Links between soilborne pathogens, plant parasitic nematodes, farm management and biophysical constraints in a southern Australian rainfed cropping system
Martin Harries A B * , Ken C. Flower B , Michael Renton B C , Sarah J. Collins D and Daniel Hüberli D
A B * , Ken C. Flower B , Michael Renton B C , Sarah J. Collins D and Daniel Hüberli D
A Department of Primary Industries and Regional Development (DPIRD), Government of Western Australia, 20 Gregory Street, Geraldton 6530, WA, Australia.
B UWA School of Agriculture and Environment and UWA Institute of Agriculture, The University of Western Australia, 35 Stirling Highway, Crawley 6009, WA, Australia.
C School of Biological Sciences, The University of Western Australia, 35 Stirling Highway, Crawley 6009, WA, Australia.
D Department of Primary Industries and Regional Development (DPIRD), Government of Western Australia, 3 Baron-Hay Court, Perth 6151, WA, Australia.
Crop & Pasture Science 73(11) 1291-1307 https://doi.org/10.1071/CP21778
Submitted: 18 November 2021 Accepted: 1 April 2022 Published: 22 June 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Rotations in rainfed farming systems of southwest Australia have shifted towards intensified cropping and it is necessary to reassess soilborne pathogens and plant parasitic nematodes within this context.
Aims: We tested the hypothesis that these recent changes in rotations and agronomy have altered the efficacy with which rotations reduce the incidence of common root pathogens and plant parasitic nematodes.
Methods: We tracked changes in common pathogen DNA in soil and the incidence and severity of crop root damage in 184 paddocks, over 6 years from 2010 to 2015, and related this to farmer practices.
Key results: Overall, severe root damage was rare, with 72% of plant samples showing no damage or only a trace and only 1% severely damaged. We found that the reduction of paddocks in pasture and resultant very low weed populations, combined with early sowing, reduced persistence of pathogens and nematode pests. But some aspects of crop management had the opposite effect: high rates of herbicide, increased frequency of cereals and canola at the expense of lupin and increased N fertiliser use.
Conclusions: Current agronomic practices and the frequency of non-host crops in rotations appear to be effective in controlling common root pathogens and plant parasitic nematodes. But the aspects of agronomic management that increased populations of pathogens should be applied cautiously.
Implications: Studies such as this that link multiple productivity constraints, such as pathogens and nematode pests, weeds and nutrients, to management practices are important to understand the sustainability of current or proposed production methods.
Keywords: agronomy, crop pathogens, crop rotation, crown rot, rainfed, rhizoctonia bare patch, root lesion nematode, take all.
Introduction
The most widespread and economically important soil root pathogens or plant parasitic nematodes of grain production in southwest Western Australia (WA) are Fusarium pseudograminearum and Fusarium culmorum complex (crown rot), Gaeumannomyces graminis var. tritici (take all), Pratylenchus neglectus, Pythium clade F. and Rhizoctonia solani Anastomosis Group (AG) 8 (rhizoctonia bare patch), (Riley and Kelly 2002; Vanstone 2002; Murray and Brennan 2009a, 2009b; Thomas et al. 2010; Khangura et al. 2013). These pathogens and nematode pests have been estimated to cause yield losses in wheat (Triticum aestivum) of AUD234 million per annum and AUD60 million per annum in barley (Hordeum vulgare) (Murray and Brennan 2009a, 2010) throughout Australia.
The 14 million hectares of mixed crop and pasture farms in southwest Australia, with a rainfed Mediterranean-type climate, supplied ∼30% of Australia’s broadacre grain over the period of this study (2010–2015) (ABS 2016).
Increasingly in dryland cropping areas throughout the world, including southwest Australia, the practise of stubble retention has been adopted. Some of the reasons this occurred were because herbicide (FAO 2020b) and nitrogen fertiliser (FAO 2020a) usage increased and tillage was utilised less for weed control and to mineralise nitrogen. This trend has continued with various forms of reduced tillage seeding machinery being widely adopted (Llewellyn et al. 2012; Llewellyn and Ouzman 2019) and due to increasing environmental concerns and regulation around the burning of crop residues, as shown by Abdurrahman et al. (2020).
Increased stubble retention has resulted in increased water use efficiency and provided a range of other benefits such as reduced soil erosion and improved soil structure (Derpsch et al. 2010; Fisher and Hobbs 2019), but has also been reported to increase the prevalence of root and stubble borne plant pathogens (Bockus and Shroyer 1998; Paulitz et al. 2002, 2006; Kirkegaard et al. 2011). There are several documented cases of minor pathogens becoming prominent under these faming systems throughout the 1970s and 1980s, i.e. R. solani (MacNish 1985; Weller et al. 1986; Pumphrey et al. 1987; Paulitz 2006), G. graminis (Cotterill and Sivasithamparam 1989), tan spot (Septoria tritici) (Bockus and Shroyer 1998) and Pythium spp. (Cook et al. 1980).
Historically crop and pasture rotations have also been a major influence on soilborne pathogens and nematode pests. A pertinent example is the southern Australian ley farming systems of the 1970s, when intensive cereal production intermixed with grassy pasture resulted in G. graminis outbreaks causing ∼40% yield loss in wheat in higher rainfall regions (>350 mm long term average growing season rainfall) (Cotterill and Sivasithamparam 1989; Kirkegaard et al. 2011). This continued until the disease cycle was broken by using grass selective herbicides and more diverse rotations, including legume break crops (Anderson et al. 2005).
More recently a shift towards cropping rather than sheep production, due to commodity price fluctuations, improved economies of scale and technological advances in crop production (Kirkegaard et al. 2011), triggered a heavy reliance on herbicides, leading to the evolution of weeds resistant to herbicides (Heap 2020). The widespread occurrence of herbicide resistant weeds in southwest Australia (Walsh et al. 2007; Owen and Powles 2009; Owen et al. 2014; Heap 2020) has made weed control more difficult to achieve and one response by farmers has been to increase production of crops in which weeds can be more effectively controlled (Harries et al. 2020).
Between 2000 and 2015, the area per farm dedicated to pasture declined by up to 30% in some agroecological zones of southwest Australia and sheep numbers declined from 26 to 14 million head (Planfarm and Bankwest 2016). The area sown to wheat, barley and canola (Brassica napus) increased in the period 2000 to 2016 by 0.7, 0.3 and 0.7 million hectares respectively, while grain legume crop area declined by 0.73 million hectares. This has resulted in substantial changes to rotations (Harries et al. 2015) and associated agronomy.
These changes in rotations not only directly impact soil pathogen populations, due to changes in the frequency of production of host crops, but also indirectly impact pathogen populations as a result of changes to other aspects of the agroecosystem, i.e. alternative host populations and nitrogen and water supply. The investigation of these interrelationships is often constrained by a lack of data linking biophysical changes to agronomic management at the field level (Lacoste 2017; Peterson et al. 2018).
We tested the hypothesis that these recent changes in rotations and agronomy have altered the efficacy with which rotations reduce the incidence of common root pathogens and nematode pests. We do this by tracking changes in common pathogen DNA in soil and the incidence and severity of root damage in paddocks, over 6 years from 2010 to 2015, and relating this to farmer practices within these paddocks.
Data sources
Data were accessed from the Focus Paddock dataset, which paired records of biophysical measurements of weeds, soilborne diseases and parasitic nematodes, and soil chemical and physical properties to land management actions from the same paddocks from 2010 to 2015. Paddocks were selected by targeting two or three soil types on each farm, which were common to the area. At face-to-face interviews instructions were given to farmers that they should identify paddocks that reflected the majority of paddocks on the farm, not to select either their poorest or best performing paddocks. In total 184 paddocks were selected, providing 1017 paddock-years, after accounting for missing data; geographically 346 in the Central Agricultural Region (CAR), 416 in the Northern Agricultural Region (NAR) and 255 in the Southern Agricultural Region (SAR) (Fig. 1).
