Field benchmarking of the critical external phosphorus requirements of pasture legumes for southern Australia
Graeme A. Sandral A , Andrew Price A , Shane M. Hildebrand A , Christopher G. Fuller A , Rebecca E. Haling B , Adam Stefanski B , Zongjian Yang B , Richard A. Culvenor B , Megan H. Ryan C D , Daniel R. Kidd C D , Simon Diffey E , Hans Lambers D F and Richard J. Simpson
C D , Simon Diffey E , Hans Lambers D F and Richard J. Simpson  B G
B G
A Department of Primary Industries, Wagga Wagga Agricultural Institute, Pine Gully Road, Wagga Wagga, NSW 2650, Australia.
B CSIRO Agriculture and Food, GPO Box 1700, Canberra, ACT 2601, Australia.
C School of Agriculture and Environment, The University of Western Australia, Crawley, WA 6009, Australia.
D Institute of Agriculture, The University of Western Australia, Crawley, WA 6009, Australia.
E Centre for Bioinformatics and Biometrics, University of Wollongong, NSW 2522, Australia.
F School of Biological Sciences, The University of Western Australia, Crawley, WA 6009, Australia.
G Corresponding author. Email: richard.simpson@csiro.au
Crop and Pasture Science 70(12) 1080-1096 https://doi.org/10.1071/CP19014
Submitted: 9 January 2019 Accepted: 2 May 2019 Published: 11 October 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
In recent decades several pasture legumes have been available in southern Australia as potential alternatives to the most widely used annual pasture legume Trifolium subterraneum. Little is known about their soil phosphorus (P) requirements, but controlled environment experiments indicate that at least some may differ in their P fertiliser requirements. In this study, pasture legume varieties, including T. subterraneum as the reference species, were grown at up to four sites in any one year over a 3-year period (in total, seven site × year experiments) to measure herbage growth responses in spring to increased soil P availability. A critical soil test P concentration (corresponding to 95% maximum yield) was estimated for 15 legumes and two pasture grasses. The critical soil P requirements of most of the legumes did not differ consistently from that of T. subterraneum, indicating their soil fertility management should follow the current soil test P guidelines for temperate Australian pastures. However, the critical P requirement of Medicago sativa was higher than that of T. subterraneum, but remains ill-defined because extractable soil P concentrations in these experiments were often not high enough to permit a critical P estimate. Three forage crop legumes (Trifolium incarnatum, Trifolium purpureum, Trifolium vesiculosum) and two pasture legumes (Ornithopus compressus, Ornithopus sativus) had lower critical soil test P concentrations. It may be feasible to manage pastures based on these species to a lower soil test P benchmark without compromising yield.
Additional keywords: critical soil test P, Ornithopus compressus, Ornithopus sativus, phosphorus fertiliser, serradella, subterranean clover, Trifolium subterraneum.
Introduction
Phosphorus (P) is an essential input for productive grazing enterprises in southern Australia because large areas used for growing dryland grass–legume pastures have soils that are deficient in P for pasture growth (Williams and Andrew 1970), can remain so even after many years of fertiliser use (Schefe et al. 2015) or require continuing applications of fertiliser to maintain optimal P availability for high production (Simpson et al. 2015). The soils are also often deficient in other macronutrients (e.g. nitrogen, sulfur and potassium) and micronutrients (e.g. molybdenum) to varying degrees. In these systems, legumes are relied on to supply nitrogen to the pasture system. Provided other nutrient deficiencies have been resolved, it is recommended that P fertiliser be applied to increase pasture yields until the critical P requirement of the pasture–soil system is achieved (i.e. the extractable soil P concentration required to achieve near-maximum yield). Thereafter, P fertiliser inputs should be moderated to maintain soil P fertility at the critical P concentration (Reuter et al. 1995; Simpson et al. 2015). Dryland pastures maintained with optimal soil fertility achieve maximum efficiency of rainfall use (e.g. Mills et al. 2006) and permit high stocking rates (Lean et al. 1997) and, consequently, high land use efficiency. The P balance efficiency of fertiliser use in grazing systems (i.e. P output expressed as a proportion of P inputs) is determined primarily by the rate at which P accumulates in moderate to high P-sorbing soils (e.g. Simpson et al. 2014, 2015), and/or by loss of P via erosion, run-off or leaching (Lewis et al. 1981). The P sorption capacity of a soil (i.e. the net capacity for phosphate to sorb to and continue to react with a soil; McLaughlin et al. 2011) usually determines whether the rate of P accumulation or rate of P loss dominates the efficiency equation. However, both components of P inefficiency are positively correlated with the extractable P concentration at which a soil is maintained by fertiliser applications (Melland et al. 2008; McDowell 2012; Simpson et al. 2014). Consequently, from both production and resource use efficiency viewpoints, the most effective use of P fertiliser occurs when a pasture soil is maintained close to the critical P requirement of the pasture–soil system.
The challenge for practical soil fertility management is knowing the critical soil test P concentration at which maximum pasture yield can be achieved. For southern Australia, there are two assessments of historical datasets that have defined the critical P requirements of temperate pastures (Gourley et al. 2007, 2019; Moody 2007). Both studies reinterpret several key experiments (e.g. Helyar and Spencer 1977), many of which explicitly examined the response to soil test P by pastures based on Trifolium subterraneum L. (subterranean clover) and Trifolium repens L. (white clover). These legumes have higher critical P requirements than the grasses with which they are grown (Ozanne et al. 1969, 1976; Helyar and Anderson 1970; Jackman and Mouat 1972; Hill et al. 2010) and, consequently, grass–legume pastures are fertilised to meet the legume’s higher P requirement, because legume N2 fixation ultimately drives the productivity of the pasture system.
Since the late 1990s, numerous alternative pasture legumes have been developed in Australia, primarily to address areas of the agricultural landscape and farming systems where T. subterraneum cannot be used reliably (Loi et al. 2005; Nichols et al. 2007, 2012). Little is known about the P requirements of these species, but controlled environment experiments indicate that at least some of the species differ in their P fertiliser requirements (Haling et al. 2016a; Sandral et al. 2018). Herein we report results from field experiments examining the growth during spring of several alternative legumes and two perennial grasses in response to six levels of soil P availability. The aim of the research was to determine the relative P requirements of the species and to estimate the critical extractable P concentration (i.e. soil test P) for near-maximum yield, under field conditions. T. subterraneum is the most commonly grown legume in permanent pastures and mixed crop–pasture systems in southern Australia and was included as a reference species.
Materials and methods
Experiment sites
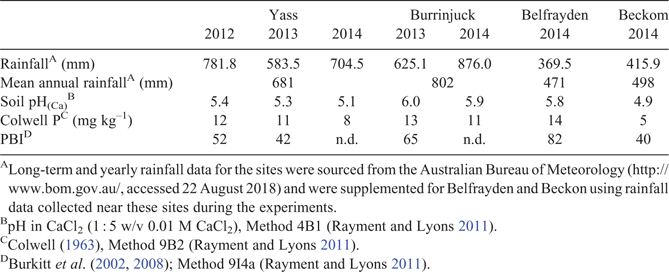
Four sites were established near Yass (–34.948, 148.930), Burrinjuck (–34.869, 148.672), Belfrayden (–35.116, 146.992) and Beckom (–34.202, 147.033) in southern New South Wales, Australia. The site characteristics are presented in Table 1. The Yass site was sown in 2012 and resown in 2013 and 2014. The Burrinjuck site was sown in 2013 and resown in 2014, whereas the Belfrayden and Beckom sites were only sown in 2014. At each site, 12 pasture cultivars were sown each year and grown at six rates of P supply with three replicates. Plot size was 10 m × 2 m.
Yass and Burrinjuck are in regions of permanent pasture-based livestock systems. Belfrayden and Beckom are in regions where pasture systems are either permanent (non-arable landscapes) or grown in sequence with crops.
Fertiliser application
Lime was applied to a site during its initial preparation if the surface soil pHCa (0–10 cm) was below 5.2. At Yass and Burrinjuck, 2 Mg lime ha–1 was applied and cultivated in to a depth of ~10 cm; at Beckom 1 Mg lime ha–1 was applied and incorporated. It was not necessary to apply lime at Belfrayden. The surface soil pHCa that was achieved is recorded in Table 1. Basal nutrients and P were then applied before sowing at each site: micronutrients (molybdenum trioxide (MoO3) 0.07 kg ha–1, boric acid (H3BO3) 1.75 kg ha–1, copper sulfate (CuSO4.5H2O) 1.75 kg ha–1 and zinc sulfate (ZnSO4.7H2O) 3.5 kg ha–1) were applied using a boom spray with 100 L ha–1 water as the carrier; and potassium sulfate (K2SO4) and magnesium sulfate (MgSO4.7H20) were applied at rates of 100 and 60 kg ha–1 respectively. P was applied to the soil surface by hand as triple superphosphate (20.7% P, 1.5% S) to establish six uniformly spread soil P levels. Rates were 0, 15, 30, 45, 60 and 80 kg P ha–1 at Yass (2012) and Burrinjuck (2013), and 0, 15, 30, 50, 65 and 85 kg P ha–1 at Belfrayden and Beckom (2014). Subsequently, maintenance P applications were applied in the same way in autumn at 0, 1, 3, 8, 15, 20 kg P ha–1 at Yass (2013 and 2014) and Burrinjuck (2014). Plots sown to perennial grasses received a total of 80 kg ha–1 year–1 nitrogen in four equal applications during May, July, August and October.
