JE (1988) Cadmium concentrations in liver, kidney and muscle in Australian sheep and cattle. Australian Journal of Experimental Agriculture 28, 291–297.
| Cadmium concentrations in liver, kidney and muscle in Australian sheep and cattle.Crossref | GoogleScholarGoogle Scholar |
Langworthy AD, Rawnsley RP, Freeman MJ, Pembleton KG, Corkrey R, Harrison MT, Lane PA, Henry DA (2018) Potential of summer-active temperate (C3) perennial forages to mitigate the detrimental effects of supraoptimal temperatures on summer home-grown feed production in south-eastern Australian dairying regions. Crop & Pasture Science 69, 808–820.
| Potential of summer-active temperate (C3) perennial forages to mitigate the detrimental effects of supraoptimal temperatures on summer home-grown feed production in south-eastern Australian dairying regions.Crossref | GoogleScholarGoogle Scholar |
Lattimore MA, McCormick L (2012) ‘Pasture varieties used in New South Wales 2012-13.’ (NSW Department of Primary Industries and the Grassland Society of NSW: Orange, NSW, Australia)
Li GD, Lodge GM, Moore GA, Craig AD, Dear BS, Boschma SP, Albertsen TO, Miller SM, Harden S, Hayes RC, Hughes SJ, Snowball R, Smith AB, Cullis BC (2008) Evaluation of perennial pasture legumes and herbs to identify species with high herbage production and persistence in mixed farming zones in southern Australia. Australian Journal of Experimental Agriculture 48, 449–466.
| Evaluation of perennial pasture legumes and herbs to identify species with high herbage production and persistence in mixed farming zones in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Li GD, Hayes RC, McCormick JI, Gardner MJ, Sandral GA, Dear BS (2014) Time of sowing and the presence of a cover-crop determine the productivity and persistence of perennial pastures in mixed farming systems. Crop & Pasture Science 65, 988–1001.
| Time of sowing and the presence of a cover-crop determine the productivity and persistence of perennial pastures in mixed farming systems.Crossref | GoogleScholarGoogle Scholar |
Linnaeus C (1753) ‘Species Plantarum: exhibentes plantas rite cognitas, ad genera relatas, cum differentiis specificis, nominibus trivialibus, synonymis selectis, locis natalibus, secundum systema sexuale digestas.’ 1st edn. (Impensis Laurentii Salvii: Stockholm, Sweden)
Lucas D (2020) Assessment of size and scope of the certified temperate pasture seeds industry. AgriFutures Australia. Available at https://www.agrifutures.com.au/wp-content/uploads/2020/11/20-104.pdf
Lutts S, Lefèvre I (2015) How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Annals of Botany 115, 509–528.
| How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas?Crossref | GoogleScholarGoogle Scholar |
Marshall DR, Broue P, Munday J (1979) Tannins in pasture legumes. Australian Journal of Experimental Agriculture 19, 192–197.
| Tannins in pasture legumes.Crossref | GoogleScholarGoogle Scholar |
Masters DG, Norman HC, Dynes RA (2001) Opportunities and limitations for animal production from saline land. Asian-Australasian Journal of Animal Sciences 14, 199–211.
Masters DG, Benes SE, Norman HC (2007) Biosaline agriculture for forage and livestock production. Agriculture, Ecosystems & Environment 119, 234–248.
| Biosaline agriculture for forage and livestock production.Crossref | GoogleScholarGoogle Scholar |
McDonald KS (2006) Strawberry clover. In ‘Bulletin 4690. Perennial pastures for Western Australia.’ (Eds GA Moore, P Sanford, T Wiley) pp. 80–82. (Department of Agriculture and Food Western Australia: Perth, WA, Australia). https://library.dpird.wa.gov.au/bulletins/1/
McDonald KS, Cocks PS, Ewing MA (2005) Genetic variation in five populations of strawberry clover (Trifolium fragiferum cv. Palestine) in Western Australia. Australian Journal of Experimental Agriculture 45, 1445–1451.