All field measurements were from a geo-referenced one-hectare area within each paddock. Farmers who managed the paddocks were interviewed annually, providing information on land use and agronomic inputs, and insights into management rationale. Wheat was grown in all paddocks in the first year of monitoring, followed by farmer-specified land uses in the following years. Climate data were obtained for each paddock using the SILO (Scientific Information for Land Owners) database (Jeffrey et al. 2001).
Soilborne pathogen DNA concentrations within soil
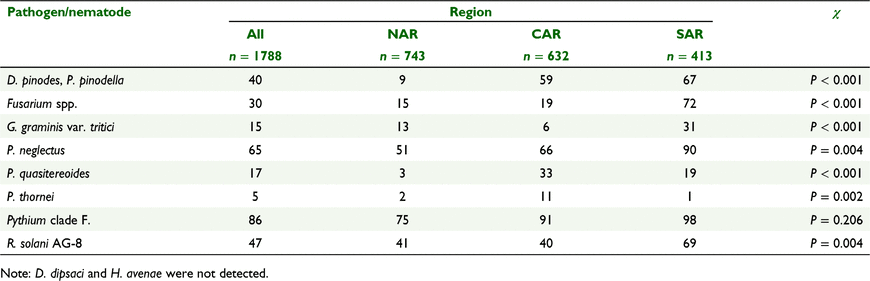
To assess levels of fungal plant pathogens and plant parasitic nematodes within the soil, the one-hectare area was divided into four nested replicates of 25 m by 100 m and soil was sampled in a zig-zag transect through each. Samples were taken on the previous crop row, where evident, twice per year, prior to seeding (February–April) and at anthesis (Zadoks 65) (August–October) (Zadoks et al. 1974), with 44 cores (11 from each replicate pooled) taken to a depth of 10 cm, without clearing the soil surface of stubble, using a 1 cm diameter Accucore® soil probe. This sampling procedure ensured that soil samples contained some plant residues and roots. Overall, 1751 DNA assays of each pathogen were matched to land uses, with 947 assays taken in autumn (pre-sowing) and 804 in spring (in crop or pasture). Samples were taken prior to 59 barley crops, 137 canola crops, 68 lupin (Lupinus angustifolius) crops, 122 pastures, 533 wheat crops and 28 other land uses, which comprised of chickpea (Cicer arietinum), faba bean (Vicia faba), fallow (chemical fallow sprayed in spring), field pea (Pisum sativum), oat (Avena sativa), oaten hay and vetch (Vicia spp.). These soil samples were air dried in a laboratory, if wet, and sent immediately to a commercial laboratory (the South Australian Research and Development Institute, Urrbrae, South Australia) for DNA analysis, using the widely tested and used PREDICTA B® assay (Hollaway et al. 2003; Ophel-Keller et al. 2008; Wicks et al. 2011; Poole et al. 2015; Hay et al. 2016). We present data of ten pathogens and nematode pests or complexes analysed for DNA content in soil: Didymella pinodes and Phoma pinodella, (black spot of field pea), Ditylenchus dipsaci (stem nematode), F. pseudograminearum and F. culmorum (crown rot), G. graminis var. tritici (take all), Heterodera avenae (cereal cyst nematode), P. neglectus, P. quasitereoides, P. thornei, Pythium Clade F., and R. solani (AG-8).
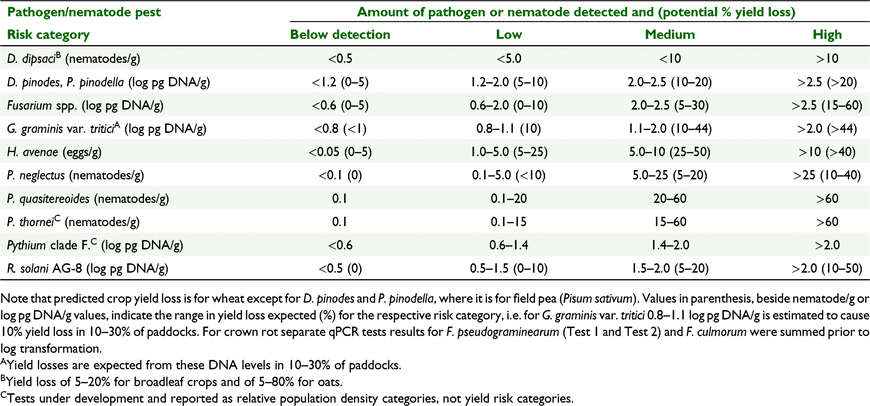
Soil DNA results are presented in three ways. First, results are reported as frequency of results within disease risk categories defined for the PREDICTA B® assay, which predicts crop yield loss based on autumn DNA concentrations in soil, log10(x + 1) transformed, for fungal pathogens and eggs of the cyst nematode, or on copies per gram of soil for root-lesion nematodes (Table 1). Second, results are shown as incidence of assays above the DNA detection limit, low risk or above (Table 1). Third, results are indicated using the DNA concentration reported from the assay, to obtain more precise analysis of pathogen DNA soil concentrations.
|
Visual assessment of pathogen damage to crop and pasture roots
To assess plant root and crown damage caused by root pathogens and nematode pests, 40 whole plants (10 per nested replicate) were taken from the hectare area in the same zig-zag pattern described above for soil samples. This was done twice each year from 2010 to 2014 inclusive: firstly in autumn or winter when plants were in the vegetative stage (Zadoks 1 for wheat), or the equivalent growth stage for other species, and secondly in spring, at anthesis (Zadoks 65 or equivalent). The number of plants from each paddock displaying root or crown damage symptomatic of Fusarium spp., G. graminis var. tritici, P. neglectus and R. solani (AG-8) were recorded. An overall rating of percentage severity of root damage (SRD) caused by all pathogens and nematode pests combined was given on a 0–5 scale: 0 (no disease), 1 = 1–5% (trace disease), 2 = 6–25% (low amount of brown lesions), 3 = 26–50% (medium amount of brown lesions, similar amounts of healthy and necrotic), 4 = 51–75% (most of the roots covered in brown lesions, little healthy root left) and 5 = 76–100% (all or nearly all roots covered in brown lesions or short brown stumps), similar to the method of McDonald and Rovira (1985).
Overall plant samples were taken from 1289 field visits, with 634 taken at the vegetative development stage from 51 barley crops, 72 canola crops, 52 lupin crops, five pastures, 439 wheat crops and 15 other land uses, and the remaining 655 samples taken at anthesis from a similar break-up of land uses.
Plant observations are presented in three ways. Firstly, the incidence of symptoms caused by Fusarium spp., G. graminis, P. neglectus and R. solani (AG-8) is reported, expressed as % of field visits from which symptoms were observed on roots of at least one of the 40 plants sampled. Secondly, the frequency of diseased plants in each 40-plant sample is shown, expressed as percentage of plants. Thirdly, the severity of disease is presented, expressed as SRD, as described above.
Statistical analysis
Binomial logistic regressions were conducted as single regressions, one predictor at a time, to test whether the incidence of positive DNA test results and incidence of paddock-years with root disease observed were related to each of the variates presented in Table 2. For the economically important pathogens and nematode pests, for which we had both DNA and root disease symptom observations (Fusarium spp., G. graminis var. tritici, P. neglectus and R. solani), binomial generalised linear models, with multiple predictors, were developed to predict the likelihood of paddocks having DNA above the detection limit or root disease symptoms in spring. We used data collected in spring for these analyses to capture effects of crop management. Optimisation of models was based on the lowest Akaike Information Criterion via the R step function. The incidence of positive DNA test results, from both the Z1 and Z65 sampling times combined, was also assessed with Chi-squared tests of goodness of fit, to determine whether the incidence of DNA assays above detection of each of these pathogens and nematode pests were the same across the three regions, and 6 years, of the survey.