Plant material
Because of the range in annual rainfall (growing season lengths) among the sites, it was not sensible to sow all the pasture varieties at each site. The cultivars of T. subterraneum were selected for use at a site using current maturity-type information (Nichols et al. 2013). Some of the alternative legumes had not been tested regionally and were without specific recommendations concerning their suitability. Subsequently, several were not resown at some of the sites because they proved to be unsuitable (see Supplementary Materials table S1, available at the journal’s website). The annual legume species (common name and cultivar) used in the experiments were as follows: Trifolium glanduliferum Boiss (gland clover cv. Prima), Trifolium hirtum All. (rose clover cv. Hykon), Trifolium incarnatum L. (crimson clover cv. Dixie), Trifolium michelianum Savi. (balansa clover cv. Bolta), Trifolium purpureum Loisel. (purple clover cv. Electra), Trifolium spumosum L. (bladder clover cv. Bartolo), T. subterraneum L. (subterranean clover cv. Leura, Narrikup, Izmir), Trifolium vesiculosum Savi (arrowleaf clover cv. Zulu II), Ornithopus compressus L. (yellow serradella cv. Santorini, Avila), Ornithopus sativus Brot. (French serradella cv. Margurita), Biserrula pelecinus L. (biserrula cv. Casbah, Mauro), Medicago truncatula Gaertn. (barrel medic cv. Sultan-SU) and Lupinus albus L. (white lupin cv. Luxor). The perennial legumes were: Medicago sativa L. (lucerne cv. SARDI 10), Trifolium ambiguum M. Bieb. (Caucasian clover cv. Kuratas), Trifolium tumens Steven ex M. Bieb. (talish clover cv. Permatas), Lotus corniculatus L. (birdsfoot trefoil line LC07AUYF) and Bituminaria bituminosa var. albomarginata (L.) C.H. Stirt. (tedera line Tedera 27). The perennial grasses were Dactylis glomerata L. (cocksfoot cv. Porto, Uplands) and Phalaris aquatica L. (phalaris cv. Advanced AT). All species are primarily used in pastures with the exception of T. incarnatum and T. purpureum (forage species) and L. albus (a crop species).
Seed was sown on 7 May (Yass 2012), 20 May (Yass and Burrinjuck 2013), 25 February (Yass and Burrinjuck 2014) and 2 April (Belfrayden and Beckom 2014). All annual legumes were resown each year at 30 kg viable seed per hectare to avoid possible differences in seed reserves and establishment density. Seeds were sown using DBS direct-drill tines (Ausplow, Cockburn Central, WA, Australia) at a row spacing of 25 cm, aiming typically for seed placement at a depth of 1 cm. Perennial legumes and perennial grasses were sown at 10 kg ha–1 and not resown annually, unless establishment was considered inadequate. The germination of all cultivars was tested and sowing rates were adjusted accordingly. Legumes were inoculated with appropriate rhizobia before sowing: Group S (WSM471 strain) for the Ornithopus spp., WSM1497 for B. pelecinus, Group G (WU425) for L. albus, Group AM (WSM1115) for M. truncatula, Group AL (RRI128) for M. sativa, SU343 for L. corniculatus, WSM4083 for B. bituminosa, Group C (WSM1325) for all the annual Trifolium spp., CC283b for T. ambiguum and Group B (TA1) for T. tumens.
Table S1 provides a full list of all of the cultivars sown at each site in each year and indicates whether the cultivar was harvested or had failed to establish. Yield and critical P results are only reported for cultivars that established and grew successfully.
Establishment and management
Yass 2012 and 2013
The perennial grasses failed to establish adequately at the Yass site in 2012 because of grass weeds (mostly Vulpia spp.) that invaded these treatments and could not be controlled. In the legume plots, grass weeds were controlled with 500 mL ha–1 of the grass-selective herbicide Select (Sumitomo Chemical Australia Pty Ltd, Epping, NSW, Australia; 240 g L–1 clethodim). T. ambiguum (cv. Kuratas), a perennial legume, established at low densities and plant numbers decreased further over the 2012–13 summer. Consequently, these plants were not harvested and were replaced with other species in autumn 2013. An experimental line of B. bituminosa var. albomarginata (Tedera 27) was sown in 2012, but winter frosts killed most seedlings. It was subsequently sown in spring (late September) 2012. However, only low numbers of B. bituminosa plants survived frosts in the 2013 winter, and peak spring growth could not be assessed adequately. The annual legumes B. pelecinus (cv. Mauro) and T. spumosum (cv. Bartolo) failed to produce adequate forage over the winter and spring of 2012 and were not assessed. Rutherglen bug (Nysius vinitor) was found on T. spumosum during spring, and was controlled by spraying with Matador (Nufarm Australia, Laverton North, Vic, Australia; 250 g L–1 lambda cyhalothrin) at 36 mL ha–1.
Yass 2014
At Yass in 2014, the annual legumes B. pelecinus (cv. Casbah), T. spumosum (cv. Bartolo) and T. hirtum (cv. Hykon) failed to produce adequate spring growth. Rutherglen bug (N. vinitor) was observed in large numbers on T. spumosum in early September and Matador was again applied at 36 mL ha–1. M. sativa (cv. SARDI 10) plant populations declined considerably over the 2013–14 summer, and consequently this species was not assessed in spring 2014 (see table S1). A Sclerotinia sp. was identified on herbage in small collapsed necrotic patches of O. sativus (cv. Margurita) and was controlled with Prosaro 420 SC (Bayer Australia, Pymble, NSW, Australia; 210 g L–1 prothioconazole and 210 g L–1 tebuconazole) at 300 mL ha–1.
Burrinjuck 2013 and 2014
At Burrinjuck in 2013, the sown perennial grasses failed due to preferential grazing by wombats (Vombatus ursinus), until they were excluded by erecting a low electrified fence wire, followed by grass weed incursions that could not be selectively removed with a herbicide. The legume plots received 500 mL ha–1 of the grass-selective herbicide Select (240 g L–1 clethodim) to control grass weeds. The legumes T. spumosum (cv. Bartolo), T. hirtum (cv. Hykon), B. pelecinus (cv. Mauro) and L. corniculatus (line LC07AUYF) failed to establish in adequate numbers and were not assessed. The likely reason for this is that these species were not competitive against Juncus bufonius L. (toad rush) that had invaded these treatments over winter. In 2014, T. spumosum (cv. Bartolo), T. hirtum (cv. Hykon), B. pelecinus (cv. Casbah), T. glanduliferum (cv. Prima), O. compressus (cv. Santorini) and T. subterraneum (cv. Narrikup) failed to establish successfully again due to invasion by J. bufonius, despite an early season application of Spinnaker (Nufarm Australia, Laverton North, Vic, Australia; 700 g kg–1 imazethapyr) at 70 g ha–1. As with the Yass site, a Sclerotinia sp. was identified on O. sativus (cv. Margurita) and controlled with Prosaro 420 SC (210 g L–1 prothioconazole and 210 g L–1 tebuconazole) at 300 mL ha–1.
Beckom and Belfrayden 2014
All species sown at these sites produced sufficient herbage in spring to enable them to be harvested. However, the spring was relatively dry with only approximately half the average rainfall at Belfrayden and two-thirds of the average rainfall at Beckom in September and October (Table 1; table S2).
Rainfall
The rainfall received over the May–October period at all sites over the 2012–2014 seasons was below average (Table 1; table S2). For example, at Yass the May–October rainfall was 93, 52.9 and 73.5 mm below the long-term (1889–2015) average for 2012, 2013 and 2014 respectively (table S2). Similarly, Burrinjuck received 65.7 and 63.5 mm below the long-term average rainfall for May–October during 2013 and 2014 respectively, whereas Belfrayden and Beckom received 89.3 and 83.3 mm below average respectively for 2014.
Although drier than average conditions were experienced over the May–October period, there was also transient waterlogging present at the Yass and Burrinjuck sites during 2013 and 2014 as a consequence of the higher than average June rainfall for these years (table S2). Waterlogging was more evident at Burrinjuck, and particularly in 2014.
Measurements
Herbage dry matter
Shoots were cut at ground level from three quadrats (200 mm × 500 mm) in each plot on: 6 November 2012, 2 October 2013 and 9 October 2014 at Yass; 23 October 2013 and 24 October 2014 at Burrinjuck; 1 October 2014 at Belfrayden; and 18 September 2014 at Beckom. These harvests were intended to assess the approximate peak period for spring growth rate. Shoots were dried at 70°C for 72 h and weighed to provide an estimate of the dry matter (DM) production for each plot.
Soil sampling
Soil P levels were determined at the time of the peak spring harvest by taking eight soil cores (depth 0–10 cm) from each of the three areas cut for DM assessment (total 24 soil cores per plot). Soil cores were broken up, mixed and dried at 40°C. Once dry, the soil samples were mixed thoroughly again and subsampled to determine the Colwell- and Olsen-extractable P concentrations (Olsen et al. 1954; Colwell 1963).
Herbage P concentrations
Herbage DM (i.e. whole shoots) harvested from four of the six P treatments (the treatment receiving no P and three other treatments selected to span the critical soil test P level of each pasture variety) was used for determination of whole-shoot P concentrations at all sites in 2014. The dry shoot material was milled and ~50 mg subsamples were ashed at 550°C. The ash was then dissolved in 2 M HCl to facilitate the colourimetric determination of P concentration using malachite green (Irving and McLaughlin 1990).
Colonisation of roots by mycorrhizal fungi
Colonisation of roots by arbuscular mycorrhizal fungi (AMF) was measured on topsoil roots of O. sativus, O. compressus and T. subterraneum grown in the unfertilised treatment at all four field sites in spring 2014 at the time of the peak spring harvest. Ten cores (diameter 2.5 cm, depth 10 cm) were taken per plot and combined to wash the roots from the soil. The roots were cleared (10% w/v KOH for 2–4 days, followed by rinsing in water and 1% v/v HCl), stained (5% v/v Schaeffer blue ink–white vinegar solution for 1 h; Vierheilig et al. 1998) and colonisation was measured using the grid-line intersect method (Giovannetti and Mosse 1980).
Root hair length
Root hair length was measured on topsoil roots (depth 0–10 cm) of O. sativus, O. compressus and T. subterraneum grown in the unfertilised treatment (i.e. 0 kg P ha–1) and 15 kg P ha–1 treatments at the Yass, Burrinjuck and Beckom field sites in May 2016. Assessments could not be made at Belfrayden because the site had been resumed for cropping. Two cores (diameter 6.5 cm, depth 10 cm) were taken per plot and combined. Roots were washed from the soil and root hairs were measured on five lengths of root selected at random from each sample. Each length of root was photographed using a Leica MZFLIII fluorescence microscope (Leica Microsystems, Sydney, NSW, Australia) fitted with a Zeiss AxioCam camera (Zeiss, Sydney, NSW, Australia). Fifteen root hairs perpendicular to the line of vision were selected on each root sample for length measurement using ImageJ v. 1.46r (U.S. National Institutes of Health, Bethesda, MD; https://imagej.nih.gov/ij/docs/guide, accessed 22 August 2018).
