Dry matter and nutritive value responses of native, naturalised and sown pasture species to soil Olsen P
M. R. McCaskill A E , M. L. Mitchell
A E , M. L. Mitchell  B , R. Zollinger A , R. D. Armstrong
B , R. Zollinger A , R. D. Armstrong  C D and D. Partington A
C D and D. Partington A
A Agriculture Victoria Research, 905 Mount Napier Road, Hamilton, Vic. 3300, Australia.
B Agriculture Victoria Research, 124 Chiltern Valley Road, Rutherglen, Vic. 3685, Australia.
C Agriculture Victoria Research, 110 Natimuk Road, Horsham, Vic. 3400, Australia.
D La Trobe University, Centre for AgriBioscience, Bundoora, Vic. 3086, Australia.
E Corresponding author. Email: malcolm.mccaskill@agriculture.vic.gov.au
Crop and Pasture Science 70(12) 1097-1109 https://doi.org/10.1071/CP18544
Submitted: 30 November 2018 Accepted: 31 May 2019 Published: 8 October 2019
Journal Compilation © CSIRO Publishing 2019 Open Access CC BY-NC-ND
Abstract
The soil phosphorus (P) requirements of 18 species that included native grasses and naturalised legumes were compared with the predominant sown species (Trifolium subterraneum, Lolium perenne and Phalaris aquatica) in a series of glasshouse and field experiments based on the Long-term Phosphate Experiment at Hamilton, Victoria. The native grasses Austrostipa scabra and Rytidosperma caespitosum had the lowest external P requirements, as measured by the Olsen P at which 90% of maximal dry matter (DM) production was obtained, but were of low nutrient value as livestock feed. The naturalised legume Lotus corniculatus had the lowest external P requirement of the legumes, but had low DM production. The highest legume DM production under low-P conditions in the field and one glasshouse experiment was obtained for T. subterraneum. This was attributed to its large seed, which enables rapid initial growth and thus captures light and nutrient resources early in the growing season. However, it forms a relatively low proportion of the pasture sward in low-P soil under grazed mixed pasture conditions in the field. This was attributed to its relatively high nutritive value, which leads to it being preferentially grazed, leaving species that are either less palatable or less accessible to grazing livestock. This work suggests that, in low-P environments, there is a much stronger selection pressure favouring low relative palatability over P efficiency. In conclusion, to maintain desirable species in temperate low-input pastures, sufficient P needs to be applied to maintain fertility above a threshold at which the less-palatable species begin to invade.
Additional keywords: dietary P, naturalised legumes, organic matter digestibility, seed size.
Introduction
The pastures that support sheep industries of south-eastern Australia depend on applied phosphorus (P) to raise the carrying capacity and ensure a high-quality diet for the growth of livestock (Richardson et al. 2009; Simpson et al. 2015). Sudden increases in the price of rock phosphate in 2008 led to concerns about the longer-term availability of P fertiliser at prices the sheep industry could afford. Pasture-based sheep production is relatively inefficient in its use of P because, on average, only 11% of applied P is removed in product annually (Weaver and Wong 2011), with most of the remainder accumulating in the topsoil as insoluble forms of organic and inorganic P (McCaskill and Cayley 2000; Schefe et al. 2015). A series of reviews recommended selecting pasture legumes for improved soil exploration by roots to improve their efficiency in P uptake, so similar levels of production can be maintained at lower soil P concentrations (Richardson et al. 2011; Simpson et al. 2011a). This would reduce the rate of both leaching and transfer from soluble to sparingly soluble forms of P, which should then enable an increase in the P efficiency of pasture-based sheep production.