| Genetic variation in five populations of strawberry clover (Trifolium fragiferum cv. Palestine) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
McGregor SE (1976) Chapter 3: Clover and some relatives – strawberry clover. In ‘Insect pollination of cultivated crop plants’. Agricultural Research Service, U.S. Department of Agriculture. https://www.ars.usda.gov/ARSUserFiles/20220500/OnlinePollinationHandbook.pdf
McVittie BJ, Hayes RC, Li GD, Sandral GA, Gardner MJ, McCormick JI, Lowrie R, Tidd J, Poile GJ (2012) Screening potential new perennial pasture legumes for tolerance to aluminium and manganese toxicities. In ‘Proceedings of the Australian legume symposium, 8–9 February, Melbourne’. (Ed. C Harris) pp. 55–57. (Australian Grasslands Association)
Mehanni AH, Repsys AP (1986) Perennial pasture production after irrigation with saline ground water in the Goulburn Valley, Victoria. Australian Journal of Experimental Agriculture 26, 319–324.
| Perennial pasture production after irrigation with saline ground water in the Goulburn Valley, Victoria.Crossref | GoogleScholarGoogle Scholar |
Milne GD (2011) Can pasture persistence be improved through the use of nonryegrass species? NZGA: Research and Practice Series 15, 157–162.
Minson DJ (1980) Nutritional differences between tropical and temperate pastures. In ‘Grazing animals.’ (Ed. FHW Morley) pp. 143–157. (Elsevier: Amsterdam, the Netherlands)
Moir J, Jordan P, Moot D, Lucas R (2016) Phosphorus response and optimum pH ranges of twelve pasture legumes grown in an acid upland New Zealand soil under glasshouse conditions. Journal of soil science and plant nutrition 16, 438–460.
| Phosphorus response and optimum pH ranges of twelve pasture legumes grown in an acid upland New Zealand soil under glasshouse conditions.Crossref | GoogleScholarGoogle Scholar |
Morley FHW (1963) The mode of pollination in strawberry clover (Trifolium fragiferum L.). Australian Journal of Experimental Agriculture 3, 5–8.
| The mode of pollination in strawberry clover (Trifolium fragiferum L.).Crossref | GoogleScholarGoogle Scholar |
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytologist 167, 645–663.
| Genes and salt tolerance: bringing them together.Crossref | GoogleScholarGoogle Scholar |
National Land and Water Resources Audit (2001) Australian dryland salinity assessment 2000 – extent, impacts, processes, monitoring and management options. p. 129. Land and Water Australia, Canberra, ACT, Australia.
Nichols PGH, Rogers ME, Craig AD, Albertsen TO, Miller SM, McClements DR, Hughes SJ, D’Antuono MF, Dear BS (2008) Production and persistence of temperate perennial grasses and legumes at five saline sites in southern Australia. Australian Journal of Experimental Agriculture 48, 536–552.
| Production and persistence of temperate perennial grasses and legumes at five saline sites in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Nichols PGH, Revell CK, Humphries AW, Howie JH, Hall EJ, Sandral GA, Ghamkhar K, Harris CA (2012) Temperate pasture legumes in Australia—their history, current use, and future prospects. Crop & Pasture Science 63, 691–725.
| Temperate pasture legumes in Australia—their history, current use, and future prospects.Crossref | GoogleScholarGoogle Scholar |
Norman HC, Humphries AW, Hulm E, Young P, Hughes SJ, Rowe T, Peck DM, Vercoe PE (2021) Productivity and nutritional value of 20 species of perennial legumes in a low-rainfall Mediterranean-type environment in southern Australia. Grass and Forage Science 76, 134–158.
| Productivity and nutritional value of 20 species of perennial legumes in a low-rainfall Mediterranean-type environment in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Norton MR, Li GD, Xu B, Price A, Tyndall P, Hayes RC (2021) Differences in dehydration tolerance affect survival of white clover (Trifolium repens) and lucerne (Medicago sativa) during a drying cycle. Crop & Pasture Science 72, 723–730.
