The extent of herbicide resistance in Lolium rigidum Gaud. (annual ryegrass) across south-eastern Australia as determined from random surveys
John Broster A * , Peter Boutsalis B , Gurjeet S. Gill B and Christopher Preston
A * , Peter Boutsalis B , Gurjeet S. Gill B and Christopher Preston  B
B
A Gulbali Institute for Agriculture Water Environment, Charles Sturt University, Boorooma Street, Wagga Wagga, NSW 2678, Australia.
B School of Agriculture, Food & Wine, University of Adelaide, Glen Osmond, SA 5064, Australia.
Crop & Pasture Science 73(11) 1308-1317 https://doi.org/10.1071/CP21753
Submitted: 5 November 2021 Accepted: 26 April 2022 Published: 24 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Annual ryegrass (Lolium rigidum) is a major weed of crop production in southern Australia that readily develops resistance to herbicides. Resistance increases both yield losses and control costs associated with this species.
Aims: This study aimed to gauge the extent and distribution of resistance to herbicides in L. rigidum across south-eastern Australian grain production systems by collecting seed from randomly selected fields.
Methods: A total of 1441 weed populations were collected through random surveys conducted over 5 years across 13 agricultural regions of four states with these samples then tested for resistance to eight herbicides from six modes of action.
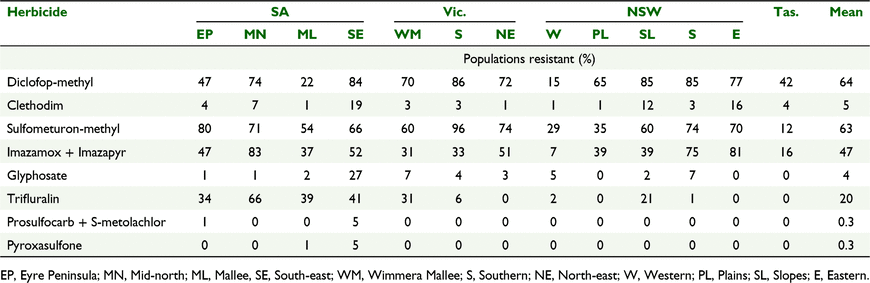
Key results: Resistance to diclofop-methyl and sulfometuron-methyl was most common, being present in 64% and 63% of populations respectively. Glyphosate resistance was present in 4% of populations collected. Only 15% of populations collected were susceptible to all herbicides tested. Large differences in resistance occurred between the 13 regions surveyed with resistance to diclofop-methyl ranging from 15% to 86% of populations and sulfometuron-methyl from 12% to 96%. Resistance to post-emergent herbicides tended to be higher than pre-emergent herbicides. Multiple resistance was common with 60% of populations collected having resistance to two or more herbicide modes of action.
Conclusions: There were significant differences in the extent of multiple resistance in L. rigidum populations collected from individual regions suggesting that the rates of resistance evolution have differed between regions.
The high incidence of herbicide resistance in L. rigidum populations randomly collected from south-eastern Australian cropping fields highlights the need for the adoption of additional weed control practices to mitigate the impact of this species on grain production systems.
Keywords: ACCase-inhibiting herbicide, ALS-inhibiting herbicide, diclofop-methyl, glyphosate, multiple resistance, pre-emergent herbicide, ryegrass, sulfometuron-methyl.
Introduction
Lolium rigidum Gaud. (annual ryegrass) is a major weed of crops in south-eastern Australia accounting for an estimated AUD93 million per annum in yield loss and considerably more in control costs (Llewellyn et al. 2016). The adoption of no-till farming across this region over the past 20 years has resulted in the reliance on herbicides for weed control (Llewellyn et al. 2012). Extensive resistance to post-emergent herbicides, especially those inhibiting the acetyl Coenzyme A carboxylase (ACCase) and acetolactate synthase (ALS) enzymes, has resulted in the widespread adoption of pre-emergent herbicides for weed control in cereal crops (Boutsalis et al. 2014). More recently, L. rigidum populations from south-eastern Australia have evolved resistance to many of these pre-emergent herbicides (Boutsalis et al. 2012; Brunton et al. 2018).
L. rigidum is well adapted to Australian winter cropping region environments and production systems. In the largely Mediterranean-type climate of this region, this weed germinates with the onset of the growing season in late autumn and sets seed at crop maturation in late spring (Gill 1996). While individual L. rigidum plants are not highly competitive, left uncontrolled this species can quickly establish large populations that can cause near complete yield loss (Reeves 1976; Lemerle et al. 1995).
One of the challenges in managing herbicide resistant populations of L. rigidum is understanding the extent of resistance present in individual fields. This can be achieved by testing for resistance by farmers; however, this is frequently not done due to impracticality of seed collection during harvest, cost and other factors (Llewellyn et al. 2002; Boutsalis and Broster 2006; Broster et al. 2019a). A more accurate and unbiased method to determine the incidence of herbicide resistance is through random surveys. Previous surveys have indicated that although resistance is extensive and widespread across south-eastern Australia, it is not uniformly distributed (Broster et al. 2011, 2012, 2013; Boutsalis et al. 2012). In some regions, resistance to one or more herbicides is much less frequent, providing farmers in these regions with greater opportunities to use herbicides for weed control.