|
Differences in concentration of DNA in soil among independent variates (Table 2) in both autumn and spring were assessed using ANOVA. To account for positive skewed distribution, DNA data for D. pinodes and P. pinodella, D. dipsaci, Fusarium spp., G. graminis var. tritici, Pythium Clade F., and R. solani (AG-8) were transformed using log10(x + 1) prior to ANOVA. If ANOVA indicated overall difference (P ≤ 0.05), t-tests and their pairwise comparisons or TukeyHSD tests were applied. Paired t-tests, based on individual paddocks, were made to determine if changes in soil DNA concentration from autumn to spring were statistically significant. Root and crown disease data were assessed in the same manner as soil DNA concentration, using ANOVA and Tukey HSD tests.
Results
Climatic conditions
Western Australia has a Mediterranean-type climate in which the grain growing season occurs between May and November. There were large differences in rainfall between years and regions with 2010 and 2011 being the years with greatest contrast (Fig. 2); annual rainfall ranging from 196 mm for the CAR in 2010, to 546 mm for the SAR in 2011 (Fig. 3).
DNA assay results
DNA concentrations within PREDICTA B risk levels
Averaged across all pathogens and nematode pests, 70% of assays were below the detection limit, 20% low, 7% medium and 3% high disease risk (Fig. 4). Because H. avenae and D. dipsaci were not detected and <1% of P. thornei and P. quasitereoides were within medium and high-risk categories, statistical analyses of these pathogens and nematode pests were limited. Incidence of DNA in the high yield loss category was greatest (13%) for D. pinodes/P. pinodella, pathogens that cause blackspot of field pea. Approximately 7% of Fusarium spp. and R. solani (AG-8) autumn DNA tests were in the high yield loss category, while all other pathogens and nematode pests were below 3.4% (Fig. 4). The incidence of paddocks with DNA below detection for each pathogen or parasitic nematodes in spring were similar to autumn (data not presented).
Incidence of DNA assays above detection limit
There were large differences in the incidence of DNA assays above detection between pathogens and nematode pests for the total of autumn and spring samples, i.e. D. dipsaci and H. avenae were not detected, compared to Pythium spp. detected in 86% of samples. Apart from D. dipsaci and H. avenae the incidence of DNA detected of each pathogen, except Pythium spp., differed between regions (Table 3). For six of the eight species detected incidence was lowest in the NAR, and for six highest incidence occurred in the SAR. Hence, in general, SAR paddocks had a higher incidence of soil DNA concentrations above detection, although there were some exceptions, including the CAR with the highest detections of P. quasitereoides and P. thornei.
The incidence of most pathogens and nematode pests remained stable over the years of the survey, with the exceptions of highly significant increases for Fusarium spp. and G. graminis var. tritici, and there was also an increasing trend for P. neglectus but this was not significant (Fig. 5).
Binomial logistic regressions of incidence of pathogen DNA in spring against environmental and management variates showed that for Fusarium spp., G. graminis var. tritici, P. neglectus and R. solani (AG-8) there were highly significant (P < 0.001) effects of air temperature and soil organic carbon (%). The negative coefficients in the regressions of temperature variates meant that lower temperature was related to a higher probability of DNA levels being above detection limit, while the positive coefficient for organic carbon indicate more organic carbon was related to higher probability of DNA above detection (Table 4).
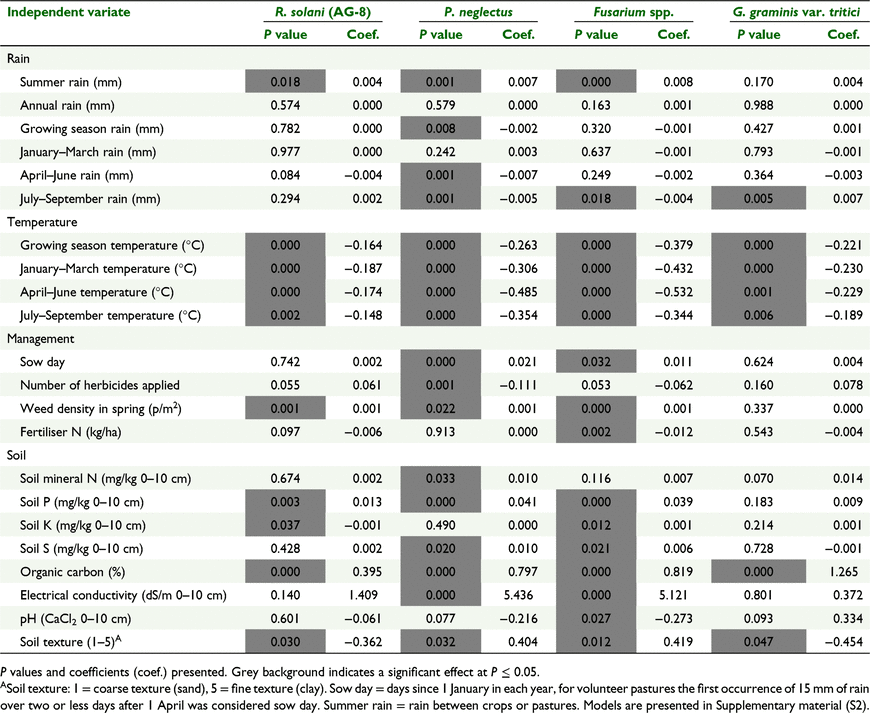
|
More summer rain, in the period between growing seasons, was associated with increased frequency of DNA of Fusarium spp., P. neglectus and R. solani (AG-8) but not G. graminis var. tritici., while January–March rain was not significant (P > 0.242). This occurred because summer rain was calculated from the date of crop maturity in the previous year and averaged 84 mm, compared to January–March rain which averaged 48 mm. Hence rain in October–December increased the frequency of DNA detection. Soil texture was also significant (P < 0.05); G. graminis var. tritici and R. solani (AG-8) DNA was more frequently detected on finer textured soils, and Fusarium spp. and P. neglectus on sandier soils. Other factors of note were that higher soil N, P and S concentration resulted in more frequent P. neglectus DNA and in paddocks with more weeds there was more frequent detection of Fusarium spp., R. solani (AG-8) and P. neglectus DNA (Table 4).
DNA concentration
When sampled in autumn the soil concentrations of all pathogens and P. neglectus were greater (P ≤ 0.05) in the SAR than the CAR and NAR (Fig. 6). The same was found from spring sampling, with the exceptions of P. neglectus in the NAR not being different to the SAR (P = 0.076) and D. pinodes/P. pinodella in the CAR not being different to the SAR (P = 0.370), data not presented. There were no differences (P > 0.05) in soil DNA concentration of G. graminis, Fusarium spp. and R. solani between the NAR and CAR in both autumn and spring. There were differences between these regions (P ≤ 0.05) for Pythium spp. and D. pinodes/P. pinodella in both autumn and spring and for P. neglectus there was a difference (P < 0.05) in autumn but not in spring.
Of all the land uses, when sampled in autumn, pastures contained the greatest DNA concentration for each pathogen assessed, with concentrations greater, (P ≤ 0.05) than some of the other land uses for all pathogens and nematode pests (Fig. 7). Interestingly for P. neglectus, canola, which is a host, was sown into paddocks with higher autumn DNA concentration than lupin, despite lupin being a non-host, as indicated by the reduction (P ≤ 0.024) of P. neglectus DNA concentration from autumn to spring within lupin crops (Fig. 7). Changes in DNA concentration from autumn to spring were mostly as expected, with increases associated with host crops, and pasture, and decreases for non-hosts, although not all changes were statistically significant. For example, within canola paddocks there were reductions from autumn to spring (P ≤ 0.05) in DNA concentration of G. graminis and R. solani (AG-8), a large reduction in Fusarium spp., which was not significant (P = 0.627), and an increase in P. neglectus which was also not significant (P = 0.243) (Fig. 7). Spring pathogens/nematode pest carried through to the following wheat crop, i.e. low DNA of Fusarium spp., R. solani (AG-8) and G. graminis var. tritici after canola and low P. neglectus eggs/g of soil after lupin (Supplementary material S1).