Experiment design and statistical analysis
The experimental design software DiGGer (Coombes 2009) was used to produce randomised complete block designs that avoided row, column and diagonal treatment duplicates. Treatments comprised the factorial of six P rates and 12 cultivars and were replicated three times.
Linear mixed-model technology was used to fit a statistical model to herbage DM measurements. The linear mixed model can be formulated in such a way that it is analogous to an analysis of variance (ANOVA). For a single trial this would be an ANOVA for a randomised complete block design where the sums of squares are partitioned into sums of squares for the overall mean, sums of squares for blocks and sums of squares for treatments (being the factorial of P rates and cultivars). Unlike ANOVA, the linear mixed model has the ability to accommodate missing values in the response and explanatory variables and can accommodate a wide range of variance models, in particular spatial models.
Spatial models for each trial were considered following the process described by Gilmour et al. (1997) for identifying and accounting for sources of field spatial variability. Spatial variability can be divided into three sources: local, global and extraneous. Local variation is based on the notion that plots closer together will be more similar than plots further apart regardless of treatment (or block) effects. The approach considered by Gilmour et al. (1997) to model local variation is based on the first-order separable autoregressive models described by Cullis and Gleeson (1991). Global variation, such as soil fertility trends, is modelled by considering the statistical significance of centred linear covariates for field row and field column. Extraneous variation, such as variation attributable to trial management operations, is modelled by considering additional terms to fit in the model, such as random field column and row effects.
Plot herbage DM outliers and poor-quality data were determined using a combination of field notes and statistical model diagnostics. Field notes included observations on grazing by wild vertebrate pests, large subsurface rocks, sclerotinia damage, root rot, waterlogged hollows and/or establishment failure. Plot values were only rejected if adverse observations coincided with a plot residual, produced after linear mixed model analysis, that was >3.
For herbage DM, all models were fitted using the statistical software package ASRemL (Butler 2009) within the R computing environment (R Core Team 2018). This analysis showed that effects of P rate and cultivar were significant (P < 0.05), but the interaction of P rate and cultivar was not. Graphs of herbage DM response to soil P fertility partitioned according to cultivar were drawn using fitted data from ASRemL (VSN International, Hemel Hempstead, UK). Plot-level fitted data from ASRemL were used initially to fit the Mitscherlich diminishing returns equation (y = A + B × Rc) using GENSTAT (16th edition; VSN International, Hemel Hempstead, UK), where y is the plot-level shoot dry matter (kg ha–1) and c is the plot-level soil test P concentration (mg kg–1) measured during spring at the time the herbage was harvested. The aim was to estimate critical soil test P requirements, maximum yields (i.e. the asymptote value, A) and the standard errors associated with the equation parameters (see table S3).
Phosphorus fertiliser application rates had been planned to exceed the anticipated P requirement of T. subterraneum, but the P requirements of the other species were unknown. We encountered problems fitting the Mitscherlich equation to species with shallow curvature (i.e. varieties with very low critical P requirements) and to species that achieved maximum yields only at the highest rates of P supply because, under these circumstances, the Mitscherlich equation overestimates maximum yield, critical P requirement and/or the error associated with these parameters. Therefore, we adopted the method of Dyson and Conyers (2013) to fit a yield response function to the plot-level data (i.e. the fitted data from ASRemL for herbage yields and the corresponding Colwell P concentrations). Critical soil test P concentrations were then determined using these yield response functions. Dyson and Conyers (2013) recognised that herbage yield and soil test values (STV) are both inexact and unknown in datasets of crop yield response to soil fertility. The relationship between plot-level fitted herbage yield data and plot-level fitted Colwell soil test P values (STV) was straightened by plotting ln(STV) against arcsine([RY%/100]0.5), where RY is relative herbage yield (i.e. yield expressed as a proportion of the maximum or asymptote value for herbage DM when P is non-limiting). The ln(STV) was plotted along the vertical axis, with arcsine([RY%/100]0.5) as the horizontal axis to permit determination of the standard error associated with a predicted y-value (i.e. the critical Colwell P concentration) using Excel 2013 (Microsoft, Redmond, WA, USA). This process required the initial determination of maximum yield and the subsequent exclusion of RY values associated with very high STV values, which may otherwise bias estimates of critical P concentrations (Dyson and Conyers 2013). The yield response of each variety was determined independently at each site in each year. Where the yield of a variety clearly plateaued at high STV, maximum yield was estimated as the average of yield values along the response plateau (e.g. varieties of Ornithopus spp.; Figs 1–3). However, when a clear yield plateau was not observed, maximum yield was assumed to be the average yield obtained from replicate plots to which the highest rates of P fertiliser had been applied (e.g. varieties of T. subterraneum; Figs 1–3). The only species where there was any doubt that this procedure would give a reasonable approximation of maximum yield was M. sativa, for which the growth response to P was often linear and did not plateau below the highest rates of P application on four of the six occasions that it was grown (Figs 1–3). When this was the case, no attempt was made to estimate maximum yield or the critical Colwell P concentration. RY values associated with very high STV were excluded as prescribed by Dyson and Conyers (2013), who used an arbitrary cut-off of twice the initial critical STV estimate. Our yield response data were considerably less variable than those of Dyson and Conyers (2013) and we reduced the cut-off to 1.5-fold the initial critical STV estimate, after determining this was likely to be the minimum multiplier without effect on the predicted critical STV.

|

|

|
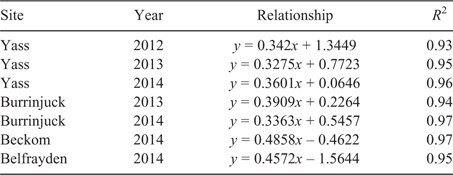
Critical Colwell P concentrations corresponding to 95% and 90% of maximum yield were estimated from the linear relationships to enable comparisons with published data that adopt different critical criteria (e.g. Gourley et al. 2007, 2019 (95%); Moody 2007 (90%)). The 95% confidence interval (CI) associated with each critical Colwell P value was estimated as the sum of twice the standard error on the high side of the estimate plus twice the standard error on the low side of the estimate (note, standard errors for values close to the asymptote are asymmetric). Critical Olsen P concentrations were subsequently determined using relationships between Colwell P and Olsen P determined using the soil samples taken in spring at each site in each year.
For each replicate of each pasture variety, herbage P concentrations were regressed against their corresponding Colwell soil test P fitted data from ASRemL to assess response of herbage P concentration to soil P fertility. The mean ± s.e.m. (n = 3) P concentration of herbage that corresponded with the critical soil test P level was estimated using these relationships.
Results
Critical soil test P concentrations
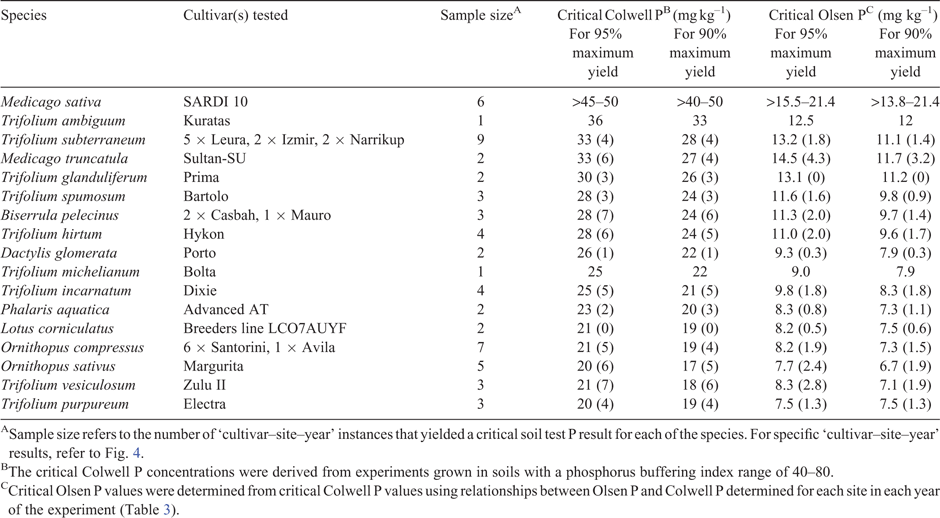
The Mitscherlich function was initially fitted to the yield response data (table S3). However, for the reasons outlined above, the Mitscherlich function was occasionally unable to adequately estimate asymptotic yield maxima and critical soil test P requirements. Consequently, the critical soil test P requirement for 90% or 95% of maximum yield was determined using the approach proposed by Dyson and Conyers (2013; Fig. 4, Table 2). Because there are regional preferences for the use of alternative soil test P methods, the corresponding critical Olsen P concentrations were derived using the relationships between Colwell P and Olsen P determined for each site in each year (Table 3). However, unless stated otherwise, results in this paper refer primarily to the critical Colwell P concentrations that correspond with a 95% maximum yield threshold.

|
|
Yass
In 2012, the critical external Colwell P requirement of T. subterraneum (cv. Leura) was 34 mg P kg–1 soil (Fig. 4). O. compressus (cv. Santorini) and L. corniculatus (line LC07AUYF) had significantly (P < 0.05) lower critical Colwell P requirements, but their maximum yields were only half or less that of T. subterraneum (Fig. 1). T. hirtum (cv. Hykon) had a similar critical P requirement and yield to that of T. subterraneum. The critical P requirement of M. sativa (cv. SARDI 10) could not be determined because a yield asymptote was not achieved within the P treatment range (0–80 kg P applied per hectare).
In 2013, T. subterraneum was the highest yielding species and had a critical Colwell P requirement of 34 mg P kg–1. Ornithopus compressus (cv. Santorini), T. purpureum (cv. Electra) and L. corniculatus (line LC07AUYF) had critical Colwell P requirements approximately half to two-thirds that of T. subterraneum. The yield of O. compressus was approximately 70% of that of T. subterraneum, but the other P-efficient species were again low yielding. The perennial grasses D. glomerata (cv. Porto) and P. aquatica (cv. Advanced AT) also had low critical Colwell P requirements, often equivalent to those of the P-efficient legumes (O. compressus, T. purpureum, L. corniculatus). However, the critical Colwell P requirements of T. hirtum (cv. Hykon), B. pelecinus (cv. Mauro), T. spumosum (cv. Bartolo) and T. ambiguum (cv. Kuratas) did not differ significantly from that of T. subterraneum. As in 2012, the critical P requirement of M. sativa could not be determined. No attempt was made to estimate the critical P requirement of B. bituminosa (line 27) because of the high loss of seedlings to frost.