The wide distribution of resistance to herbicides, particularly to post-emergent herbicides, in L. rigidum in Australia and the subsequent lack of in-crop selective herbicides with alternative modes of action, has resulted in the widespread adoption of recently released pre-emergent herbicides for the control of this weed (Boutsalis et al. 2014). These herbicides, prosulfocarb + S-metolachlor introduced in 2008 and pyroxasulfone introduced in 2012, have become commonly used for L. rigidum control in cereals in many regions across south-eastern Australia (Brunton et al. 2019). Due to this increased selection pressure, these herbicides are under considerable threat from resistance evolution.
The aim of this study was firstly to gauge the extent and distribution of resistance to herbicides in L. rigidum in south-eastern Australia by collecting seed from randomly selected fields, and secondly to compare the findings to those of previous surveys. Populations collected were tested with eight herbicides, from six modes of action, that are registered for the control of L. rigidum in Australian winter cropping systems, including pre-emergent herbicides introduced since previous surveys were conducted.
Materials and methods
Seed collection
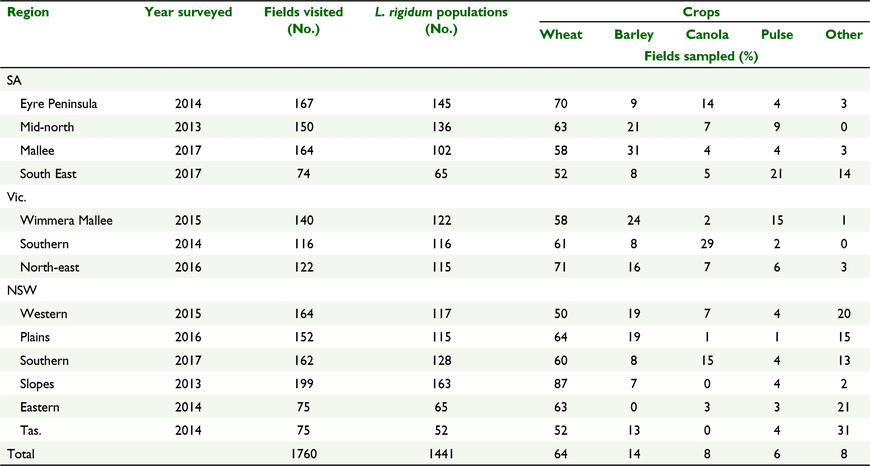
Weed seed collection surveys of South Australia (SA), Victoria (Vic.), Tasmania (Tas.) and southern New South Wales (NSW) cropping regions were conducted at crop maturity in the 2013–2017 growing seasons (Fig. 1). Fields were selected by travelling for a predetermined distance (5 or 10 km depending upon the size of surveyed area). At each stop, a single field was surveyed with sampling commencing 10 m in from the edge of the crop and continued in a zig zag pattern on a transect that covered at least one ha of the field. The sampling location (longitude and latitude) was recorded using a global positioning system (GPS) navigational unit. After collection the populations were kept in a covered enclosure where they were subjected to fluctuating summer temperatures for 4 months, after which time the seed was threshed from the heads and stored at room temperature until tested. A full description of the surveying methodology is described in Boutsalis et al. (2012).
As L. rigidum becomes mature at a similar time across this very large area, it is impractical to survey it in a single year. Therefore, the area was divided into 13 regions and two to three regions were surveyed each year (Fig. 1). There was a target of assessing at least 120 crop fields in each region, collecting seed from L. rigidum populations if present.
Plant growth and treatment
Populations collected from SA and Vic. were tested at the Waite Campus, University of Adelaide. Populations collected from Tas. and NSW were tested at Charles Sturt University (CSU), Wagga Wagga. Each testing centre used their own standard susceptible population (Adelaide – SLR4 and VLR1; CSU – 120327a), which was included in every test. A standard resistant population was also included where one was available for that herbicide.
Seed planting, seedling maintenance and herbicide screening procedures are described in Boutsalis et al. (2012) and Broster et al. (2011). In brief, 50–60 seeds (0.2 grams) of seed were sown into pots (three replicates at CSU, one replicate at Adelaide), with germination of plants to be treated with post-emergent herbicides counted before treatment; at this stage populations at CSU were thinned to 20 plants (not thinned at Adelaide). At CSU seeds were sown into plastic trays (150 mm × 100 mm × 60 mm) filled with either a 50:50 peat:sand mix (diclofop-methyl, clethodim and glyphosate) or garden loam (all other herbicides) while at Adelaide 0.55 L pots containing a coco peat mix were used. Seeds treated with pre-emergent herbicides were sown on the soil surface, sprayed and then covered with either 5 mm of garden loam (CSU) or coco peat mix (Adelaide). At CSU trials were conducted in a temperature-controlled glasshouse (10°C−25°C) while at Adelaide they were conducted outdoors under natural autumn and winter growing conditions. At both locations the trials were watered daily and fertilised as required.