Plant observations
Incidence of field visits with disease observed
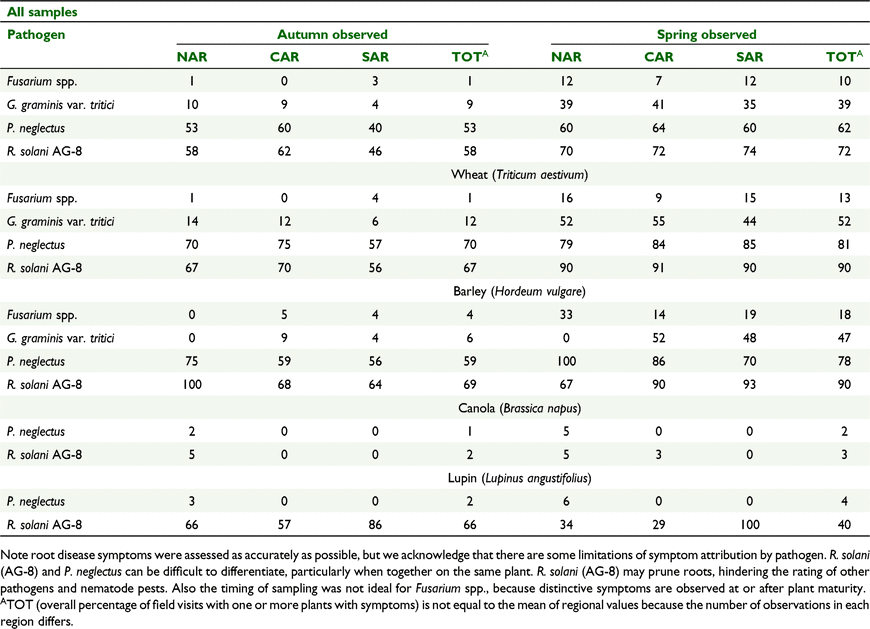
Plants displaying root disease symptoms (autumn and spring visits combined) of Fusarium spp. were observed from 6% of visits, G. graminis 24%, P. neglectus 58% and R. solani (AG-8) 65%. Root damage from all pathogens and nematode pests was more frequent in spring: Fusarium spp. observed in 10% of spring samples, G. graminis 39%, P. neglectus 62% and R. solani (AG-8) 72% (Table 5).
|
For the binomial logistic regression of root disease symptoms (presence/absence) fewer variate/pathogen combinations with a P value < 0.05 than for binomial logistic regressions of DNA. In common with the DNA analysis there were highly significant (P < 0.001) effects of air temperature; greater incidence of plant root disease with lower temperatures for all pathogens/nematode pests except Fusarium spp. Higher soil N, P and S content resulted in greater frequency of paddocks with root damage from P. neglectus. In contrast to the DNA, less rainfall in various time periods, associated with negative coefficients in the regressions of rain variates, meant that lower rainfall was related to increased frequency of paddocks containing plants with damage symptoms of each pathogen. Also summer rain only affected the frequency of paddocks with plant damage caused by G. graminis var. tritici (Table 6).

|
Frequency of diseased plants in each 40 plant sample
In autumn the proportion of plants within the 40 plant sample with root or crown symptoms was 0%, 1.1%, 18.7% and 9.7% for Fusarium spp., G. graminis, P. neglectus and R. solani (AG-8) respectively. Root or crown damage occurred on more plants in spring; 10%, 39%, 62% and 72% of the 40 plant sample for Fusarium spp., G. graminis, P. neglectus and R. solani (AG-8) respectively. Hence for all pathogens few samples contained a large proportion of plants displaying symptoms (Supplementary material S3). The percentage of plants with symptoms of Fusarium spp., P. neglectus and R. solani (AG-8) increased with the number of wheat crops grown in succession, consequently the incidence of plants with symptoms of at least one root pathogen also increased when paddocks were successively sown to wheat (Fig. 8).
Severity of root disease (SRD)
Most samples were rated as having low disease severity: 25% displayed no disease symptoms, 47% a trace, with only 1% with most roots diseased and none with all roots diseased, as per the 0–5 rating scale. Mean SRD from all samples (autumn and spring visits) was 0.69. The severity of root diseased was greater in wheat (0.84 ± s.e.m. 0.03) and barley (0.84 ± 0.09) than canola (0.11 ± 0.03) and lupin (0.22 ± 0.04); lower in the SAR (0.52 ± 0.05) than the CAR (0.73 ± 0.04) and NAR (0.74 ± 0.03); and declined over the survey period, with a reduction in each subsequent year: 2010 (1.5 ± 0.03), 2011 (0.95 ± 0.12), 2012 (0.63 ± 0.04), 2013 (0.43 ± 0.04) and 2014 (0.24 ± 0.02). Severity of root damage did not increase in the second or third wheat crops in succession (Fig. 8).
Discussion
Overall incidence and severity of disease
Overall, our results showed that farmers are managing soilborne pathogens and plant parasitic nematodes effectively, with few instances of severe root disease. However, these organisms were distributed widely throughout the survey area, particularly R. solani (AG-8) and P. neglectus, and recent changes in rotations and agronomy have altered the efficacy with which rotations can be used to manage common soil pathogens.
The incidence of each pathogen or nematode pest are reflective of documented changes in their prominence across southwest Australia, with reports of reduced prominence of G. graminis var. tritici, and H. avenae (Cotterill and Sivasithamparam 1989; Vanstone et al. 2008; Kirkegaard et al. 2011) and increased frequency of R. solani (AG-8) and P. neglectus (Vanstone et al. 2008; Khangura et al. 2013). The incidences of DNA above detection were mostly similar to those previously found in this region: H. avenae and stem nematode undetected (Flower et al. 2019), P. neglectus 70% (Vanstone 2002) and Fusarium spp. (41%), but lower for R. solani (AG-8) (81%) (Khangura et al. 2013). The number of plants per sample with root or crown symptoms of these pathogens and nematodes also reflected the current prominence of these organisms, overall ∼15% of plants with R. solani (AG-8) and P. neglectus symptoms, compared to ∼1% for G. graminis var. tritici and Fusarium spp.
Pathogen dynamics by land use
Host/non-host relationships were identified by comparing autumn and spring pathogen DNA concentrations in soil. These matched known host ranges for each pathogen, although not always at statistically significant levels. An exception to this was the significant reduction in R. solani (AG-8) DNA over the growing season (autumn to spring) in paddocks sown to canola, which was accompanied by a low proportion (5%) of canola plants with symptoms of R. solani (AG-8) damage compared to the other crops (∼67%). This contradicts previous management guidelines, that R. solani (AG-8) has such a wide host range that it cannot be controlled using rotations (MacLeod et al. 2008), and adds to a growing body of evidence suggesting canola is a poor host of R. solani AG-8 (Gupta et al. 2010; Babiker et al. 2013; Hüberli et al. 2013; Flower et al. 2019). Interestingly P. neglectus DNA increased within canola crops and this carried through to following wheat crops, but only a small proportion of canola plants (2%) showed P. neglectus symptoms, which indicates canola is a tolerant host, as previously reported (Smiley et al. 2014; Flower et al. 2019) and visual assessment of symptoms is not a good indicator of susceptibility.
Rotations
Cereals accounted for 66% of paddock-years in the Focus Paddock dataset (Harries et al. 2020) and remain the most frequently grown crops in southwest Australia, but importantly canola production has increased from 0.4 to 1.4 million hectares over the period 2005 to 2015 (ABS 2016) and inclusion of canola at the expense of lupin is likely to result in the build-up of P. neglectus throughout the survey area (Riley and Kelly 2002). Indeed, paddocks sown to canola had higher P. neglectus soil DNA concentration than those sown to lupin, indicating that this may have already occurred. Conversely, inclusion of canola in place of pasture reduces the inoculum load of Fusarium spp. and G. graminis var. tritici, R. solani (AG-8) and D. pinodes/P. pinodella, within subsequent wheat and barley crops. In contrast, the recent increase in frequency of cereal production needs to be managed carefully because we found increased incidence of plant symptoms caused by several pathogens and increasing overall incidence of root disease symptoms in longer sequences of wheat monoculture and increased incidence of DNA assays of Fusarium spp. and G. graminis var. tritici over the period of the survey.