In 2014, the critical Colwell P requirement of T. subterraneum was 36 mg P kg–1. O. compressus (cvv. Avila, Santorini), O. sativus (cv. Margurita) and T. purpureum (cv. Electra) had critical Colwell P requirements that were 45–55% of that of T. subterraneum. O. sativus (cv. Margurita) and O. compressus (cv. Santorini) yielded as well as T. subterraneum, but the yield of O. compressus (cv. Avila) was only half that of T. subterraneum. P. aquatica (cv. Advanced AT) again had a low critical P requirement, similar to that of the more P-efficient legumes. However, D. glomerata (cv. Porto) yielded poorly and had a critical P requirement that was not significantly lower than that of T. subterraneum. The experimental mixture of L. albus and T. subterraneum had a low critical Colwell P requirement (similar in magnitude to that of the Ornithopus spp.), but also had a maximum yield that was 18% lower than that of T. subterraneum in monoculture.
Burrinjuck
In 2013, the critical Colwell P requirement of T. subterraneum (cv. Leura) was 34 mg P kg–1 (Fig. 2). Trifolium purpureum (cv. Electra), O. sativus (cv. Margurita) and O. compressus (cv. Santorini) had critical Colwell P requirements that were numerically approximately two-thirds that of T. subterraneum (Fig. 4). However, only the critical P requirement of T. purpureum was significantly (P < 0.05) lower. The maximum herbage yields of T. purpureum and O. sativus equalled that of T. subterraneum, but the yield of O. compressus (cv. Santorini) was only approximately 70% of T. subterraneum. The forage legume T. incarnatum yielded at least 30% more than any other species. The yield of M. sativa (cv. SARDI 10) did not plateau within the P treatment range (0–80 kg P applied per hectare) and the critical P requirement could not be determined.
In 2014, spring DM yields were considerably higher than those measured in 2013. Although 2014 was below average for rainfall, the soil at the Burrinjuck was relatively wet during spring. J. bufonius is favoured by wet soil conditions and germinated in high density in several plots, causing establishment failures for all but the most competitive species. The critical Colwell P requirement of T. subterraneum was 30 mg P kg–1. O. sativus (cv. Margurita) again yielded as well as T. subterraneum, with a significantly lower critical Colwell P requirement of 20 mg P kg–1. T. vesiculosum (cv. Zulu II) also had a significantly lower critical P requirement (19 mg P kg–1), but yielded relatively poorly. The critical P requirements of T. michelianum and T. incarnatum (cv. Dixie) were not significantly different from that of T. subterraneum. However, T. michelianum (cv. Bolta) exceeded the yield of T. subterraneum by 16%. M. sativa (cv. SARDI 10) achieved a yield plateau in this season. The critical Colwell P requirement of M. sativa was 45 mg kg–1, significantly greater than that of any of the other legumes.
Belfrayden and Beckom
The late-maturing T. subterraneum cv. Leura was not grown at Beckom and Belfrayden because it is not adapted to the climate at these locations. The better-adapted (mid-season) cultivars Izmir and Narrikup were grown instead.
At Beckom, the critical Colwell P estimates for the T. subterraneum cultivars Narrikup and Izmir were 28 and 32 mg P kg–1, respectively, but the two values were not significantly different (Fig. 4). O. sativus (cv. Margurita), O. compressus (cv. Santorini) and T. vesiculosum (Zulu II) had critical P requirements half or less than that of the T. subterraneum cultivars. T. subterraneum cv. Narrikup out-yielded cv. Izmir (Fig. 3). T. vesiculosum was high yielding. The maximum yields of O. sativus and O. compressus were not significantly different to that of T. subterraneum (cv. Izmir), but were substantially less than that of cv. Narrikup. T. spumosum (cv. Bartolo), T. hirtum (cv. Hykon), M. truncatula (Sultan-SU) and B. pelecinus (cv. Casbah) had critical P requirements and maximum yields that were not different from those of the high-yielding cv. Narrikup. The yield of M. sativa did not plateau, and its critical P requirement could not be determined.
At Belfrayden, the critical Colwell P requirements of the T. subterraneum cultivars Narrikup and Izmir were 30 and 40 mg P kg–1, respectively, but the two values were not significantly different (Fig. 4). However, cv. Narrikup again out-yielded cv. Izmir (by ~23%; Fig. 3). Only T. incarnatum (cv. Dixie) and T. hirtum (cv. Hykon) had critical P requirements that were significantly lower than that of T. subterraneum cv. Izmir, but their critical P requirements were not significantly lower than that of T. subterraneum cv. Narrikup. The maximum yield of O. sativus (cv. Margurita) was equivalent to that of T. subterraneum cv. Izmir. O. compressus (cv. Santorini) yielded relatively poorly. The critical P requirement of M. sativa was 45 mg P kg–1 and significantly (P < 0.05) higher than that of T. vesiculosum (Zulu II), M. truncatula (Sultan-SU), O. sativus (cv. Margurita), O. compressus (cv. Santorini), T. incarnatum (cv. Dixie) and T. hirtum (cv. Hykon), but not significantly higher than that of either of the T. subterraneum cultivars.
Repeatability of critical P estimates
The repeatability of critical soil test P estimates for the pasture species was examined by assuming that each ‘cultivar–site–year’ experiment was an independent assessment of a species’ critical P requirement (Table 2). This was clearly the case when considering critical P estimates among sites, but was also considered to be reasonable when considering critical P estimates among years and within sites because the weather differed from year to year, and the experiment plots were oversown each year with additional seed to ensure plant densities did not decline and confound yield responses to soil P fertility. The number of ‘site–year’ experiments in which each species was represented varied for the reasons described above, so repeatability of the critical P estimate was determined by calculating the standard deviation associated with each estimate of average critical P value. By this measure, the critical P estimates for some pasture varieties (e.g. L. corniculatus, D. glomerata, P. aquatica) were highly repeatable, whereas the repeatability of critical P estimates for some other varieties (e.g. B. pelecinus, T. versiculosum) was relatively poor.
Herbage P concentrations
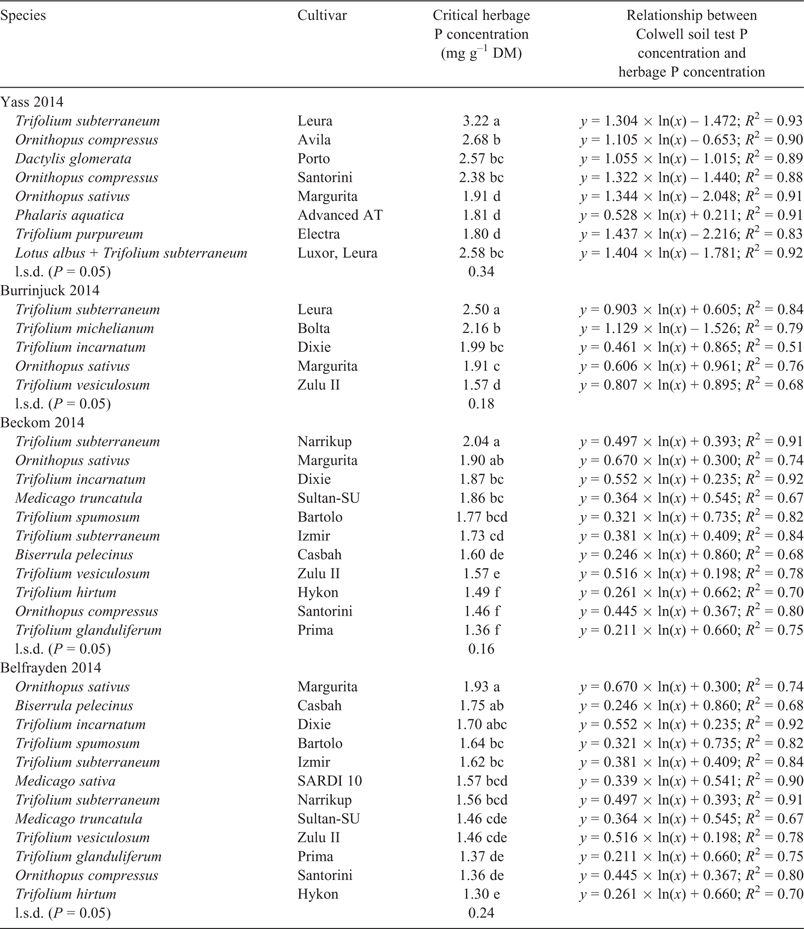
Herbage P concentrations were determined for all species grown in the final year of the experiments (2014). Natural log functions described the positive relationships between topsoil Colwell P and herbage P concentrations (Table 4) and were used to estimate the herbage P concentration of plants when grown at their critical external P supply level. Within each site, critical herbage P concentrations ranged up to 1.5-fold among the legumes. However, the rankings of many genotypes were often not consistent among the sites. Plant biomass P concentrations are promoted as potentially indicative of plant P status. For example, biomass P concentrations have been most extensively studied in T. subterraneum and the consensus critical range for whole shoots near flowering is expected to be 2.8–3.2 mg P g–1 DM (Pinkerton et al. 1997). The P concentrations of whole shoots of T. subterraneum fell within or close to this range at Yass and Burrinjuck, but values were lower at Beckom and Belfrayden, prompting us to examine whether dry late spring weather conditions were associated with relatively low herbage P concentrations. Only T. subterraneum and the Ornithopus spp. were grown at all four sites in 2014. Their critical (internal) herbage P concentrations were negatively correlated with the prevailing October rainfall at each site (Fig. 5a). However, critical (external) soil test P concentrations were not affected consistently by dry late-season conditions (Fig. 5b). Interpretation of critical herbage P concentrations is subject to many qualifications, because biomass P concentrations vary with cultural conditions, plant age, prevailing environment and genotype (Smith and Loneragan 1997). The present results serve to reinforce the caution that must be used when relying on biomass P testing as an indicator of soil fertility.