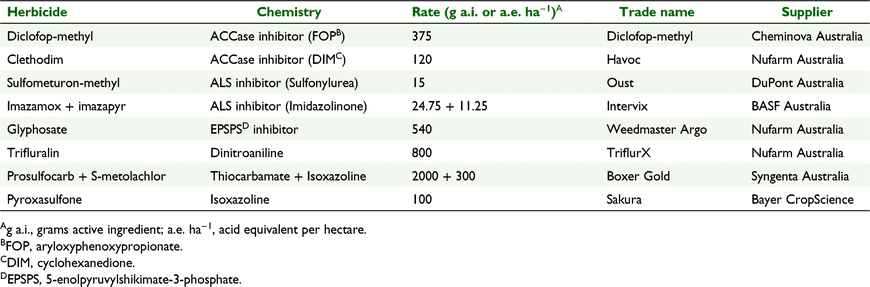
The herbicides and rates used in the testing are listed in Table 1. Sulfometuron-methyl was used as the sulfonylurea herbicide in this study, as it provided more reliable results in pots than did other sulfonylurea herbicides.
|
Plants were counted as survivors of the herbicide if they had emerged and developed to the two-leaf stage 28 days after treatment with pre-emergent herbicides or if they had new shoot growth 21 days after treatment with post-emergent herbicides. Following Boutsalis et al. (2012), populations from each field were considered resistant only if 20% or more of the individuals in that population had survived the herbicide application.
Statistical analysis
The frequency of resistance to each herbicide in each region was compared to the average frequency across all regions using an exact binomial test (McDonald 2014) with the null hypothesis being that resistance was equally distributed across regions. The same test was used to compare frequencies of resistance in SA, Vic., Tas., NSW Slopes and NSW Plains regions with previous surveys with the null hypothesis that resistance frequencies had not changed between surveys.
For ACCase- and ALS-inhibiting herbicides, where two different herbicides were tested within each mode of action, due to the large number of samples a multinomial goodness of fit test (G statistic) was used to test if the frequency of resistance to these two modes of action was the same (McDonald 2014) with the null hypothesis being that resistance occurred equally across the mode of action. This test was also used to compare the distribution of resistance to cumulative numbers of modes of action in each region with the average distribution across all the populations (McDonald 2014). In this case the null hypothesis was that resistance in each region had evolved at similar rates and so the proportion of populations with resistance to different numbers of modes of action should be similar.
Results
Populations collected and crop distribution
Over the period 2013–2017, 1760 crop fields were surveyed and 1441 L. rigidum populations collected, with L. rigidum present in 82% of fields surveyed. Wheat was the dominant crop in all the cropping regions, occurring on average in 64% of the surveyed fields. The next most commonly occurring crop was barley in 14% of fields, then canola in 8% and pulse crops (field peas, lentils, faba beans, lupins and chickpeas) in 6% of fields (Table 2).
|
Resistance to post-emergent selective herbicides
Resistance to ACCase inhibiting herbicides was present in L. rigidum populations collected in all regions (Table 3). Sixty four percent of populations across the surveyed area were resistant to the ACCase inhibitor diclofop-methyl with differences between regions. The NSW Western region (15%, P < 0.0001), SA Mallee region (22%, P < 0.0001) and SA Eyre Peninsula (47%, P < 0.0001) had fewer resistant populations compared to the average, whereas SA Mid-north (74%, P = 0.015), NSW Eastern region (77%, P = 0.038), SA South-east region (84%, P < 0.0001), NSW Slopes region (85%, P < 0.0001), NSW Southern region (85%, P < 0.0001) and Vic. Southern region (86%, P < 0.0001) all had more resistant populations compared to the average (Table 3).
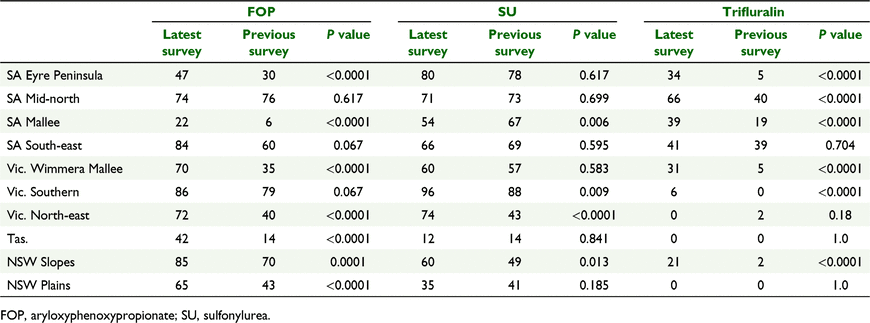
Compared to previous surveys of resistance in L. rigidum in SA, Vic., Tas., NSW Slopes region and NSW Plains region, the recent survey shows increases in the frequency of resistance to ACCase-inhibiting herbicides in many of the regions surveyed (Broster et al. 2011, 2012, 2013; Boutsalis et al. 2012). For example, in 2005 35% of populations had resistance to diclofop-methyl in Vic. Wimmera Mallee region (Boutsalis et al. 2012) compared to 70% of populations in 2015 (P < 0.0001) (Table 4). Likewise, resistance to this herbicide was detected in 14% of populations from Tas. in 2010 (Broster et al. 2012) compared to 42% in 2014 (P < 0.0001). Resistance to diclofop-methyl also increased in the other regions except SA Mid-north region (P = 0.617) and SA South-east region (P = 0.067), where resistance was already high (Boutsalis et al. 2012) (Table 5).
|
|
Although clethodim is also an ACCase inhibitor, the frequency of resistant populations (5% overall) to this herbicide was lower than to diclofop-methyl (G = 2304; P < 0.0001). The incidence of resistance to clethodim also varied between regions with the NSW Slopes (12%, P = 0.0016), NSW Eastern (16%, P = 0.0012) and SA South-east regions (19%, P < 0.0001) all having more resistant populations than the average (Table 3). Despite the high frequency of resistance to diclofop-methyl, clethodim remains commonly used for control of L. rigidum in canola and pulse crops in southern Australia due to a much lower frequency of resistant populations (Saini et al. 2015b).