Management factors
Weed density and herbicide applications
One reason for the increased use of canola is the excellent weed control obtained in this crop. Harries et al. (2020) conducted detailed analyses of weed dynamics with the Focus Paddocks observing that glyphosate tolerant canola crops averaged 4 grass weeds/m2 in spring compared to 14/m2 for wheat and 562/m2 for pasture. The low density of weeds/alternative pathogen hosts in canola may be an important factor in the reduction in R. solani (AG-8) DNA within the canola years that we reported. This idea is supported by the fact that logistic regression indicated more weeds per square metre were associated with increased incidence of Fusarium spp., P. neglectus and R. solani (AG-8) DNA and incidence of R. solani (AG-8) root disease. This shows weeds are hosting pathogens, as has been previously reported in WA (Vanstone and Russ 2001a, 2001b) and nematode pests and/or weed competition may be impacting root growth and soil pathogen interactions; stunting root growth so that roots take longer to grow into the sub-soil, beyond the soil depth where pathogens and nematodes are most prolific. Additionally, longer sequences of monoculture wheat led to greater grass weed populations; with mean weed density in the fourth successive wheat crop (35 grass weeds/m2) triple that of the first wheat (Harries et al. 2020). Hence, the reduced frequency of pasture and increase in canola, a non-host crop in which weeds can be controlled, are important factors keeping cereal pathogens and nematode pests in check within the current cereal dominated rotations.
While low grass weed numbers are a benefit for suppression of cereal diseases the use of herbicides can stunt plant growth and predispose plants to pathogens and nematode pests. This may explain why in our optimised models for Fusarium spp., G. graminis var. tritici and R. solani (AG-8) we found the incidence of root damage increased if more herbicides were applied to a paddock. Several studies document increased R. solani (AG-8) damage in cereals and soybean with the application of ALS (acetolactate synthase) inhibitor herbicides (Rovira 1986; Bradley et al. 2002; Lee et al. 2012; Rose et al. 2016). ALS-inhibitors were some of the most commonly used herbicides within the Focus Paddock data set (Harries et al. 2020).
Plant nutrition
Plant nutrition was also an influential management factor, although more so for P. neglectus than the other pathogens. Greater soil concentration of one or more of the macro nutrients (N, P, K and S) resulted in a greater incidence of Fusarium spp., P. neglectus and R. solani (AG-8) DNA and P. neglectus root damage. Also, our optimised models of P. neglectus included increasing fertiliser N as a predictor of increased incidence of both DNA above detection and root disease. This is noteworthy because mean amount of N fertiliser applied to rainfed wheat in Australia increased from 30 kg N/ha to 45 kg N/ha from 2000 to 2017 (Angus 2001; Angus and Grace 2017), type and amount of nitrogen fertiliser is documented to impact Fusarium spp. (Duffy and Défago 1999) and increasing rates of N fertiliser have been reported to increase nematode population densities and favour plant parasitic species such as Pratylenchus spp. (Todd 1996; Sarathchandra et al. 2001; Thompson et al. 2008; Forge et al. 2020). These findings are consistent with N being a common constraint in dryland farming systems (Farooq and Siddique 2017); the logical consequence of ameliorating the N constraint is an increase in root and shoot biomass and in species that parasitise these roots and crowns, as was reported by Wilkinson et al. (2018). It should also be acknowledged that fertiliser inputs generally increase yield in dryland farming systems and this needs to be balanced against our findings of associated increase in disease.
Sowing date
Sowing later in the year, increasing sow day, increased the incidence of root disease caused by Fusarium spp., R. solani (AG-8), P. neglectus and G. graminis var. tritici, with sow day a variate within the optimised models for each of these pathogens, except G. graminis var. tritici. The median sowing date became earlier over the survey period, being 25th of May in 2010 compared to 11th of May in 2014 (Harries et al. 2020), a continuing trend reported from other surveys (Stephens and Lyons 1998; Fletcher et al. 2015; Fletcher et al. 2016; Anderson et al. 2017). The reduced root damage from earlier sowing is likely to be due to more rapid root growth in warmer soil, as evidenced by the negative coefficients in most temperature variates for each pathogen in the binomial logistic regressions.
Environmental factors
The strong geographic difference in pathogen/nematode pest DNA incidence and concentration in soil, where SAR had the greatest and NAR least for most organisms tested, was not evident in plant root observations. For the DNA assays, lower temperature was related to higher probability of DNA levels being above detection limit. Likewise, root disease incidence increased as temperature decreased, but also as rain decreased. Hence, in environmental conditions with greater water stress, the higher latitude NAR and CAR regions (Harries et al. 2021), there was more root damage despite less soil DNA compared to the SAR. This is consistent with findings of Poole et al. (2015) who analysed a sub-set of the Focus Paddock data to report that temperature and rainfall parameters explained most of the variation in root health but this was not always strongly correlated to soil DNA levels.
Several factors driven by climate and soil differences between regions may be involved, i.e. we found greater rainfall and more fertile soils in the SAR meant plants produced more above ground biomass (Harries et al. 2021), and presumably below ground biomass although this was not measured, which may enable plants to cope with higher background pathogens and nematode pests DNA levels. Also, soils vary in suppressiveness of soilborne disease and nematode pest symptoms. For example, greater rotational diversity has been associated with increased microbiota biomass, diversity and suppression of crop pathogens and plant parasitic nematode levels (Postma et al. 2008; McDaniel et al. 2014), although this will depend on crops used and pathogen/nematode pest issues (Flower et al. 2019). However, we did not have the data to test these relationships.
We found that the climate influenced nematode pest and soilborne disease expression. Given reports of substantial and continuing climate change in southwest Australia, with increased summer fallow rainfall, reduced growing season rainfall and increased temperatures (BOM 2018; Scanlon and Doncon 2020), it is important to investigate these effects on the interaction between soil pathogen/nematode pest and their host plants, across the whole of the cropping region of southwest WA.
Conclusions
Overall, the inclusion of non-host crops at the current level coupled with current agronomic practices meant severe root damage was rare. We found that the reduction of paddocks in pasture and resultant very low weed populations, combined with early sowing will, in general, reduce the persistence of the soil pathogens and nematode pests in paddocks of southwest Australia. Some aspects of management had the opposite effect, including increased frequency of herbicide use, cereals and canola replacing lupin and increased N fertiliser use, and these must be applied cautiously.
These agronomic changes have mostly been made in response to production constraints other than root pathogens and nematode pests, for example to reduce soil erosion, improve soil water conservation and yield potential and improve weed control and soil fertility. Studies like ours, that link management practices to multiple productivity constraints, such as pathogens, nematode pests, weeds, and nutrients, are important to understand the sustainability of current or proposed production methods.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study were obtained from the Grains Research and Development Corporation (GRDC) and the Department of Primary Industries and Regional Development (DPIRD) by permission. Data will be shared upon reasonable request to the corresponding author with permission from GRDC and DPIRD.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study was financially supported by the Western Australian Department of Primary Industries and Regional Development and the Grains Research and Development Corporation (GRDC) through projects DAW00213 and DAW00201.
Acknowledgements
We acknowledge the support of the farmers who hosted Focus Paddocks, and staff from DPIRD, the Mingenew–Irwin Group, the Liebe Group, Western Australian No-tillage Farmers Association and the Facey Group, who contributed to field monitoring and collation of farmer records. In particular we thank Bill MacLeod and Shahajahan Miyan for coordinating plant root assessments and Alan McKay, Grant Poole and Russell Burns for coordination of PREDICTA B assays.