Mycorrhizal colonisation
In 2014, colonisation of roots by AMF was measured on topsoil roots (depth 0–10 cm) of O. sativus, O. compressus and T. subterraneum grown in the unfertilised treatment at all four field sites. The roots of all three species were heavily colonised by AMF (Table 5). Colonisation ranged from 44% to 57% of root length at the Yass, Beckom and Belfrayden sites, and from 27% to 46% at the Burrinjuck site.
Root hair length
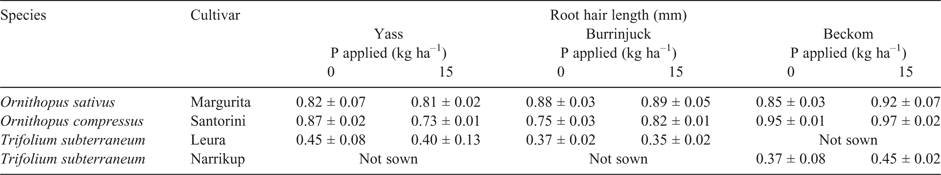
In 2016, root hair length was measured on topsoil roots (depth 0–10 cm) of O. sativus, O. compressus and T. subterraneum from the unfertilised (i.e. 0 kg P ha–1) and 15 kg P ha–1 treatments at the Yass, Burrinjuck and Beckom sites. Root hairs on the Ornithopus spp. were typically 0.73–0.97 mm long, whereas those on T. subterraneum were typically 0.37–0.45 mm long (Table 6). There was no significant effect (P > 0.05) of P treatment on root hair length. The density of root hairs per unit root length was higher on the Ornithopus spp. than on T. subterraneum (data not shown).
Discussion
Critical external P requirement and yield potential of pasture legumes
Critical soil test P concentrations in the top 10 cm of soil profiles that indicate 90% (Moody 2007) or 95% (Gourley et al. 2007, 2019) of maximum yield have been developed for pasture management in the temperate Mediterranean climatic areas of southern Australia based on analysis of empirical data from numerous field experiments. The experiments were conducted during spring, when growth rates of clover-based pastures are usually at or near their maximum, so that the soil test P benchmark represents the requirement for high-yielding pasture. The definition of critical soil test P requirements at this time of year also fits well with the common farm practice of soil testing in spring to determine fertiliser application rates for late summer–autumn, before the opening seasonal rainfall. Widespread application of the P benchmarks assumes that pasture legumes are present and fixing nitrogen, that temperatures and soil moisture conditions during spring are conducive for rapid growth rates (e.g. Cullen et al. 2008), that other nutrients and soil physical and/or chemical conditions are non-limiting for pasture growth or will be corrected concurrently (e.g. Trotter et al. 2014) and that plant-available P is predominantly concentrated in the uppermost layer of the soil profile. The latter assumption is reasonable for many southern Australian soils with moderate to high P sorption capacity. For example, Simpson et al. (2015) found, after 14 years of optimal and supra-optimal P fertiliser applications at a site near Canberra in the Australian Capital Territory (phosphorus-buffering index (PBI) = 50), that 70% of change in soil total P had occurred in the top 10 cm of the soil, with the remaining change confined to the 10- to 20-cm layer. Similarly, after 100 years of P fertiliser use near Rutherglen in Victoria (PBI = 120), all the increase in soil total P concentration was confined to the top 20 cm of the soil, with 80–84% of the change occurring in the topmost 10 cm of the soil profile (Schefe et al. 2015). However, in light-textured soils with very low P-buffering capacity (e.g. PBI < 15), where soil P can leach from the uppermost soil layer (e.g. Lewis et al. 1981; Ritchie and Weaver 1993), it is likely that critical topsoil P benchmarks will be less reliable indicators of pasture yield potential.
Different soil test protocols extract different amounts of P. Consequently, critical P concentrations vary with the soil test being used, and may vary among soil types. Two bicarbonate extracts of topsoil (depth 0–10 cm) are the most widely used extractable soil P tests in southern Australia (Olsen et al. 1954; Colwell 1963). The critical Olsen P value for pasture production is claimed to be independent of soil type (Gourley et al. 2007, 2019), whereas the critical Colwell P value varies with a soil’s P-buffering capacity (Helyar and Spencer 1977). Consequently, different critical Colwell P concentrations are recommended for soils based on results of the PBI test (Burkitt et al. 2002, 2008) of the soil (Gourley et al. 2007, 2019; Moody 2007), and the two tests are typically offered concurrently by soil testing services.
In the present study we determined the critical external P requirement and yield potential of a range of pasture legumes that are used, or are of potential use, in southern Australia at four sites (PBI range 40–80; Table 1) where we can expect that surface applications of P fertiliser will not be leached from the uppermost soil layers (e.g. Schefe et al. 2015; Simpson et al. 2015).
At all sites and in every season, a range in critical soil test P requirements was observed among the pasture varieties. We surmise that the reference species T. subterraneum would have a critical P requirement that reflected the critical soil test P guidelines for soil fertility management of clover-based pastures (Gourley et al. 2007, 2019), because they were derived in many cases from experiments with pastures based on T. subterraneum and T. repens, and T. subterraneum and T. repens have similar critical P requirements (e.g. Helyar and Anderson 1970, 1971). The current recommended critical P concentration for 95% of maximum yield falls within the range 28–32 mg Colwell P kg–1 (i.e. for soils with PBI 40–80), or is 15 mg Olsen P kg–1 soil (all soil types). The average critical P concentrations from all ‘site–year’ measurements for T. subterraneum in the present study were close to these benchmark values (e.g. 33 mg Colwell P kg–1 and 13.2 mg Olsen P kg–1; Fig. 4; Table 2).
Differences in the maximum yield of cultivars within a species were observed on the few occasions that such a comparison was made (Figs 1–4). However, there were no significant differences in the critical P requirements among cultivars from a single species. In contrast, significant differences in critical P concentrations were found among some legume species. Nine of the legumes were compared with T. subterraneum on three or more ‘cultivar–site–year’ occasions (Fig. 4). Of this group, five species had a significantly lower critical P requirement than T. subterraneum in most of the comparisons: O. compressus in five of seven comparisons; O. sativus in three of five comparisons; T. purpureum in three of three comparisons; and T. incarnatum and T. vesiculosum in two of three comparisons (Fig. 4). In every instance, the exceptions occurred at Burrinjuck in 2013 or Belfrayden in 2014. On these occasions, the species putatively identified as having a lower critical P requirement always ranked below T. subterraneum, and the inability to demonstrate differences in these specific ‘site–year’ instances suggests there was higher variance due to site or seasonal conditions. The average critical P concentrations of the low critical-P legumes was 20–21 mg Colwell P kg–1 (7.5–8.2 mg Olsen P kg–1) for O. compressus, O. sativus, T. purpureum and T. vesiculosum, and 25 mg Colwell P kg–1 (9.8 mg Olsen P kg–1) for T. incarnatum. The results for Ornithopus spp. are consistent with those of other studies that have reported O. compressus and O. sativus to have critical applied P requirements that are less than that of T. subterraneum, and more comparable to those of grasses (Blair and Cordero 1978; de Ruiter 1981; Bolland and Paynter 1992, 1994; Moir et al. 2014).
L. corniculatus was only tested twice because it did not persist over the dry summer periods at the Yass site, and resowing became impossible due to the low availability of seed. However, it also appeared to have a low critical P requirement, similar to that of the Ornithopus spp. A low applied P requirement has been noted previously (e.g. Acuňa 2008), and further assessment, in a perennial growing season environment, may be justified.
We deduced that M. sativa is likely to have a critical P requirement that is substantially higher than that of T. subterraneum (Fig. 4). The highest P application rate used in the experiments was not high enough on four of six occasions to observe a plateau in the growth response of M. sativa to soil P fertility. On one of the two occasions that a yield plateau was achieved, the critical P requirement of M. sativa was significantly higher than that of T. subterraneum. Consequently, we suspect that the critical P requirement for 95% maximum yield of M. sativa will be greater than 45–50 mg Colwell P kg–1 (greater than 16–21 mg Olsen P kg–1). M. sativa is a globally important forage species prized for its high yields and high-quality forage (Annicchiarico et al. 2015), yet we are unaware of any study that has determined its critical soil test P requirement under field conditions. Limited data are available from glasshouse experiments, and they support the hypothesis that M. sativa has a substantially higher critical P requirement than T. subterraneum (Helyar and Anderson 1970, 1971). The high P requirement of M. sativa may also be exacerbated by the plant’s susceptibility to aluminium toxicity, even in moderately acid soils (pH 5.0–5.5; Munns 1965; Crocker et al. 1985).
Implications for management of soil P status
Superficially, there appeared to be a wide range in critical P requirements among the varieties tested in the field experiments (Table 2), as found in glasshouse and controlled environment experiments (Haling et al. 2016a; Sandral et al. 2018). However, in the glasshouse, a much wider range in critical P application rates was found than observed for critical soil test P concentrations in the field. The glasshouse experiments (Sandral et al. 2018) also identified five statistically significant (P < 0.05) critical P groupings among many of the species examined in the present field experiments, namely T. subterraneum = T. spumosum > T. hirtum = M. truncatula = T. purpureum = T. incarnatum > T. michelianum = T. vesiculosum = T. glanduliferum > B. pelecinus > O. compressus = O. sativus. However, the present field experiments only supported the notion that the critical P requirement of T. subterraneum was significantly greater than that of T. incarnatum, T. purpureum, T. vesiculosum, O. compressus and O. sativus in most instances. The experiments have also indicated that M. sativa has a substantially higher critical P requirement than T. subterraneum.