Across the surveyed area 63% of populations were resistant to the sulfonylurea herbicide, sulfometuron-methyl with significant differences between regions. Tas. (12%, P < 0.0001), NSW Western region (29%, P < 0.0001) and NSW Plains region (35%, P < 0.0001) all had fewer resistant populations to sulfometuron-methyl than the average whereas NSW Southern region (74%, P = 0.002), Vic. North-east region (74%, P = 0.012), SA Eyre Peninsula region (80%, P < 0.0001) and Vic. Southern region (96%, P < 0.0001) all had more resistant populations than the average (Table 3). Imazamox + imazapyr is a common herbicide mix used for weed control in imidazolinone tolerant (Clearfield®) crops in southern Australia and is a different class of ALS-inhibiting herbicide to the sulfonylurea herbicides (Tranel and Wright 2002). Resistance to imazamox + imazapyr (47% overall) was lower (G = 150; P < 0.0001) than for the sulfonylurea herbicides. Resistance to imazamox + imazapyr varied across the regions (Table 3). The NSW Western region (7%, P < 0.0001), Tas. (16%, P < 0.0001) Vic. Wimmera Mallee region (31%, P = 0.001) and Vic. Southern region (33%, P = 0.0038) all had fewer resistant populations to this herbicide mixture compared to the average. NSW Southern region (75%, P < 0.0001), NSW Eastern region (81%, P < 0.0001) and SA Mid-north region (83%, P < 0.0001) all had more resistant populations than the average (Table 3).
In previous surveys, resistance to sulfonylurea herbicides was high in most regions (Broster et al. 2011, 2012; Boutsalis et al. 2012). In the current survey, frequencies of resistance to sulfonylurea herbicides remained similar to previous surveys in most regions with the exception of SA Mallee (P = 0.006) where the frequency decreased and, Vic. North-east (P < 0.0001), Vic. Southern (P = 0.009) and NSW Slopes (P = 0.013), where resistance frequency had increased (Table 4). Weeds resistant to the sulfonylurea herbicides often remain susceptible to imidazolinone herbicides (Yu and Powles 2014). However, resistance to the imidazolinone herbicides in L. rigidum has reached high levels across much of south-eastern Australia (Table 3). In some regions, the level of resistance to imidazolinone herbicides was much lower than resistance to sulfonylureas but in other regions, L. rigidum populations were equally resistant to both herbicides.
Resistance to glyphosate
Across the surveyed area only 4% of populations were glyphosate resistant (Table 3). There was variation in resistance across the regions with the SA South-east region having more resistant populations to glyphosate (27%, P < 0.0001) compared to the average, while several regions (NSW Plains, NSW Eastern, Tas.) had no resistant populations (Table 3).
Resistance to pre-emergent herbicides
Resistance to the pre-emergent herbicides was present in 20% of populations; however, the frequency of resistant populations was variable between regions (Table 3). Resistance to trifluralin was higher than the average in the SA Mid-north region (66%, P < 0.0001), SA South-east region (41%, P < 0.0001), SA Mallee region (39%, P < 0.0001), SA Eyre Peninsula region (34%, P < 0.0001) and Vic. Wimmera Mallee region (31%, P = 0.002) (Table 3). All other regions, except NSW Slopes, had fewer resistant populations than the average, with no resistance detected in Vic. North-east, NSW Western, NSW Plains or Tas. regions (Table 3). The incidence of resistance to the two newer pre-emergent herbicides, prosulfocarb + S-metolachlor and pyroxasulfone, was low, between 0 and 5% of populations. Resistance to these two herbicides was only found in two of the 12 surveyed regions, with only the SA South-east region having more resistant populations to both herbicides compared with the average (P = 0.001 for both herbicides) (Table 3).
Trifluralin resistance increased from 2% in NSW Slopes in 2007 (Broster et al. 2011) compared with 21% in this survey (P < 0.0001). Likewise, trifluralin resistance increased from 5% of populations from the SA Eyre Peninsula in 2009 (Boutsalis et al. 2012) to 47% in 2014 (P < 0.0001) (Table 4). However, trifluralin resistance had not changed in SA South-east (P = 0.704), Vic. North-east (P = 0.18), NSW Plains (P = 1) and Tas. regions (P = 1) compared with previous surveys (Boutsalis et al. 2012; Broster et al. 2012, 2013) (Table 4).