References
Abdurrahman MI, Chaki S, Saini G (2020) Stubble burning: effects on health & environment, regulations and management practices. Environmental Advances 2, 100011| Stubble burning: effects on health & environment, regulations and management practices.Crossref | GoogleScholarGoogle Scholar |
ABS (2016) Agricultural commodities, Australia and state/territory – 2015–16, Cat. no. 7121. Australian Bureau of Statistics, Canberra, ACT. Available at https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/7121.02015-16?OpenDocument [Accessed 8 October 2020]
Anderson WK, Hamza MA, Sharma DL, D’Antuono MF, Hoyle FC, Hill N, Shackley BJ, Amjad M, Zaicou-Kunesch C (2005) The role of management in yield improvement of the wheat crop – a review with special emphasis on Western Australia. Australian Journal of Agricultural Research 56, 1137–1149.
| The role of management in yield improvement of the wheat crop – a review with special emphasis on Western Australia.Crossref | GoogleScholarGoogle Scholar |
Anderson WK, Stevens D, Siddique KHM (2017) Dryland agriculture in Australia: experiences and innovations. In ‘Innovations in dryland agriculture’. (Eds M Farooq, KH Siddique) (Springer). Available at https://www.springer.com/gp/book/9783319479279 [Accessed 1 December 2020]
Angus JF (2001) Nitrogen supply and demand in Australian agriculture. Australian Journal of Experimental Agriculture 41, 277–288.
| Nitrogen supply and demand in Australian agriculture.Crossref | GoogleScholarGoogle Scholar |
Angus JF, Grace PR (2017) Nitrogen balance in Australia and nitrogen use efficiency on Australian farms. Soil Research 55, 435–450.
| Nitrogen balance in Australia and nitrogen use efficiency on Australian farms.Crossref | GoogleScholarGoogle Scholar |
Babiker EM, Hulbert SH, Schroeder KL, Paulitz TC (2013) Evaluation of Brassica species for resistance to Rhizoctonia solani and binucleate Rhizoctonia (Ceratobasidum spp.) under controlled environment conditions. European Journal of Plant Pathology 136, 763–772.
| Evaluation of Brassica species for resistance to Rhizoctonia solani and binucleate Rhizoctonia (Ceratobasidum spp.) under controlled environment conditions.Crossref | GoogleScholarGoogle Scholar |
Bockus WW, Shroyer JP (1998) The impact of reduced tillage on soilborne plant pathogens. Annual Review of Phytopathology 36, 485–500.
| The impact of reduced tillage on soilborne plant pathogens.Crossref | GoogleScholarGoogle Scholar | 15012510PubMed |
BOM (2018) State of the climate 2018. Bureau of Meteorology, Australian Government. Available at http://www.bom.gov.au/state-of-the-climate/State-of-the-Climate-2018.pdf [Accessed 8 October 2020]
Bradley CA, Hartman GL, Wax LM, Pedersen WL (2002) Influence of herbicides on Rhizoctonia root and hypocotyl rot of soybean. Crop Protection 21, 679–687.
| Influence of herbicides on Rhizoctonia root and hypocotyl rot of soybean.Crossref | GoogleScholarGoogle Scholar |
Cook RJ, Sitton JW, Waldher JT (1980) Evidence for Pythium as a pathogen of direct-drilled wheat in the Pacific Northwest. Plant Disease 64, 102–103.
| Evidence for Pythium as a pathogen of direct-drilled wheat in the Pacific Northwest.Crossref | GoogleScholarGoogle Scholar |
Cotterill PJ, Sivasithamparam K (1989) An autecological study of the take-all fungus (Gaeumannomyces graminis var. tritici) in Western Australia. Australian Journal of Agricultural Research 40, 229–240.
| An autecological study of the take-all fungus (Gaeumannomyces graminis var. tritici) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Derpsch R, Friedrich T, Kassam A, Hongwen L (2010) Current status of adoption of no-till farming in the world and some of its main benefits. International Journal of Agricultural and Biological Engineering 3, 1–25.
| Current status of adoption of no-till farming in the world and some of its main benefits.Crossref | GoogleScholarGoogle Scholar |
Duffy BK, Défago G (1999) Macro-and microelement fertilizers influence the severity of Fusarium crown and root rot of tomato in a soilless production system. HortScience 34, 287–291.
| Macro-and microelement fertilizers influence the severity of Fusarium crown and root rot of tomato in a soilless production system.Crossref | GoogleScholarGoogle Scholar |
FAO (2020a) ‘Fertiliser by nutrient database’, (FAOSTAT, FAO: Rome, Italy) Available at https://www.fao.org/faostat/en/#data/RFN
FAO (2020b) ‘Pesticide use database’, (FAOSTAT, FAO: Rome, Italy) Available at http://www.fao.org/faostat/en/#data/RP
Farooq M, Siddique K (2017) Research and developmental issues in dryland agriculture. In ‘Innovations in dryland agriculture’. (Eds M Farooq, K Siddique) (Springer)
Fisher T, Hobbs P (2019) Tillage: global update and prospects. In ‘Australian agriculture in 2020: from conservation to automation’. (Eds JE Pratley, J Kirkegaard) pp. 3–20. (Agronomy Australia and Charles Sturt University: Wagga Wagga, NSW, Australia)
Fletcher AL, Robertson MJ, Abrecht DG, Sharma DL, Holzworth DP (2015) Dry sowing increases farm level wheat yields but not production risks in a Mediterranean environment. Agricultural Systems 136, 114–124.
| Dry sowing increases farm level wheat yields but not production risks in a Mediterranean environment.Crossref | GoogleScholarGoogle Scholar |
Fletcher A, Lawes R, Weeks C (2016) Crop area increases drive earlier and dry sowing in Western Australia: implications for farming systems. Crop & Pasture Science 67, 1268–1280.
| Crop area increases drive earlier and dry sowing in Western Australia: implications for farming systems.Crossref | GoogleScholarGoogle Scholar |
Flower KC, Hüberli D, Collins SJ, Thomas G, Ward PR, Cordingley N (2019) Progression of plant-parasitic nematodes and foliar and root diseases under no-tillage with different crop rotations. Soil and Tillage Research 191, 18–28.
| Progression of plant-parasitic nematodes and foliar and root diseases under no-tillage with different crop rotations.Crossref | GoogleScholarGoogle Scholar |
Forge T, Ehret D, Messiga A, Dorais M (2020) Influences of nitrogen inputs on nematode populations under highbush blueberry. Journal of Nematology 52, 1–14.
| Influences of nitrogen inputs on nematode populations under highbush blueberry.Crossref | GoogleScholarGoogle Scholar | 32628827PubMed |
Gupta VVSR, McKay A, Diallo S, Smith D, Cook A, Kirkegaard J, Ophel-Keller K, Roget DK (2010) Temporal dynamics of Rhizoctonia solani AG8 inoculum in Australian soils, In ‘Proceedings of the 6th Australian Soilborne Diseases Symposium’. 9–11 August 2010. (Australasian Plant Pathology Society) Available at https://publications.csiro.au/rpr/download?pid=csiro:EP101928&dsid=DS4
Harries M, Anderson G, Hüberli D (2015) Crop sequences in Western Australia: what are they and are they sustainable? Findings of a four-year survey. Crop & Pasture Science 66, 634–647.
| Crop sequences in Western Australia: what are they and are they sustainable? Findings of a four-year survey.Crossref | GoogleScholarGoogle Scholar |
Harries M, Flower KC, Scanlan CA (2021) Sustainability of nutrient management in grain production systems of south-west Australia. Crop & Pasture Science 72, 197–212.
| Sustainability of nutrient management in grain production systems of south-west Australia.Crossref | GoogleScholarGoogle Scholar |
Harries M, Flower KC, Scanlan CA, Rose MT, Renton M (2020) Interactions between crop sequences, weed populations and herbicide use in Western Australian broadacre farms: findings of a six-year survey. Crop & Pasture Science 71, 491–505.
| Interactions between crop sequences, weed populations and herbicide use in Western Australian broadacre farms: findings of a six-year survey.Crossref | GoogleScholarGoogle Scholar |
Hay FS, Herdina , Ophel-Keller K, Hartley DM, Pethybridge SJ (2016) Prediction of potato tuber damage by root-knot nematodes using quantitative DNA assay of soil. Plant Disease 100, 592–600.