We expect that seasonal conditions in the field will not always permit the expression of differences in critical P requirements. For example, dry soil conditions such as experienced at Belfrayden in 2014, where rainfall received during September and October was only half the average for that time of year, are likely to restrict P acquisition (e.g. Pinkerton and Simpson 1986). This is because the diffusion path for P in a drying surface soil is attenuated and/or broken as soil moisture content declines. We found that dry late spring conditions were associated with lower herbage P concentrations and assume that this was a reflection of the increased need for P mobilisation from older leaves to support the growth of young leaves as P acquisition became constrained by a drying topsoil. We contend that the general compression of the range in critical external P requirement among these species in the field (i.e. compared with that in the well-watered and pasteurised soil conditions of a glasshouse experiment) may also be a consequence of the many challenges to root growth in a natural soil environment, such as hard soils (Passioura 2002), soil acidity (Munns 1965) and root diseases (Simpson et al. 2011). In addition, high levels of colonisation of roots by AMF in the field (Table 5) may have a similar effect. Schweiger et al. (1995) have shown, in glasshouse experiments, that T. subterraneum gains a greater P acquisition benefit when colonised by AMF than do the Ornithopus spp. These authors hypothesised that this was because T. subterraneum has short root hairs, whereas the root hairs of Ornithopus spp. are long. Indeed, the lengths of root hairs on these two species in the field (Table 6) were short (0.40 mm) and long (0.86 mm) respectively, and similar to their contrasting lengths of root hairs observed in glasshouse experiments (Haling et al. 2016b; Yang et al. 2017; Sandral et al. 2018).
The critical soil test P concentrations recommended for pasture management in southern Australia (Gourley et al. 2007) do not recognise that there may be differences in P requirements among legumes that underpin different pasture types. The data from the present field experiments confirm the suitability of current recommendations for T. subterraneum pastures, and indicate that the benchmark critical P concentrations for T. subterraneum are suitable for most of the pasture legumes that were examined. However, a lower soil P availability benchmark is feasible for pastures based on species such as O. compressus and O. sativus, and forage crops using T. incarnatum, T. purpureum or T. vesiculosum. A much higher soil test P benchmark is potentially needed for M. sativa pastures. However, further field experiments will be necessary to determine the appropriate critical soil test P concentration for this legume.
Reducing the requirement for P fertiliser in grass–legume pastures
O. compressus and O. sativus showed the greatest potential to improve P efficiency in pasture systems because of their low critical P requirements. Their use, as alternatives to T. subterraneum, would reduce the critical soil test P concentration for pasture production to a P level similar to that required by key companion grasses (e.g. P. aquatica, D. glomerata), but it may still be greater than that required for other P-efficient grasses (e.g. Lolium rigidum Gaudin, Vulpia spp., Rytidosperma richardsonii (Cashmore) Connor and Edgar, Holcus lanatus L.; Hill et al. 2005). Compared with T. subterraneum, the Ornithopus spp. have higher specific root lengths and longer root hairs that allow them to explore the soil for P more effectively (Haling et al. 2016b).
It has been estimated previously that if the soil test P benchmark for pasture production were reduced from 15 mg Olsen P kg–1 (i.e. that recommended for T. subterraneum) to 10 mg Olsen P kg–1 (i.e. suitable for Ornithopus spp.), the annual P fertiliser input for soil fertility maintenance may be reduced by approximately 30% (Simpson et al. 2014). In moderate to high P-sorbing soils, P accumulates after fertiliser application at a rate that is positively correlated with the soil test P concentration at which the soil is being maintained. Reducing the soil test P concentration of a soil is expected to drive efficiency in P use because the rate at which P accumulates in the soil would also be reduced.
The Ornithopus spp. are already used successfully on sandy, acid soils in parts of Western Australia, northern New South Wales and as a pasture ley in crop rotations in Western Australia and the Riverina region of New South Wales (Freebairn 1996; Nichols et al. 2007; Hackney et al. 2013). Further work is now required to investigate the potential to use Ornithopus spp. in pastures across a wider area of southern Australia. There is limited evidence to suggest that they may not persist well in some pasture environments (Hayes et al. 2015); consequently, there is a need to investigate the factors that may limit wider use (e.g. flowering time, hard-seededness, seed softening, waterlogging tolerance, sensitivity to aluminium or manganese in acid soils).
An experimental mixture of L. albus and T. subterraneum cv. Leura was sown at Yass in 2014 to determine whether T. subterraneum could benefit from companion planting with a species that releases high amounts of citrate from its roots and has been shown to mobilise ‘sparingly available’ P (Gardner et al. 1982; Johnson et al. 1996; Neumann et al. 2000). T. subterraneum relies on ‘nutrient foraging’ to acquire P by proliferating root length in nutrient patches when growing in low-P soil (Haling et al. 2016b) and has very low amounts of organic anions, such as citrate, in its rhizosphere compared with L. albus (Kidd et al. 2016). Combining species with complementary P acquisition strategies such as these may result in an improved yield outcome (Gardner and Boundy 1983; Hinsinger et al. 2011). Indeed, the combined species did achieve a significantly lower critical soil test P requirement than T. subterraneum cv. Leura alone. On this basis, the treatment seemed surprisingly successful, because the critical soil test P of the mixture was as low as that achieved by the Ornithopus spp. However, the maximum yield of the mixture was also 18% lower than that of the T. subterraneum monoculture. It is unclear whether the lower maximum yield was due to competitive interference within the mixture, or an artefact of the experimental protocol (e.g. Lupinus seed was sod seeded into established T. subterraneum pasture plots, inevitably causing a certain amount of disturbance). Although the mixed sward did appear to have some yield advantage in moderately P-deficient soil, the main factor reducing the estimate of critical P concentration was the lower maximum yield. Consequently, the value or otherwise of mixing these species with complementary root traits for P acquisition was not resolved and warrants further examination.
Conclusions
The series of field experiments reported in this paper demonstrates differences in the critical soil test P requirements among pasture legume species that are either used currently or are of potential use for southern Australia. The results indicate that for practical P fertiliser management of grass–legume pastures on soils with PBI 40–80, pastures based on T. subterraneum, T. spumosum, T. hirtum, M. truncatula, T. michelianum, T. glanduliferum and B. pelecinus should be fertilised to the current recommended soil P fertility benchmarks (e.g. Gourley et al. 2007, 2019). However, the forage species T. incarnatum, T. purpureum and T. vesiculosum and two pasture species O. compressus and O. sativus had lower critical soil test P requirements than T. subterraneum in most instances, suggesting they may be fertilised to a lower soil test P concentration without compromising yield. The data also indicate that M. sativa has a substantially higher P requirement than T. subterraneum, but further work is required to define the soil test P concentration to which this species should be fertilised for maximum yield. The Ornithopus spp., which already have some use as permanent pasture varieties (Loi et al. 2005; Nichols et al. 2012), may have the potential to underpin development of productive pasture systems that require less P fertiliser than is presently needed for pastures based on T. subterraneum in southern Australia.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors thank Edward Storey and family of ‘Werong’ near Yass, Peter and Faye Southwell, Alison Southwell and Dan Trigg of ‘Fairview’ near Burrinjuck, Gnadbro Pastoral Co. of ‘Lyndoch’ near Belfrayden and Peter Carmody of ‘Wybimbie’ near Beckom for providing land for these experiments. The authors also thank Brent Henderson and Eric Zurcher (CSIRO) for their comments on the mathematical and statistical treatment of the data. This work was predominantly funded by Meat and Livestock Australia (MLA) and Australian Wool Innovation Limited (AWI) as part of ‘Phosphorus-Efficient Legume Pasture Systems’ (B.PUE.0104), with data analysis continuing under ‘RnD4P-15-02-016 Phosphorus Efficient Pastures’, a project supported by funding from the Australian Government Department of Agriculture and Water Resources as part of its Rural R&D for Profit program and from MLA, Dairy Australia and AWI. Megan H. Ryan was funded by an Australian Research Council Future Fellowship (FT140100103).
References
Acuňa H (2008) Responses to phosphorus, potassium and sulphur application on the productivity of Lotus spp. in two soil groups of central Chile. Lotus Newsletter 8, 1–6.Annicchiarico P, Barrett BE, Brummer EC, Julier B, Marshall AH (2015) Achievements and challenges in improving temperate perennial forage legumes. Critical Reviews in Plant Sciences 34, 327–380.
| Achievements and challenges in improving temperate perennial forage legumes.Crossref | GoogleScholarGoogle Scholar |
Blair GJ, Cordero S (1978) Phosphorus efficiency of three annual legumes. Plant and Soil 50, 387–398.
| Phosphorus efficiency of three annual legumes.Crossref | GoogleScholarGoogle Scholar |
Bolland MDA, Paynter BH (1992) Comparative responses of annual pasture legume species to superphosphate applications in medium and high rainfall areas of Western Australia. Fertilizer Research 31, 21–33.
| Comparative responses of annual pasture legume species to superphosphate applications in medium and high rainfall areas of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Bolland MDA, Paynter BH (1994) Critical phosphorus concentrations for burr medic, yellow serradella, subterranean clover, and wheat. Communications in Soil Science and Plant Analysis 25, 385–394.
| Critical phosphorus concentrations for burr medic, yellow serradella, subterranean clover, and wheat.Crossref | GoogleScholarGoogle Scholar |
Burkitt LL, Moody PW, Gourley CJP, Hannah MC (2002) A simple phosphorus buffering index for Australian soils. Australian Journal of Soil Research 40, 1–18.
Burkitt LL, Sale PWG, Gourley CJP (2008) Soil phosphorus buffering measures should not be adjusted for current phosphorus fertility. Australian Journal of Soil Research 46, 676–685.
| Soil phosphorus buffering measures should not be adjusted for current phosphorus fertility.Crossref | GoogleScholarGoogle Scholar |
Butler D, Cullis BR, Gilmour AR, Gogel BJ (2009) ASReml-R reference manual. Available at: https://asreml.kb.vsni.co.uk/wp-content/uploads/sites/3/2018/02/ASReml-R-2-Reference-Manual.pdf (accessed 22 August 2018).
Colwell JD (1963) The estimation of the phosphorus fertiliser requirements of wheat in southern New South Wales by soil analysis. Australian Journal of Experimental Agriculture 3, 190–197.
| The estimation of the phosphorus fertiliser requirements of wheat in southern New South Wales by soil analysis.Crossref | GoogleScholarGoogle Scholar |
Coombes N (2009) DiGGer design search tool in R. Available at: http://www.austatgen.org/software/ (accessed 22 August 2018).