Multiple resistance
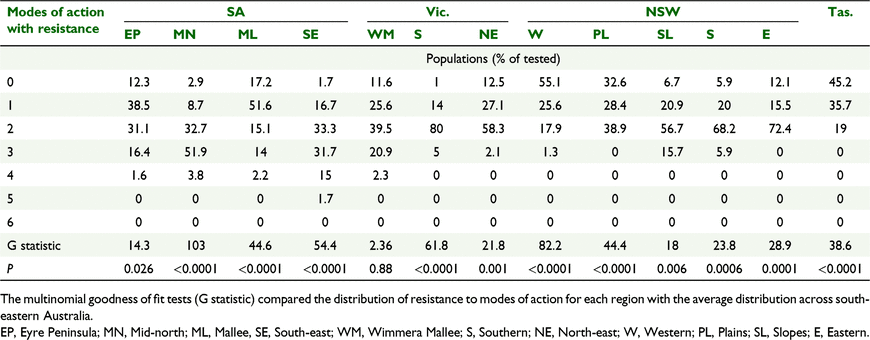
Across south-eastern Australia, 15% of the populations were susceptible to all herbicides tested. A further 25% of populations were resistant to a single mode of action and the remaining 60% of populations were resistant to multiple herbicide modes of action (Fig. 2). Most common was resistance to two modes of action in 45% of populations (Fig. 2). No population had resistance to all six herbicide modes of action tested; however, there was one population with resistance to five modes of action and 18 populations with resistance to four modes of action.
The extent of multiple resistance was variable across the 13 regions sampled (Table 4). SA Mid-north, SA South-east, Vic. Southern, NSW Southern and NSW Slopes regions all had less than 10% of populations with susceptibility to all herbicides. In contrast, 55% of populations from NSW Western region were completely susceptible. The amount of multiple resistance varied from 19% in Tas. and NSW Western region to 88% in SA Mid-north region. SA South-east region had one sample with resistance to five modes of action and all regions of SA and Vic. Wimmera Mallee region had populations with resistance to at least four modes of action, compared with none of the regions in NSW or Tas. (Table 5). The multinomial goodness of fit test (Table 5) confirmed the differences between regions with only the Vic. Wimmera Mallee region having a distribution similar to south-eastern Australia as a whole.
Overall, 60% of L. rigidum populations from south-eastern Australia displayed resistance to two or more herbicide modes of action (Fig. 2). This is a modest increase in multiple resistance from 51% detected a decade previously (Boutsalis et al. 2012). In NSW, the level of multiple resistance had increased in NSW Plains region (19% to 39% P ≤ 0.0001) (Broster et al. 2013), but there was little change for NSW Slopes (72% this survey P = 0.314) as multiple resistance was already at 76% of populations previously (Broster et al. 2011).
Discussion
The random collection and herbicide screening of more than 1400 L. rigidum populations, across an estimated 9.5 million ha, represents an extensive herbicide resistance survey of south-eastern Australian cropping regions. L. rigidum resistance to herbicides registered for control of this weed in cropping systems is widespread. The proportion of populations that were resistant to at least one herbicide ranged from 54% in Tas. to 99% in Vic. Southern. Resistance to the post-emergent ALS and ACCase-inhibiting herbicides was most common; however, resistance was also present to the three pre-emergent herbicides tested. The extent of resistance detected provides a challenge for both current and future control of L. rigidum in the regions’ cropping systems.
Lolium spp. overseas are also prone to developing resistance. A survey of 75 L. perenne ssp. multiflorum (Lam.) Husnot (Italian ryegrass) populations from Oregon identified resistance to diclofop-methyl in 59% of populations, to the sulfonylurea herbicides in 27% of populations and resistance to the imidazolinone herbicides in 11% of populations (Rauch et al. 2010). This would be expected as similar crops are grown in both regions (Oregon and south-eastern Australia); therefore it would be expected that herbicide options and use would also be similar.
While diclofop-methyl resistance has increased, clethodim resistance is still below 10% of populations in the majority of regions (Table 3). This is consistent with other studies, in Australia and overseas, that have shown populations of L. rigidum resistant to diclofop-methyl and other FOP herbicides are often controlled by DIM herbicides (Yu et al. 2007; Rauch et al. 2010; Saini et al. 2015a).
A 2015 survey of 348 L. rigidum populations from Western Australia identified 79% with resistance to diclofop-methyl, 92% with resistance to sulfometuron-methyl and 14% resistant to clethodim (Owen and Powles 2018) compared with the average for south-eastern Australia of 64% resistant to diclofop-methyl, 63% resistant to sulfometuron-methyl and 5% resistant to clethodim (Table 3). In contrast, the incidence of resistance to trifluralin was 20% across south-eastern Australia, compared to 1% in Western Australia (Owen et al. 2014; Owen and Powles 2018). These differences reflect interactions between soil type, management practices and crops grown, all of which influence the choice of herbicides and frequency of use of the different herbicides (Broster et al. 2019b).
In comparison with trifluralin the frequency of resistance to prosulfocarb and pyroxasulfone is lower (≤5%). This was expected as trifluralin has a longer history of use across the surveyed area (Boutsalis et al. 2006); however the detection of resistance to prosulfocarb and pyroxasulfone after a relatively short exposure is a cause for concern. Therefore, it is recommended that growers should consider adopting management practices (e.g. herbicide mixtures) that can slow down resistance evolution, prolonging the life of these herbicides.
The 5-enolpyruvylshikimate-3-phosphate synthase inhibitor glyphosate is one of the most important herbicides used for weed control globally and in Australia (Duke and Powles 2008). Despite the widespread use of glyphosate in farming systems of south-eastern Australia, glyphosate resistance was rare, except in SA South-east region, where it was present in 27% of fields. Glyphosate resistance was also identified in 1% of L. rigidum populations found in Western Australia (Owen et al. 2014).