| Differences in dehydration tolerance affect survival of white clover (Trifolium repens) and lucerne (Medicago sativa) during a drying cycle.Crossref | GoogleScholarGoogle Scholar |
Oram RN (1993) The case for increasing biodiversity in the legume component of southern Australian pastures. In ‘Alternative pasture legumes. Proceedings of the second national alternative pasture legumes workshop’. Technical Report 219. (Eds DL Michalk, AD Craig, WJ Collins) pp. 199–202. (Primary Industries South Australia: Adelaide, SA, Australia)
Oregon State University (2022) Strawbery clover Trifolium fragiferum L. Oregon State University, Corallis, OR, USA. Available at https://forages.oregonstate.edu/forages/strawberry-clover [Accessed 8 August 2022]
Pires AL, Ahirichs JL, Rhykerd CL (1992) Response of eleven forage species to treatment of acid soil with calcitic and dolomitic lime. Communications in Soil Science and Plant Analysis 23, 541–558.
| Response of eleven forage species to treatment of acid soil with calcitic and dolomitic lime.Crossref | GoogleScholarGoogle Scholar |
Quesenberry KH, Baltensperger DD, Dunn RA (1986) Screening Trifolium spp. for response to Meloidogyne spp. Crop Science 26, 61–64.
| Screening Trifolium spp. for response to Meloidogyne spp.Crossref | GoogleScholarGoogle Scholar |
Raguse CA, Henderson DW, Hull JL (1971) Perennial irrigated pastures. I. Plant, soil water, and animal responses under rotational and continuous grazing1. Agronomy Journal 63, 306–308.
| Perennial irrigated pastures. I. Plant, soil water, and animal responses under rotational and continuous grazing1.Crossref | GoogleScholarGoogle Scholar |
Real D, Oldham CM, Nelson MN, Croser J, Castello M, Verbyla A, Pradhan A, Van Burgel A, Méndez P, Correal E, Teakle NL, Revell CK, Ewing MA (2014) Evaluation and breeding of tedera for Mediterranean climates in southern Australia. Crop & Pasture Science 65, 1114–1131.
| Evaluation and breeding of tedera for Mediterranean climates in southern Australia.Crossref | GoogleScholarGoogle Scholar |
Reed KFM (2016) Fertility of herbivores consuming phytoestrogen-containing Medicago and Trifolium Species. Agriculture 6, 35
| Fertility of herbivores consuming phytoestrogen-containing Medicago and Trifolium Species.Crossref | GoogleScholarGoogle Scholar |
Refshauge G, Newell MT, Hopkins DL, Holman BWB, Morris S, Hayes RC (2022) The plasma and urine mineral status of lambs offered diets of perennial wheat or annual wheat, with or without lucerne. Small Ruminant Research 209, 106639
| The plasma and urine mineral status of lambs offered diets of perennial wheat or annual wheat, with or without lucerne.Crossref | GoogleScholarGoogle Scholar |
Rigg JL, Webster AT, Harvey DM, Orgill SE, Galea F, Dando AG, Collins DP, Harris CA, Newell MT, Badgery WB, Hayes RC (2021) Cross-host compatibility of commercial rhizobial strains for new and existing pasture legume cultivars in south-eastern Australia. Crop & Pasture Science 72, 652–665.
| Cross-host compatibility of commercial rhizobial strains for new and existing pasture legume cultivars in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Roberts RJ, Ridsdill-Smith TJ, Milner RJ, Walters PJ (1979) Insect pests of pastures. In ‘Pasture research on the Northern Tablelands of New South Wales’. (Ed. R Parkin) pp. 91–98. (Department of Agriculture New South Wales: Orange, NSW, Australia)
Rogers AL, Bailey ET (1963) Salt tolerance trials with forage plants in south-western Australia. Australian Journal of Experimental Agriculture and Animal Husbandry 3, 125–130.
| Salt tolerance trials with forage plants in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Rogers M, West D (1993) The effects of rootzone salinity and hypoxia on shoot and root growth in Trifolium species. Annals of Botany 72, 503–509.
| The effects of rootzone salinity and hypoxia on shoot and root growth in Trifolium species.Crossref | GoogleScholarGoogle Scholar |
Rogers ME, Noble CL, Pederick RJ (1997a) Identifying suitable temperate forage legume species for saline areas. Australian Journal of Experimental Agriculture 37, 639–645.
| Identifying suitable temperate forage legume species for saline areas.Crossref | GoogleScholarGoogle Scholar |
Rogers ME, Noble CL, Pederick RJ (1997b) Identifying suitable temperate forage legume species for saline areas. Australian Journal of Experimental Agriculture 37, 639–645.