Crocker GJ, Sheridan AKP, Holford ICR (1985) Lucerne responses to lime and interactions with other nutrients on granitic soils. Australian Journal of Experimental Agriculture 25, 337–346.
| Lucerne responses to lime and interactions with other nutrients on granitic soils.Crossref | GoogleScholarGoogle Scholar |
Cullen BR, Eckard RJ, Callow MN, Johnson IR, Chapman DF, Rawnsley RP, Garcia SC, White T, Snow VO (2008) Simulating pasture growth rates in Australian and New Zealand grazing systems. Australian Journal of Agricultural Research 59, 761–768.
| Simulating pasture growth rates in Australian and New Zealand grazing systems.Crossref | GoogleScholarGoogle Scholar |
Cullis BR, Gleeson AC (1991) Spatial analysis of field experiments – an extension to two dimensions. Biometrics 47, 1449–1460.
| Spatial analysis of field experiments – an extension to two dimensions.Crossref | GoogleScholarGoogle Scholar |
de Ruiter JM (1981) The phosphate response of eight Mediterranean annual and perennial legumes. New Zealand Journal of Agricultural Research 24, 33–36.
| The phosphate response of eight Mediterranean annual and perennial legumes.Crossref | GoogleScholarGoogle Scholar |
Dyson CB, Conyers MK (2013) Methodology for online biometric analysis of soil test–crop response datasets. Crop & Pasture Science 64, 435–441.
| Methodology for online biometric analysis of soil test–crop response datasets.Crossref | GoogleScholarGoogle Scholar |
Freebairn RD (1996) The history of serradella (Ornithopus spp.) in NSW – a miracle pasture for light soils. Agdex 137, NSW Agriculture and Fisheries, Sydney.
Gardner WK, Boundy KA (1983) The acquisition of phosphorus by Lupinus albus L. IV. The effect of interplanting wheat and white lupin on the growth and mineral composition of the two species. Plant and Soil 70, 391–402.
| The acquisition of phosphorus by Lupinus albus L. IV. The effect of interplanting wheat and white lupin on the growth and mineral composition of the two species.Crossref | GoogleScholarGoogle Scholar |
Gardner WK, Parbery DG, Barber DA (1982) The acquisition of phosphorus by Lupinus albus L. II. The effect of varying phosphorus supply and soil type on some characteristics of the soil/root interface. Plant and Soil 68, 33–41.
| The acquisition of phosphorus by Lupinus albus L. II. The effect of varying phosphorus supply and soil type on some characteristics of the soil/root interface.Crossref | GoogleScholarGoogle Scholar |
Gilmour AR, Cullis BR, Verbyla AP (1997) Accounting for natural and extraneous variation in the analysis of field experiments. Journal of Agricultural Biological & Environmental Statistics 2, 269–293.
| Accounting for natural and extraneous variation in the analysis of field experiments.Crossref | GoogleScholarGoogle Scholar |
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytologist 84, 489–500.
| An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots.Crossref | GoogleScholarGoogle Scholar |
Gourley CJP, Melland AR, Waller RA, Awty IM, Smith AP, Peverill KI, Hannah MC (2007) Making better fertiliser decisions for grazed pastures in Australia. (Victorian Government Department of Primary Industries: Melbourne) Available at: http://www.asris.csiro.au/downloads/BFD/Making%20Better%20Fertiliser%20Decisions%20for%20Grazed%20Pastures%20in%20Australia.pdf (accessed 22 August 2018).
Gourley CJP, Weaver DM, Simpson RJ, Aarons SR, Hannah MM, Peverill KI (2019) The development and application of functions describing pasture yield responses to phosphorus, potassium and sulfur in Australia using meta-data analysis and derived soil-test calibration relationships. Crop & Pasture Science 70, in press
Hackney B, Rodham C, Piltz J (2013) ‘Using French serradella to increase crop and livestock production.’ (Meat and Livestock Australia: Sydney)
Haling RE, Yang ZJ, Shadwell N, Culvenor RA, Stefanski A, Ryan MH, Sandral GA, Kidd DR, Lambers H, Simpson RJ (2016a) Growth and root dry matter allocation by pasture legumes and a grass with contrasting external critical phosphorus requirements. Plant and Soil 407, 67–79.
| Growth and root dry matter allocation by pasture legumes and a grass with contrasting external critical phosphorus requirements.Crossref | GoogleScholarGoogle Scholar |
Haling RE, Yang ZJ, Shadwell N, Culvenor RA, Stefanski A, Ryan MH, Sandral GA, Kidd DR, Lambers H, Simpson RJ (2016b) Root morphological traits that determine phosphorus-acquisition efficiency and critical external phosphorus requirement in pasture species. Functional Plant Biology 43, 815–826.
| Root morphological traits that determine phosphorus-acquisition efficiency and critical external phosphorus requirement in pasture species.Crossref | GoogleScholarGoogle Scholar |
Hayes RC, Sandral GA, Simpson R, Price A, Stefanksi A, Newell NT (2015) A preliminary evaluation of alternative annual legume species under grazing on the Southern Tablelands of NSW. In ‘Building productive, diverse and sustainable landscapes. Proceedings of the 17th Australian Society of Agronomy Conference’. Hobart, Australia. (Eds T Acuña, C Moeller, D Parsons, M Harrison) p. 4. http://www.agronomyaustraliaproceedings.org/images/sampledata/2015_Conference/pdf/agronomy2015final00332.pdf (accessed 22 August 2018)
Helyar KR, Anderson AJ (1970) Responses of five pasture species to phosphorus, lime and nitrogen on an infertile acid soil with high phosphate sorption capacity. Australian Journal of Agricultural Research 21, 677–692.
| Responses of five pasture species to phosphorus, lime and nitrogen on an infertile acid soil with high phosphate sorption capacity.Crossref | GoogleScholarGoogle Scholar |
Helyar KR, Anderson AJ (1971) Effects of lime on the growth of five species, on aluminium toxicity, and on phosphorus availability. Australian Journal of Agricultural Research 22, 707–721.
| Effects of lime on the growth of five species, on aluminium toxicity, and on phosphorus availability.Crossref | GoogleScholarGoogle Scholar |
Helyar KR, Spencer K (1977) Sodium bicarbonate soil test values and the phosphate buffering capacity of soils. Australian Journal of Soil Research 15, 263–273.
| Sodium bicarbonate soil test values and the phosphate buffering capacity of soils.Crossref | GoogleScholarGoogle Scholar |
Hill JO, Simpson RJ, Wood JT, Moore AD, Chapman DF (2005) The phosphorus and nitrogen requirements of temperate pasture species and their influence on grassland botanical composition. Australian Journal of Agricultural Research 56, 1027–1039.
| The phosphorus and nitrogen requirements of temperate pasture species and their influence on grassland botanical composition.Crossref | GoogleScholarGoogle Scholar |
Hill JO, Simpson RJ, Ryan MH, Chapman DF (2010) Root hair morphology and mycorrhizal colonisation of pasture species in response to phosphorus and nitrogen nutrition. Crop & Pasture Science 61, 122–131.
| Root hair morphology and mycorrhizal colonisation of pasture species in response to phosphorus and nitrogen nutrition.Crossref | GoogleScholarGoogle Scholar |
Hinsinger P, Betencourt E, Bernard L, Brauman A, Plassard C, Shen J, Tang X, Zhang F (2011) P for two, sharing a scarce resource: soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiology 156, 1078–1086.
| P for two, sharing a scarce resource: soil phosphorus acquisition in the rhizosphere of intercropped species.Crossref | GoogleScholarGoogle Scholar | 21508183PubMed |
Irving GCJ, McLaughlin MJ (1990) A rapid and simple field-test for phosphorus in Olsen and Bray No 1 extracts of soil. Communications in Soil Science and Plant Analysis 21, 2245–2255.
| A rapid and simple field-test for phosphorus in Olsen and Bray No 1 extracts of soil.Crossref | GoogleScholarGoogle Scholar |
Jackman RH, Mouat MCH (1972) Competition between grass and clover for phosphate. I. Effect of browntop (Agrostis tenuis Sibth.) on white clover (Trifolium repens L.). Growth and nitrogen fixation. New Zealand Journal of Agricultural Research 15, 653–666.
| Competition between grass and clover for phosphate. I. Effect of browntop (Agrostis tenuis Sibth.) on white clover (Trifolium repens L.). Growth and nitrogen fixation.Crossref | GoogleScholarGoogle Scholar |
Johnson JF, Allan DL, Vance CP, Weiblen G (1996) Root carbon dioxide fixation by phosphorus-deficient Lupinus albus (contribution to organic acid exudation by proteoid roots). Plant Physiology 112, 19–30.
| Root carbon dioxide fixation by phosphorus-deficient Lupinus albus (contribution to organic acid exudation by proteoid roots).Crossref | GoogleScholarGoogle Scholar | 12226371PubMed |
Kidd DR, Ryan MH, Haling RE, Lambers H, Sandral GA, Yang Z, Culvenor RA, Cawthray GR, Stefanski A, Simpson RJ (2016) Rhizosphere carboxylates and morphological root traits in pasture legumes and grasses. Plant and Soil 402, 77–89.
| Rhizosphere carboxylates and morphological root traits in pasture legumes and grasses.Crossref | GoogleScholarGoogle Scholar |
Lean GR, Vizard AL, Webb Ware JK (1997) Changes in productivity and profitability of wool-growing farms that follow recommendations from agricultural and veterinary studies. Australian Veterinary Journal 75, 726–731.
| Changes in productivity and profitability of wool-growing farms that follow recommendations from agricultural and veterinary studies.Crossref | GoogleScholarGoogle Scholar | 9406631PubMed |
Lewis DC, Clarke AL, Hall WB (1981) Factors affecting the retention of phosphorus applied as superphosphate to the sandy soils in south-eastern South Australia. Australian Journal of Soil Research 19, 167–174.
| Factors affecting the retention of phosphorus applied as superphosphate to the sandy soils in south-eastern South Australia.Crossref | GoogleScholarGoogle Scholar |
Loi A, Howieson JG, Nutt BJ, Carr SJ (2005) A second generation of annual pasture legumes and their potential for inclusion in Mediterranean-type farming systems. Australian Journal of Experimental Agriculture 45, 289–299.
| A second generation of annual pasture legumes and their potential for inclusion in Mediterranean-type farming systems.Crossref | GoogleScholarGoogle Scholar |
McDowell RW (2012) Minimising phosphorus losses from the soil matrix. Current Opinion in Biotechnology 23, 860–865.
| Minimising phosphorus losses from the soil matrix.Crossref | GoogleScholarGoogle Scholar | 22464284PubMed |
McLaughlin M, McBeath T, Smernik R, Stacey S, Ajiboye B, Guppy C (2011) The chemical nature of P accumulation in agricultural soils – implications for fertiliser management and design: an Australian perspective. Plant and Soil 349, 69–87.
| The chemical nature of P accumulation in agricultural soils – implications for fertiliser management and design: an Australian perspective.Crossref | GoogleScholarGoogle Scholar |
Melland AR, McCaskill MR, White RE, Chapman DF (2008) Loss of phosphorus and nitrogen in runoff and subsurface drainage from high and low input pastures grazed by sheep in Australia. Australian Journal of Soil Research 46, 161–172.
| Loss of phosphorus and nitrogen in runoff and subsurface drainage from high and low input pastures grazed by sheep in Australia.Crossref | GoogleScholarGoogle Scholar |
Mills A, Moot DJ, McKenzie BA (2006) Cocksfoot pasture production in relation to environmental variables. Proceedings of the New Zealand Grassland Association 68, 89–94.