While L. rigidum in Australia was globally the first weed species to evolve resistance to glyphosate in 1996 (Powles et al. 1998; Pratley et al. 1999), glyphosate resistance in Australia is still lower than in several weed species of North America (Heap and Duke 2018). A survey of Kochia scoparia (L) Roth (kochia) populations in Alberta found 13 glyphosate resistant populations out of 300 sites sampled (Hall et al. 2014). In contrast, a survey of Amaranthus rudis Sauer (waterhemp) populations in Missouri found 30% resistant to glyphosate (Schultz et al. 2015). Canola is the only glyphosate resistant winter crop grown across the surveyed area, and during the period reported in this paper comprised no more than 17% of either NSW or Victorian canola crops (Agricultural Biotechnology Council of Australia 2022), with it unable to be grown in South Australia or Tasmania due to government moratoria. This compares with over 90% of canola crops in Canada having herbicide resistant cultivars, mainly glyphosate resistant, in 2006 (O’Donovan et al. 2006) and 91% of soybeans being herbicide tolerant by 2007 (Bonny 2008). As such, it would be expected that there would be both less applications of glyphosate and more of other herbicides, reducing selection pressure for the development of glyphosate resistance.
There were differences between the regions in resistance to multiple herbicide modes of action (Table 4), indicating that resistance evolution is not occurring at the same rates across the regions. These differences are likely to result from differences in crop rotations, including pastures (Table 2), and management practices reducing reliance on individual herbicide modes of action for L. rigidum control (Broster et al. 2019b). While multiple resistance often occurs through the sequential use of different herbicides, it can also occur through cross-resistance, predominantly through enhanced metabolism of herbicides (Han et al. 2016). Multiple resistance was also common in L. rigidum populations from Western Australia (Owen et al. 2014). However, for Italian ryegrass from Oregon only about 35% of populations had multiple resistance (Rauch et al. 2010). Multiple resistance to herbicides was also identified in resistance surveys for Avena fatua L. (wild oat) in Canada (Beckie et al. 2013) Raphanus raphanistrum L. (wild radish) in Western Australia (Owen et al. 2015) and for A. rudis L. (waterhemp) in Missouri (Schultz et al. 2015).
Conclusion
The current extensive survey of L. rigidum from fields across south-eastern Australia has demonstrated that herbicide resistance is widespread in this species and increasing in frequency. Despite this, some regions maintain high levels of susceptibility to some herbicides, offering users the advantage of still being able to use a wider range of herbicides. However, the continuing reliance on herbicides for L. rigidum control in crops will inevitably lead to selection for additional resistance. This is evident in the current survey, where resistance was identified to prosulfocarb + S-metolachlor and pyroxasulfone, which have been recently introduced (Boutsalis et al. 2014). Despite the availability of newer herbicides, reliance solely on herbicides for control of L. rigidum is not sustainable and other practices will be required, such as harvest weed seed control (Walsh et al. 2017).
Data availability
The data that support this study are available in the article and upon request to the corresponding author.
Conflicts of interest
Christopher Preston is an Associate Editor of Crop & Pasture Science, but was blinded from the peer-review process for this paper.
Declaration of funding
This research was funded by the Grains Research and Development Corporation grant number UCS00020. The GRDC had no involvement in study design, collection, analysis or interpretation of data, or in writing the manuscript.
Acknowledgements
The authors would like to thank Alicia Merriam, David Brunton, Geetha Velappan, Ruwan Lenorage and Allison Chambers and casual staff and students at Charles Sturt University for technical assistance.
References
Agricultural Biotechnology Council of Australia (2022) GM canola growth in Australia. Available at https://www.abca.com.au/wp-content/uploads/2020/07/GMCanolaGrowthinAustralia_Tables_UpdatedJune2020.pdf [Accessed 11 February 2022]Beckie HJ, Lozinski C, Shirriff S, Brenzil CA (2013) Herbicide-resistant weeds in the Canadian Prairies: 2007 to 2011. Weed Technology 27, 171–183.
| Herbicide-resistant weeds in the Canadian Prairies: 2007 to 2011.Crossref | GoogleScholarGoogle Scholar |
Bonny S (2008) Genetically modified glyphosate-tolerant soybean in the USA: adoption factors, impacts and prospects. A review. Agronomy for Sustainable Development 28, 21–32.
| Genetically modified glyphosate-tolerant soybean in the USA: adoption factors, impacts and prospects. A review.Crossref | GoogleScholarGoogle Scholar |
Boutsalis P, Broster JC (2006) Herbicide resistance testing of Lolium rigidum by commercial institutions. In ‘Proceedings of the 15th Australian weeds conference’. Adelaide, SA. (Eds C Preston, JH Watts, ND Crossman) pp. 448–490 (Weed Management Society of South Australia: Adelaide, SA)
Boutsalis P, Preston C, Broster JC (2006) Management of trifluralin resistance in annual ryegrass (Lolium rigidum Gaudin) in southern Australia. In ‘Proceedings of the 15th Australian Weeds Conference’. Adelaide, SA. (Eds C Preston, JH Watts, ND Crossman) pp. 507–510. (Weed Management Society of South Australia: Adelaide, SA)
Boutsalis P, Gill GS, Preston C (2012) Incidence of herbicide resistance in rigid ryegrass (Lolium rigidum) across southeastern Australia. Weed Technology 26, 391–398.
| Incidence of herbicide resistance in rigid ryegrass (Lolium rigidum) across southeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Boutsalis P, Gill GS, Preston C (2014) Control of rigid ryegrass in Australian wheat production with pyroxasulfone. Weed Technology 28, 332–339.
| Control of rigid ryegrass in Australian wheat production with pyroxasulfone.Crossref | GoogleScholarGoogle Scholar |
Broster JC, Koetz EA, Wu H (2011) Herbicide resistance levels in annual ryegrass (Lolium rigidum Gaud.) in southern New South Wales. Plant Protection Quarterly 26, 22–28.