| Identifying suitable temperate forage legume species for saline areas.Crossref | GoogleScholarGoogle Scholar |
Rogers ME, Craig AD, Munns RE, Colmer TD, Nichols PGH, Malcolm CV, Barrett-Lennard EG, Brown AJ, Semple WS, Evans PM, Cowley K, Hughes SJ, Snowball R, Bennett SJ, Sweeney GC, Dear BS, Ewing MA (2005) The potential for developing fodder plants for the salt-affected areas of southern and eastern Australia: an overview. Australian Journal of Experimental Agriculture 45, 301–329.
| The potential for developing fodder plants for the salt-affected areas of southern and eastern Australia: an overview.Crossref | GoogleScholarGoogle Scholar |
Rogers ME, Colmer TD, Frost K, Henry D, Cornwall D, Hulm E, Hughes S, Nichols PGH, Craig AD (2009) The influence of NaCl salinity and hypoxia on aspects of growth in Trifolium species. Crop & Pasture Science 60, 71–82.
| The influence of NaCl salinity and hypoxia on aspects of growth in Trifolium species.Crossref | GoogleScholarGoogle Scholar |
Rumball W, Claydon RB (2005) ‘GAO153’ turf type strawberry clover (Trifolium fragiferum L.). New Zealand Journal of Agricultural Research 48, 421–422.
| ‘GAO153’ turf type strawberry clover (Trifolium fragiferum L.).Crossref | GoogleScholarGoogle Scholar |
Rumball W, Claydon RB, Miller JE (1991a) ‘Grasslands Onward’ strawberry clover (Trifolium fragiferum L.). New Zealand Journal of Agricultural Research 34, 131–133.
| ‘Grasslands Onward’ strawberry clover (Trifolium fragiferum L.).Crossref | GoogleScholarGoogle Scholar |
Rumball W, Claydon RB, Miller JE (1991b) ‘Grasslands Upward’ strawberry clover (Trifolium fragiferum L.). New Zealand Journal of Agricultural Research 34, 135–136.
| ‘Grasslands Upward’ strawberry clover (Trifolium fragiferum L.).Crossref | GoogleScholarGoogle Scholar |
Rumbaugh MD, Pendery BM, James DW (1993) Variation in the salinity tolerance of strawberry clover (Trifolium fragiferum L.). Plant and Soil 153, 265–271.
| Variation in the salinity tolerance of strawberry clover (Trifolium fragiferum L.).Crossref | GoogleScholarGoogle Scholar |
Russell JS (1976) Comparative salt tolerance of some tropical and temperate legumes and tropical grasses. Australian Journal of Experimental Agriculture 16, 103–109.
| Comparative salt tolerance of some tropical and temperate legumes and tropical grasses.Crossref | GoogleScholarGoogle Scholar |
Sandral GA, Price A, Hildebrand SM, Fuller CG, Haling RE, Stefanski A, Yang Z, Culvenor RA, Ryan MH, Kidd DR, Diffey S, Lambers H, Simpson RJ (2019) Field benchmarking of the critical external phosphorus requirements of pasture legumes for southern Australia. Crop & Pasture Science 70, 1080–1096.
| Field benchmarking of the critical external phosphorus requirements of pasture legumes for southern Australia.Crossref | GoogleScholarGoogle Scholar |
Schachtman DP, Kelman WM (1991) Potential of Lotus germplasm for the development of salt, aluminium and manganese tolerant pasture plants. Australian Journal of Agricultural Research 42, 139–149.
| Potential of Lotus germplasm for the development of salt, aluminium and manganese tolerant pasture plants.Crossref | GoogleScholarGoogle Scholar |
Shipton WA (1967) Diseases of clovers in Western Australia. Journal of Agriculture, Western Australia 8, 289–292.