| Prediction of potato tuber damage by root-knot nematodes using quantitative DNA assay of soil.Crossref | GoogleScholarGoogle Scholar | 30688598PubMed |
Heap I (2020) International survey of herbicide resistant weeds. Available at www.weedscience.org [Accessed 5 February 2020]
Hollaway GJ, Ophel-Keller KM, Taylor SP, Burns RA, McKay AC (2003) Effect of soil water content, sampling method and sample storage on the quantification of root lesion nematodes (Pratylenchus spp.) by different methods. Australasian Plant Pathology 32, 73–79.
| Effect of soil water content, sampling method and sample storage on the quantification of root lesion nematodes (Pratylenchus spp.) by different methods.Crossref | GoogleScholarGoogle Scholar |
Hüberli D, Connor M, Miyan S, MacLeod W, Desbiolles J, Bogacki P, McKay A (2013) Integrated disease management options to control rhizoctonia bare-patch in cereals. In ‘2013 Agribusiness crop updates’. 25–26 February, Perth, Western Australia. (Grains industry association of Western Australia) Available at https://researchrepository.murdoch.edu.au/id/eprint/13686/
Jeffrey SJ, Carter JO, Moodie KB, Beswick AR (2001) Using spatial interpolation to construct a comprehensive archive of Australian climate data. Environmental Modelling & Software 16, 309–330.
| Using spatial interpolation to construct a comprehensive archive of Australian climate data.Crossref | GoogleScholarGoogle Scholar |
Khangura RK, MacNish GC, MacLeod WJ, Vanstone VA, Hanbury CD, Loughman R, Speijers JE (2013) Current status of cereal root diseases in Western Australia under intensive cereal production and their comparison with the historical survey conducted during 1976–1982. Journal of Phytopathology 161, 828–840.
| Current status of cereal root diseases in Western Australia under intensive cereal production and their comparison with the historical survey conducted during 1976–1982.Crossref | GoogleScholarGoogle Scholar |
Kirkegaard JA, Peoples MB, Angus JF, Unkovich MJ (2011) Diversity and evolution of rainfed farming systems in southern Australia. In ‘Rainfed farming systems’. (Eds P Tow, I Cooper, I Partridge, C Birch) pp. 715–754. (Springer: Dordrecht, Netherlands)
Lacoste M (2017) Assessing the performance of ‘comparative agriculture’ methods to determine regional diversity in Australian farming systems: methodological relevance and application in the Western Australian wheatbelt. PhD Thesis, University of Western Australia, Perth, WA, Australia.
Lee H, Ullrich SE, Burke IC, Yenish J, Paulitz TC (2012) Interactions between the root pathogen Rhizoctonia solani AG-8 and acetolactate-synthase-inhibiting herbicides in barley. Pest Management Science 68, 845–852.
| Interactions between the root pathogen Rhizoctonia solani AG-8 and acetolactate-synthase-inhibiting herbicides in barley.Crossref | GoogleScholarGoogle Scholar | 22307918PubMed |
Llewellyn R, Ouzman J (2019) Conservation Agriculture in Australia: 30 years on. In ‘Australian agriculture in 2020: from conservation to automation’. (Eds JE Pratley, J Kirkegaard) pp. 21–33. (Agronomy Australia and Charles Sturt University: Wagga Wagga, NSW, Australia)
Llewellyn RS, D’Emden FH, Kuehne G (2012) Extensive use of no-tillage in grain growing regions of Australia. Field Crops Research 132, 204–212.
| Extensive use of no-tillage in grain growing regions of Australia.Crossref | GoogleScholarGoogle Scholar |
MacLeod B, Vanstone V, Khangura R, Beard C (2008) Root disease under intensive cereal production systems. Western Austrlian Agricultural Authority, Perth, WA. Available at https://researchlibrary.agric.wa.gov.au/cgi/viewcontent.cgi?article=1085&context=bulletins [Accessed 10 November 2020]
MacNish GC (1985) Methods of reducing rhizoctonia patch of cereals in Western Australia. Plant Pathology 34, 175–181.
| Methods of reducing rhizoctonia patch of cereals in Western Australia.Crossref | GoogleScholarGoogle Scholar |
McDaniel MD, Tiemann LK, Grandy AS (2014) Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta-analysis. Ecological Applications 24, 560–570.
| Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta-analysis.Crossref | GoogleScholarGoogle Scholar | 24834741PubMed |
McDonald HJ, Rovira AD (1985) Development of inoculation technique for Rhizoctonia solani and its application to screening cereal cultivars for resistance. In ‘Ecology and management of soilborne plant disease’. (Eds CA Parker, AD Rovira, KJ Moore, PT Wong, JF Kollmorgen) pp. 174–176. (American Phytopathology Society: St Paul, MN, USA)
Murray GM, Brennan JP (2009a) The current and potential costs from diseases of wheat in Australia. Grains Research and Development Corporation Canberra Report for GRDC, Canberra, Australia. Available at https://grdc.com.au/resources-and-publications/all-publications/publications/2009/10/the-current-and-potential-costs-from-diseases-of-wheat-in-australia.
Murray GM, Brennan JP (2009b) Estimating disease losses to the Australian wheat industry. Australasian Plant Pathology 38, 558–570.
| Estimating disease losses to the Australian wheat industry.Crossref | GoogleScholarGoogle Scholar |
Murray GM, Brennan JP (2010) Estimating disease losses to the Australian barley industry. Australasian Plant Pathology 39, 85–96.
| Estimating disease losses to the Australian barley industry.Crossref | GoogleScholarGoogle Scholar |
Ophel-Keller K, McKay A, Hartley D Ophel-Keller K, McKay A, Hartley D (2008) Development of a routine DNA-based testing service for soilborne diseases in Australia. Australasian Plant Pathology 37, 243–253.
| Development of a routine DNA-based testing service for soilborne diseases in Australia.Crossref | GoogleScholarGoogle Scholar |
Owen MJ, Powles SB (2009) Distribution and frequency of herbicide-resistant wild oat (Avena spp.) across the Western Australian grain belt. Crop & Pasture Science 60, 25–31.
| Distribution and frequency of herbicide-resistant wild oat (Avena spp.) across the Western Australian grain belt.Crossref | GoogleScholarGoogle Scholar |
Owen MJ, Martinez NJ, Powles SB (2014) Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt. Weed Research 54, 314–324.
| Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt.Crossref | GoogleScholarGoogle Scholar |
Paulitz TC (2006) Low input no-till cereal production in the Pacific Northwest of the U.S.: the challenges of root diseases. European Journal of Plant Pathology 115, 271–281.
| Low input no-till cereal production in the Pacific Northwest of the U.S.: the challenges of root diseases.Crossref | GoogleScholarGoogle Scholar |
Paulitz TC, Smiley RW, Cook RJ (2002) Insights into the prevalence and management of soilborne cereal pathogens under direct seeding in the Pacific Northwest, U.S.A. Canadian Journal of Plant Pathology 24, 416–428.
| Insights into the prevalence and management of soilborne cereal pathogens under direct seeding in the Pacific Northwest, U.S.A.Crossref | GoogleScholarGoogle Scholar |
Paulitz TC, Okubara PA, Schillinger WF (2006) First report of damping-off of canola caused by Rhizoctoniasolani AG 2-1 in Washington State. Plant Disease 90, 829
| First report of damping-off of canola caused by Rhizoctoniasolani AG 2-1 in Washington State.Crossref | GoogleScholarGoogle Scholar | 30781257PubMed |
Peterson CA, Eviner VT, Gaudin ACM (2018) Ways forward for resilience research in agroecosystems. Agricultural Systems 162, 19–27.
| Ways forward for resilience research in agroecosystems.Crossref | GoogleScholarGoogle Scholar |
Planfarm and Bankwest (2016) Planfarm Bankwest benchmarks 2015–16. Planfarm Pty Ltd & Bankwest Agribusiness Centre, Perth, WA, Australia. Available at http://agric.firstsoftwaresolutions.com/attachments/1215/Planfarm%20Bankwest%20Benchmarks%202015-2016%20full-report.pdf. [Accessed 5 February 2020]
Poole GJ, Harries M, Hüberli D, Miyan S, MacLeod WJ, Lawes R, McKay A (2015) Predicting cereal root disease in Western Australia using soil DNA and environmental parameters. Phytopathology 105, 1069–1079.