Broster JC, Koetz EA, Wu H (2012) Herbicide resistance frequencies in ryegrass (Lolium spp.) and other grass species in Tasmania. Plant Protection Quarterly 27, 36–42.
Broster JC, Koetz EA, Wu H (2013) Herbicide resistance levels in annual ryegrass (Lolium rigidum Gaud.) and wild oat (Avena spp.) in southwestern New South Wales. Plant Protection Quarterly 28, 126–132.
Broster JC, Pratley JE, Ip RHL, Ang L, Seng KP (2019a) A quarter of a century of monitoring herbicide resistance in Lolium rigidum in Australia. Crop & Pasture Science 70, 283–293.
| A quarter of a century of monitoring herbicide resistance in Lolium rigidum in Australia.Crossref | GoogleScholarGoogle Scholar |
Broster JC, Pratley JE, Ip RHL, Ang L, Seng KP (2019b) Cropping practices influence incidence of herbicide resistance in annual ryegrass (Lolium rigidum) in Australia. Crop & Pasture Science 70, 77–84.
| Cropping practices influence incidence of herbicide resistance in annual ryegrass (Lolium rigidum) in Australia.Crossref | GoogleScholarGoogle Scholar |
Brunton DJ, Boutsalis P, Gill G, Preston C (2018) Resistance to multiple PRE herbicides in a field-evolved rigid ryegrass (Lolium rigidum) population. Weed Science 66, 581–585.
| Resistance to multiple PRE herbicides in a field-evolved rigid ryegrass (Lolium rigidum) population.Crossref | GoogleScholarGoogle Scholar |
Brunton DJ, Boutsalis P, Gill G, Preston C (2019) Resistance to very-long-chain fatty-acid (VLCFA)-inhibiting herbicides in multiple field-selected rigid ryegrass (Lolium rigidum) populations. Weed Science 67, 267–272.
| Resistance to very-long-chain fatty-acid (VLCFA)-inhibiting herbicides in multiple field-selected rigid ryegrass (Lolium rigidum) populations.Crossref | GoogleScholarGoogle Scholar |
Duke SO, Powles SB (2008) Glyphosate: a once-in-a-century herbicide. Pest Management Science 64, 319–325.
| Glyphosate: a once-in-a-century herbicide.Crossref | GoogleScholarGoogle Scholar | 18273882PubMed |
Gill G (1996) Ecology of annual ryegrass. Plant Protection Quarterly 11, 195–198.
Hall LM, Beckie HJ, Low R, Shirriff SW, Blackshaw RE, Kimmel N, Neeser C (2014) Survey of glyphosate-resistant kochia (Kochia scoparia L. Schrad.) in Alberta. Canadian Journal of Plant Science 94, 127–130.
| Survey of glyphosate-resistant kochia (Kochia scoparia L. Schrad.) in Alberta.Crossref | GoogleScholarGoogle Scholar |
Han H, Yu Q, Owen MJ, Cawthray GR, Powles SB (2016) Widespread occurrence of both metabolic and target-site herbicide resistance mechanisms in Lolium rigidum populations. Pest Management Science 72, 255–263.
| Widespread occurrence of both metabolic and target-site herbicide resistance mechanisms in Lolium rigidum populations.Crossref | GoogleScholarGoogle Scholar | 25703739PubMed |
Heap I, Duke SO (2018) Overview of glyphosate-resistant weeds worldwide. Pest Management Science 74, 1040–1049.
| Overview of glyphosate-resistant weeds worldwide.Crossref | GoogleScholarGoogle Scholar | 29024306PubMed |
Lemerle D, Verbeek B, Coombes N (1995) Losses in grain yield of winter crops from Lolium rigidum competition depend on crop species, cultivar and season. Weed Research 35, 503–509.
| Losses in grain yield of winter crops from Lolium rigidum competition depend on crop species, cultivar and season.Crossref | GoogleScholarGoogle Scholar |
Llewellyn RS, Lindner RK, Pannell DJ, Powles SB (2002) Resistance and the herbicide resource: perceptions of Western Australian grain growers. Crop Protection 21, 1067–1075.
| Resistance and the herbicide resource: perceptions of Western Australian grain growers.Crossref | GoogleScholarGoogle Scholar |
Llewellyn RS, D’Emden FH, Kuehne G (2012) Extensive use of no-tillage in grain growing regions of Australia. Field Crops Research 132, 204–212.
| Extensive use of no-tillage in grain growing regions of Australia.Crossref | GoogleScholarGoogle Scholar |
Llewellyn R, Ronning D, Clarke M, Mayfield A, Walker S, Ouzman J (2016) Impact of Weeds on Australian Grain Production: the cost of weeds to Australian grain growers and the adoption of weed management and tillage practices. Grains Research and Development Corporation, Canberra, Australia.