Moir JL, Schwass MJ, Moot DJ (2014) Lime, phosphorus and sulphur response of French serradella (Ornithopus sativus) grown in an acid upland soil. Journal of International Scientific Publications: Agriculture and Food 2, 125–139.
Moody PW (2007) Interpretation of a single-point P buffering index for adjusting critical levels of the Colwell soil P test. Australian Journal of Soil Research 45, 55–62.
| Interpretation of a single-point P buffering index for adjusting critical levels of the Colwell soil P test.Crossref | GoogleScholarGoogle Scholar |
Munns DN (1965) Soil acidity and growth of a legume. I. Interactions of lime with nitrogen and phosphate on growth of Medicago sativa L. and Trifolium subterraneum L. Australian Journal of Agricultural Research 16, 733–741.
| Soil acidity and growth of a legume. I. Interactions of lime with nitrogen and phosphate on growth of Medicago sativa L. and Trifolium subterraneum L.Crossref | GoogleScholarGoogle Scholar |
Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Romheld V, Martinoia E (2000) Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Annals of Botany 85, 909–919.
| Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.).Crossref | GoogleScholarGoogle Scholar |
Nichols PGH, Loi A, Nutt BJ, Evans PM, Craig AD, Pengelly BC, Dear BS, Lloyd DL, Revell CK, Nair RM, Ewing MA, Howieson JG, Auricht GA, Howie JH, Sandral GA, Carr SJ, de Koning CT, Hackney BF, Crocker GJ, Snowball R, Hughes EJ, Hall EJ, Foster KJ, Skinner PW, Barbetti MJ, You MP (2007) New annual and short-lived perennial pasture legumes for Australian agriculture – 15 years of revolution. Field Crops Research 104, 10–23.
| New annual and short-lived perennial pasture legumes for Australian agriculture – 15 years of revolution.Crossref | GoogleScholarGoogle Scholar |
Nichols PGH, Revell CK, Humphries AW, Howie JH, Hall EJ, Sandral GA, Ghamkhar K, Harris CA (2012) Temperate pasture legumes in Australia – their history, current use, and future prospects. Crop & Pasture Science 63, 691–725.
| Temperate pasture legumes in Australia – their history, current use, and future prospects.Crossref | GoogleScholarGoogle Scholar |
Nichols PGH, Foster KJ, Piano E, Pecetti L, Kaur P, Ghamkhar K, Collins WJ (2013) Genetic improvement of subterranean clover (Trifolium subterraneum L.). 1. Germplasm, traits and future prospects. Crop & Pasture Science 64, 312–346.
| Genetic improvement of subterranean clover (Trifolium subterraneum L.). 1. Germplasm, traits and future prospects.Crossref | GoogleScholarGoogle Scholar |
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture Circular No. 939. Available at: https://archive.org/details/estimationofavai939olse (accessed 22 August 2018).
Ozanne PG, Keay J, Biddiscombe EF (1969) Comparative applied phosphate requirements of eight annual pasture species. Australian Journal of Agricultural Research 20, 809–818.
| Comparative applied phosphate requirements of eight annual pasture species.Crossref | GoogleScholarGoogle Scholar |
Ozanne PG, Howes KMW, Petch A (1976) Comparative phosphate requirements of four annual pastures and two crops. Australian Journal of Agricultural Research 27, 479–488.
| Comparative phosphate requirements of four annual pastures and two crops.Crossref | GoogleScholarGoogle Scholar |
Passioura JB (2002) Soil conditions and plant growth. Plant, Cell & Environment 25, 311–318.
| Soil conditions and plant growth.Crossref | GoogleScholarGoogle Scholar |
Pinkerton A, Simpson JR (1986) Interactions of surface drying and subsurface nutrients affecting plant growth on acidic soil profiles from an old pasture. Australian Journal of Experimental Agriculture 26, 681–689.
| Interactions of surface drying and subsurface nutrients affecting plant growth on acidic soil profiles from an old pasture.Crossref | GoogleScholarGoogle Scholar |
Pinkerton A, Smith FW, Lewis DC (1997) Pasture species. In ‘Plant analysis: an interpretation manual’. (Eds DJ Reuter, JB Robinson) pp. 287–346. (CSIRO Publishing: Melbourne)
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available at: https://www.R-project.org/ (accessed 22 August 2018).
Rayment GE, Lyons D (2011) ‘Soil chemical methods – Australasia.’ (CSIRO Publishing: Melbourne)
Reuter DJ, Dyson CB, Elliott DE, Lewis DC, Rudd CL (1995) An appraisal of soil phosphorus testing data for crops and pastures in South Australia. Australian Journal of Experimental Agriculture 35, 979–995.
| An appraisal of soil phosphorus testing data for crops and pastures in South Australia.Crossref | GoogleScholarGoogle Scholar |
Ritchie GSP, Weaver DM (1993) Phosphorus retention and release from sandy soils of the Peel–Harvey catchment. Fertilizer Research 36, 115–122.
| Phosphorus retention and release from sandy soils of the Peel–Harvey catchment.Crossref | GoogleScholarGoogle Scholar |
Sandral GA, Haling RE, Ryan MH, Price A, Pitt WM, Hildebrand SM, Fuller CG, Kidd DR, Stefanksi A, Lambers H, Simpson RJ (2018) Intrinsic capacity for nutrient foraging predicts critical external phosphorus requirement of 12 pasture legumes. Crop & Pasture Science 69, 174–182.
| Intrinsic capacity for nutrient foraging predicts critical external phosphorus requirement of 12 pasture legumes.Crossref | GoogleScholarGoogle Scholar |
Schefe CR, Barlow KM, Robinson NA, Crawford DM, McLaren TI, Smernik RJ, Croatto G, Walsh RD, Kitching M (2015) 100 years of superphosphate addition to pasture in an acid soil – current nutrient status and future management. Soil Research 53, 662–676.
| 100 years of superphosphate addition to pasture in an acid soil – current nutrient status and future management.Crossref | GoogleScholarGoogle Scholar |
Schweiger PF, Robson AD, Barrow NJ (1995) Root hair length determines beneficial effect of a Glomus species on shoot growth of some pasture species. New Phytologist 131, 247–254.
| Root hair length determines beneficial effect of a Glomus species on shoot growth of some pasture species.Crossref | GoogleScholarGoogle Scholar |
Simpson RJ, Richardson AE, Riley IT, McKay AC, McKay SF, Ballard RA, Ophel-Keller K, Hartley D, O’Rourke TA, Barbetti MJ, Sivasithamparam K, Li H, Ryan MH (2011) Damage to roots of Trifolium subterraneum L. (subterranean clover), failure of seedlings to establish and the presence of root pathogens during autumn-winter. Grass and Forage Science 66, 585–605.
| Damage to roots of Trifolium subterraneum L. (subterranean clover), failure of seedlings to establish and the presence of root pathogens during autumn-winter.Crossref | GoogleScholarGoogle Scholar |
Simpson RJ, Richardson AE, Nichols SN, Crush JR (2014) Pasture plants and soil fertility management to improve the efficiency of phosphorus fertiliser use in temperate grassland systems. Crop & Pasture Science 65, 556–575.
| Pasture plants and soil fertility management to improve the efficiency of phosphorus fertiliser use in temperate grassland systems.Crossref | GoogleScholarGoogle Scholar |
Simpson RJ, Stefanski A, Marshall DJ, Moore AD, Richardson AE (2015) Management of soil phosphorus fertility determines the phosphorus budget of a temperate grazing system and is the key to improving phosphorus-balance efficiency. Agriculture, Ecosystems & Environment 212, 263–277.
| Management of soil phosphorus fertility determines the phosphorus budget of a temperate grazing system and is the key to improving phosphorus-balance efficiency.Crossref | GoogleScholarGoogle Scholar |
Smith FW, Loneragan JF (1997) Interpretation of plant analysis: concepts and principles. In ‘Plant analysis: an interpretation manual’. (Eds DJ Reuter, JB Robinson) pp. 1–33. (CSIRO Publishing: Melbourne)
Trotter M, Guppy C, Haling R, Trotter T, Edwards C, Lamb D (2014) Spatial variability in pH and key soil nutrients: is this an opportunity to increase fertiliser and lime-use efficiency in grazing systems? Crop & Pasture Science 65, 817–827.
| Spatial variability in pH and key soil nutrients: is this an opportunity to increase fertiliser and lime-use efficiency in grazing systems?Crossref | GoogleScholarGoogle Scholar |
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Microbiology 64, 5004–5007.
Williams CH, Andrew CS (1970) Mineral nutrition of pastures. In ‘Australian grasslands’. (Ed. R Milton Moore) pp. 321–338. (Australian National University Press: Canberra)
Yang Z, Culvenor RA, Haling RE, Stefanski A, Ryan MH, Sandral GA, Kidd DR, Lambers H, Simpson RJ (2017) Variation in root traits associated with nutrient foraging among temperate pasture legumes and grasses. Grass and Forage Science 72, 93–103.
| Variation in root traits associated with nutrient foraging among temperate pasture legumes and grasses.Crossref | GoogleScholarGoogle Scholar |