Singh DK, Sale PWG (1997a) Defoliation frequency and the response by white clover to increasing phosphorus supply 2. Non-structural carbohydrate concentrations in plant parts. Australian Journal of Agricultural Research 48, 119–124.
| Defoliation frequency and the response by white clover to increasing phosphorus supply 2. Non-structural carbohydrate concentrations in plant parts.Crossref | GoogleScholarGoogle Scholar |
Singh DK, Sale PWG (1997b) Defoliation frequency and the response by white clover to increasing phosphorus supply 1. Leaf dry matter yield and plant morphology responses. Australian Journal of Agricultural Research 48, 111–118.
| Defoliation frequency and the response by white clover to increasing phosphorus supply 1. Leaf dry matter yield and plant morphology responses.Crossref | GoogleScholarGoogle Scholar |
Somerville D (2019) ‘Honey and pollen flora of south-eastern Australia.’ (New South Wales Department of Primary Industries: Paterson, NSW, Australia)
Stafford AD, Anderson CWN, Hedley MJ, McDowell RW (2016) Cadmium accumulation by forage species used in New Zealand livestock grazing systems. Geoderma Regional 7, 11–18.
| Cadmium accumulation by forage species used in New Zealand livestock grazing systems.Crossref | GoogleScholarGoogle Scholar |
Stevenson K, Zhou MX, Smith RW (2017) Screening for waterlogging tolerance in strawberry clover and other perennial legumes. In ‘Doing more with less. 18th Australian society of agronomy conference’. Ballarat, Vic., Australia. 24–28 September 2017. (Eds GJ O’Leary, RD Armstrong, L Hafner) Available at http://agronomyaustraliaproceedings.org/images/sampledata/2017/165_ASA2017_Stevenson_Kristy_Final.pdf
Striker GG, Colmer TD (2017) Flooding tolerance of forage legumes. Journal of Experimental Botany 68, 1851–1872.
| Flooding tolerance of forage legumes.Crossref | GoogleScholarGoogle Scholar |
Stutz RS, De Faveri J, Culvenor RA (2023) Legumes for lambs: perennial options for summer finishing pastures in southern New South Wales. Crop & Pasture Science. (Under review – This Issue).
Teakle N, Flowers T, Real D, Colmer T (2007) Lotus tenuis tolerates the interactive effects of salinity and waterlogging by ‘excluding’ Na+ and Cl− from the xylem. Journal of Experimental Botany 58, 2169–2180.
| Lotus tenuis tolerates the interactive effects of salinity and waterlogging by ‘excluding’ Na+ and Cl− from the xylem.Crossref | GoogleScholarGoogle Scholar |
Tiver NS (1954) Strawberry clover. Journal of the Department of Agriculture of South Australia 57, 317–325.
Townsend CE (1985) Miscellaneous perennial clovers. In ‘Clover science and technology’. (Ed. NL Taylor) pp. 563–578. (American Society of Agronomy)
Van Keuren RW, Hoveland CS (1985) Clover management and utilization. In ‘Clover science and technology’. Agronomy Monograph No. 25. (Ed. NL Taylor) pp. 325–354. (ASA-CSSA-SSSA: Madison, WI, USA)
Ward PR, Micin SF (2006) The capacity of dryland lucerne for groundwater uptake. Australian Journal of Agricultural Research 57, 483–487.
| The capacity of dryland lucerne for groundwater uptake.Crossref | GoogleScholarGoogle Scholar |
Wheeler DM, Dodd MB (1995) The effect of aluminium on the growth of a range of temperate legume species and cultivars: a summary of results. In ‘Plant–soil interactions at low pH: principles and management. Proceedings of the third international symposium on plant-soil interactions at low pH’. Brisbane, Qld, Australia. 12–16 September 1993. (Eds RA Date, NJ Grundon, GE Rayment, ME Probert) pp. 433–437. (Springer Netherlands: Dordrecht, the Netherlands)
Whittet JN (1964a) Pasture plant introductions. In ‘Pastures.’ pp. 491–514. (Department of Agriculture, New South Wales: Sydney, NSW)
Whittet JN (1964b) Pastures under irrigation. In ‘Pastures.’ pp. 352–399. (Department of Agriculture: New South Wales: Sydney, NSW)
Williams RD (1931) Welsh PI. Breed. Stn Bull. Ser. H. 12, 2.
Wright DSC (1964) Self- and cross-fertility in strawberry clover (Trifolium fragiferum L.). New Zealand Journal of Agricultural Research 7, 32–36.
| Self- and cross-fertility in strawberry clover (Trifolium fragiferum L.).Crossref | GoogleScholarGoogle Scholar |
Zohary M, Heller D (1984) ‘The genus Trifolium.’ (The Israel Academy of Sciences and Humanities: Jerusalem, Israel)