| Predicting cereal root disease in Western Australia using soil DNA and environmental parameters.Crossref | GoogleScholarGoogle Scholar | 25822184PubMed |
Postma J, Schilder MT, Bloem J, van Leeuwen-Haagsma WK (2008) Soil suppressiveness and functional diversity of the soil microflora in organic farming systems. Soil Biology and Biochemistry 40, 2394–2406.
| Soil suppressiveness and functional diversity of the soil microflora in organic farming systems.Crossref | GoogleScholarGoogle Scholar |
Pumphrey FV, Wilkins DE, Hane DC, Smiley RW (1987) Influence of tillage and nitrogen fertilizer on Rhizoctonia root rot (bare patch) of winter wheat. Plant Disease 71, 125–127.
| Influence of tillage and nitrogen fertilizer on Rhizoctonia root rot (bare patch) of winter wheat.Crossref | GoogleScholarGoogle Scholar |
Riley IT, Kelly SJ (2002) Endoparasitic nematodes in cropping soils of Western Australia. Australian Journal of Experimental Agriculture 42, 49–56.
| Endoparasitic nematodes in cropping soils of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Rose MT, Cavagnaro TR, Scanlan CA, Rose TJ, Vancov T, Kimber S, Kennedy IR, Kookana RS, Van Zwieten L (2016) Impact of herbicides on soil biology and function. Advances in Agronomy 136, 133–220.
| Impact of herbicides on soil biology and function.Crossref | GoogleScholarGoogle Scholar |
Rovira AD (1986) Effects of the herbicide chlorsulfuron on rhizoctonia bare patch and take-all of barley and wheat. Plant Disease 70, 879
| Effects of the herbicide chlorsulfuron on rhizoctonia bare patch and take-all of barley and wheat.Crossref | GoogleScholarGoogle Scholar |
Sarathchandra SU, Ghani A, Yeates GW, Burch G, Cox NR (2001) Effect of nitrogen and phosphate fertilisers on microbial and nematode diversity in pasture soils. Soil Biology and Biochemistry 33, 953–964.
| Effect of nitrogen and phosphate fertilisers on microbial and nematode diversity in pasture soils.Crossref | GoogleScholarGoogle Scholar |
SARDI (2020) PreDictaB research: risk categories. South Australian Research and Development Institute. Available at https://pir.sa.gov.au/__data/assets/pdf_file/0020/320834/Crop_research_risk_categories.pdf [Accessed 11 December 2020]
Scanlon TT, Doncon G (2020) Rain, rain, gone away: decreased growing-season rainfall for the dryland cropping region of the south-west of Western Australia. Crop & Pasture Science 71, 128–133.
| Rain, rain, gone away: decreased growing-season rainfall for the dryland cropping region of the south-west of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Schoknecht NR, Pathan S (2013) Soil groups of Western Australia: a simple guide to the main soils of Western Australia. Department of Primary Industries and Regional Development, Perth, WA. Available at http://researchlibrary.agric.wa.gov.au/cgi/viewcontent.cgi?article=1347&context=rmtr [Accessed 4 August 2021]
Smiley RW, Yan G, Gourlie JA (2014) Selected Pacific Northwest crops as hosts of Pratylenchus neglectus and P. thornei. Plant Disease 98, 1341–1348.
| Selected Pacific Northwest crops as hosts of Pratylenchus neglectus and P. thornei.Crossref | GoogleScholarGoogle Scholar | 30703934PubMed |
Stephens DJ, Lyons TJ (1998) Variability and trends in sowing dates across the Australian wheatbelt. Australian Journal of Agricultural Research 49, 1111–1118.
| Variability and trends in sowing dates across the Australian wheatbelt.Crossref | GoogleScholarGoogle Scholar |
Thomas GJ, MacLeod WJ, Sweetingham MW (2010) Incidence of root and hypocotyl diseases in lupin crops in Western Australia between 1986 and 2005. Crop & Pasture Science 61, 241–246.
| Incidence of root and hypocotyl diseases in lupin crops in Western Australia between 1986 and 2005.Crossref | GoogleScholarGoogle Scholar |
Thompson JP, Owen KJ, Stirling GR, Bell MJ (2008) Root-lesion nematodes (Pratylenchus thornei and P. neglectus): a review of recent progress in managing a significant pest of grain crops in northern Australia. Australasian Plant Pathology 37, 235–242.
| Root-lesion nematodes (Pratylenchus thornei and P. neglectus): a review of recent progress in managing a significant pest of grain crops in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Todd TC (1996) Effects of management practices on nematode community structure in tallgrass prairie. Applied Soil Ecology 3, 235–246.
| Effects of management practices on nematode community structure in tallgrass prairie.Crossref | GoogleScholarGoogle Scholar |
Vanstone V (2002) Impact and management of root lesion nematodes in Western Australia. Grains Research and Development Corporation (GRDC), Canberra. Available at https://grdc.com.au/research/reports/report?id=5014. [Accessed 9 November 2020]
Vanstone VA, Russ MH (2001a) Ability of weeds to host the root lesion nematodes Pratylenchus neglectus and P. thornei I. Grass weeds. Australasian Plant Pathology 30, 245–250.
| Ability of weeds to host the root lesion nematodes Pratylenchus neglectus and P. thornei I. Grass weeds.Crossref | GoogleScholarGoogle Scholar |
Vanstone VA, Russ MH (2001b) Ability of weeds to host the root lesion nematodes Pratylenchus neglectus and P. thornei II*. Broad-leaf weeds. Australasian Plant Pathology 30, 251–258.
| Ability of weeds to host the root lesion nematodes Pratylenchus neglectus and P. thornei II*. Broad-leaf weeds.Crossref | GoogleScholarGoogle Scholar |
Vanstone VA, Hollaway GJ, Stirling GR (2008) Managing nematode pests in the southern and western regions of the Australian cereal industry: continuing progress in a challenging environment. Australasian Plant Pathology 37, 220–234.
| Managing nematode pests in the southern and western regions of the Australian cereal industry: continuing progress in a challenging environment.Crossref | GoogleScholarGoogle Scholar |
Walsh MJ, Owen MJ, Powles SB (2007) Frequency and distribution of herbicide resistance in Raphanus raphanistrum populations randomly collected across the Western Australian wheatbelt. Weed Research 47, 542–550.
| Frequency and distribution of herbicide resistance in Raphanus raphanistrum populations randomly collected across the Western Australian wheatbelt.Crossref | GoogleScholarGoogle Scholar |
Weller DM, Cook RJ, MacNish G, Bassett EN, Powelson RL, Petersen RR (1986) Rhizoctonia root rot of small grains favored by reduced tillage in the Pacific Northwest. Plant Disease 70, 70–73.
| Rhizoctonia root rot of small grains favored by reduced tillage in the Pacific Northwest.Crossref | GoogleScholarGoogle Scholar |
Wicks T, Walker G, Pederick S, Anstis S (2011) Onion stunting in South Australia associated with Rhizoctoniasolani AG 8. Australasian Plant Pathology 40, 126–132.
| Onion stunting in South Australia associated with Rhizoctoniasolani AG 8.Crossref | GoogleScholarGoogle Scholar |
Wilkinson CJ, Butler AA, Kelly SJ, Collins SJ (2018) Can a change in nitrogen reduce plant parasitic nematodes (Pratylenchus quasitereoides) in Western Australian wheat crops? In ‘10th Australasian Soilborne Diseases Symposium’. 4–8 September, Adelaide, Australia. (Eds VVSR Gupta, S Barnett, S Kroker) pp. 11–12. Available at https://www.appsnet.org/publications/proceedings/ASDS%202018%20Proceedings.pdf [Accessed 15 October 2021]
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Research 14, 415–421.
| A decimal code for the growth stages of cereals.Crossref | GoogleScholarGoogle Scholar |