McDonald JH (2014) ‘Handbook of biological statistics.’ 3rd edn. (Sparky House Publishing: Baltimore, MD, USA)
O’Donovan JT, Harker KN, Clayton GW, Blackshaw RE (2006) Comparison of a glyphosate-resistant Canola (Brassica napus L.) system with traditional herbicide regimes. Weed Technology 20, 494–501.
| Comparison of a glyphosate-resistant Canola (Brassica napus L.) system with traditional herbicide regimes.Crossref | GoogleScholarGoogle Scholar |
Owen MJ, Powles SB (2018) Current levels of herbicide resistance in key weed species in the WA grain belt. GRDC Update Papers 26 February 2018. Grains Research and Development Corporation, Canberra, ACT. Available at https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2018/02/current-levels-of-herbicide-resistance-in-key-weed-species.
Owen MJ, Martinez NJ, Powles SB (2014) Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt. Weed Research 54, 314–324.
| Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt.Crossref | GoogleScholarGoogle Scholar |
Owen MJ, Martinez NJ, Powles SB (2015) Multiple herbicide-resistant wild radish (Raphanus raphanistrum) populations dominate Western Australian cropping fields. Crop & Pasture Science 66, 1079–1085.
| Multiple herbicide-resistant wild radish (Raphanus raphanistrum) populations dominate Western Australian cropping fields.Crossref | GoogleScholarGoogle Scholar |
Powles SB, Lorraine-Colwill DF, Dellow JJ, Preston C (1998) Evolved resistance to glyphosate in rigid ryegrass (Lolium rigidum) in Australia. Weed Science 46, 604–607.
| Evolved resistance to glyphosate in rigid ryegrass (Lolium rigidum) in Australia.Crossref | GoogleScholarGoogle Scholar |
Pratley J, Urwin N, Stanton R, Baines P, Broster J, Cullis K, Schafer D, Bohn J, Kruger R (1999) Resistance to glyphosate in Lolium rigidum. I. Bioevaluation. Weed Science 47, 405–411.
| Resistance to glyphosate in Lolium rigidum. I. Bioevaluation.Crossref | GoogleScholarGoogle Scholar |
Rauch TA, Thill DC, Gersdorf SA, Price WJ (2010) Widespread occurrence of herbicide-resistant Italian ryegrass (Lolium multiflorum) in Northern Idaho and Eastern Washington. Weed Technology 24, 281–288.
| Widespread occurrence of herbicide-resistant Italian ryegrass (Lolium multiflorum) in Northern Idaho and Eastern Washington.Crossref | GoogleScholarGoogle Scholar |
Reeves TG (1976) Effect of annual ryegrass (Lolium rigidum Gaud.) on yield of wheat. Weed Research 16, 57–63.
| Effect of annual ryegrass (Lolium rigidum Gaud.) on yield of wheat.Crossref | GoogleScholarGoogle Scholar |
Saini RK, Malone J, Preston C, Gill G (2015a) Target enzyme-based resistance to clethodim in Lolium rigidum populations in Australia. Weed Science 63, 946–953.
| Target enzyme-based resistance to clethodim in Lolium rigidum populations in Australia.Crossref | GoogleScholarGoogle Scholar |
Saini RK, Kleemann SGL, Preston C, Gill GS (2015b) Alternative herbicides for the management of clethodim-resistant rigid ryegrass (Lolium rigidum) in faba bean (Vicia faba L.) in Southern Australia. Weed Technology 29, 578–586.
| Alternative herbicides for the management of clethodim-resistant rigid ryegrass (Lolium rigidum) in faba bean (Vicia faba L.) in Southern Australia.Crossref | GoogleScholarGoogle Scholar |
Schultz JL, Chatham LA, Riggins CW, Tranel PJ, Bradley KW (2015) Distribution of herbicide resistances and molecular mechanisms conferring resistance in Missouri waterhemp (Amaranthus rudis Sauer) populations. Weed Science 63, 336–345.
| Distribution of herbicide resistances and molecular mechanisms conferring resistance in Missouri waterhemp (Amaranthus rudis Sauer) populations.Crossref | GoogleScholarGoogle Scholar |
Tranel PJ, Wright TR (2002) Resistance of weeds to ALS-inhibiting herbicides: what have we learned? Weed Science 50, 700–712.
| Resistance of weeds to ALS-inhibiting herbicides: what have we learned?Crossref | GoogleScholarGoogle Scholar |
Walsh MJ, Aves C, Powles SB (2017) Harvest weed seed control systems are similarly effective on rigid ryegrass. Weed Technology 31, 178–183.
| Harvest weed seed control systems are similarly effective on rigid ryegrass.Crossref | GoogleScholarGoogle Scholar |
Yu Q, Powles SB (2014) Resistance to AHAS inhibitor herbicides: current understanding. Pest Management Science 70, 1340–1350.
| Resistance to AHAS inhibitor herbicides: current understanding.Crossref | GoogleScholarGoogle Scholar | 24338926PubMed |
Yu Q, Collavo A, Zheng MQ, Owen M, Sattin M, Powles SB (2007) Diversity of acetyl-coenzyme A carboxylase mutations in resistant Lolium populations: evaluation using clethodim. Plant Physiology 145, 547–558.
| Diversity of acetyl-coenzyme A carboxylase mutations in resistant Lolium populations: evaluation using clethodim.Crossref | GoogleScholarGoogle Scholar | 17720757PubMed |