Although selection for these traits is a useful long-term objective, more immediate progress may be possible by implementing fertility and grazing management that favours P-efficient species already present in pastures. There is evidence of considerable variation in plant response to increased fertility both between and within species (e.g. Gourley et al. 1993; Hill et al. 2010; Maxwell et al. 2013; Haling et al. 2016a, 2016b, 2018). The largest differences are in growth potential under non-limiting nutrition, but there are also differences in the critical P level at which 90% of maximum yield is achieved. Although pasture development in south-eastern Australia has been traditionally based on species of high growth potential, such as subterranean clover (Trifolium subterraneum L.), perennial ryegrass (Lolium perenne L.) and phalaris (Phalaris aquatica L.), a wide range of native and naturalised species is found on areas of low and moderate soil fertility. Among the higher-quality native perennial grasses are species of weeping grass (Microlaena stipoides (Labill.) R.Br.) and wallaby grasses (Rytidosperma spp. Steud.). Naturalised legumes include suckling clover (Trifolium dubium Sibth.), clustered clover (Trifolium glomeratum L.), hop clover (Trifolium campestre Schreb.) and birds-foot trefoil (Lotus corniculatus L.). In the early days of fertiliser application, these naturalised legumes were the species that responded to P before the widespread sowing of subterranean clover (Griffith Davies et al. 1934; Moore 1970). These species are often found in seed surveys (Fortune et al. 1995) and as volunteers in pastures (Kemp et al. 2003). Although their dry matter (DM) production may be lower than the sown species, they would contribute DM and fix nitrogen for use by the grass.
Fertility responses are most commonly measured by applying the limiting nutrient at various rates to a soil of low fertility. However, this does not test the capacity of plants to access sparingly soluble forms of P. In pasture systems, nearly all fertiliser is surface applied and P builds up in the topsoil (McCaskill and Cayley 2000), mostly in sparingly soluble inorganic forms, but also in organic forms (Schefe et al. 2015). A regime to compare the capacity of plants to access these forms of P needs to account for the chemical forms and physical distribution of such P. One of the few sites in south-eastern Australia where these forms and distributions have become equilibrated over a long period, at a wide range of P application rates and in close proximity is the Long-Term Phosphate Experiment (LTPE) at Hamilton, Victoria, where P has been applied at six rates averaging between 0 and 29 kg P ha–1 year–1 since 1979 (Cayley et al. 1999). This represented an ideal testing environment for a combination of field and glasshouse experimentation.
Although agronomic studies typically define critical values for the production of DM, increases in nutritive value are another important component in the response of grazing systems to fertiliser (Saul et al. 1999). This occurs primarily through an increase in the proportion of species with a higher nutritive value, but also because higher soil fertility is associated with a higher nutritive value within a species. On the LTPE, the more nutritious sown species persist down to a P application rate of 8 kg P ha–1 year–1, below which naturalised species have invaded (Cayley et al. 1999). Selective grazing of the more nutritious species by sheep would be expected to favour the species of lower nutritive value, particularly at the lower fertility margins of persistence (e.g. Cook et al. 1978). Grazing management can also affect the persistence of species, such as the differences between set-stocked and rotational grazing (Sanford et al. 2003). A sown species can only be expected to persist at lower levels of fertility if it is not preferentially grazed by livestock. In southern Australia this leads to preferential grazing of the more palatable legume over the associated grass, whereas in northern Australia the relatively unpalatable sown legumes dominate at low soil fertility and sown grasses dominate at higher fertility (Coates et al. 1990).
Despite being the dominant pasture legume in southern Australia, subterranean clover has a high external requirement for P relative to other species that are normally within the pasture, such as phalaris, native grasses and weed species (Hill et al. 2005). This is related to its root architecture, consisting of relatively thick roots (Hill et al. 2006) and a short root hair radius (Yang et al. 2017). Across a wide range of pasture legume species, the foraging capacity for P has been related to the volume of soil enclosed within the root hair radius (Haling et al. 2016a, 2016b, 2018; Sandral et al. 2018). The most promising pasture legume for a low external P requirement was yellow serradella (Ornithopus compressus L.). However, these studies were conducted in controlled environments using pasteurised, reconstituted soils to minimise root diseases (Simpson et al. 2011b), with measurements conducted on young plants. Although many of the legume species tested had much lower external P requirements than subterranean clover, there are other limitations in their agronomy that have so far prevented them from becoming as widespread as subterranean clover. To complement these controlled environment studies, we compared the external P requirements of 18 naturalised and native species that are already widespread in the pastures of southern Australia using both field and glasshouse experiments. Our first hypothesis was that the native and naturalised species that are often found in low-fertility situations have lower external P requirements than subterranean clover. Our second hypothesis was that within each species, low soil fertility is associated with lower forage nutrient value.
Materials and methods
A series of four experiments was conducted: one where test species were sown into weed matting in the field, two of which used undisturbed soil cores grown in the glasshouse and one where botanical composition was assessed across a plot boundary on the LTPE.
Site
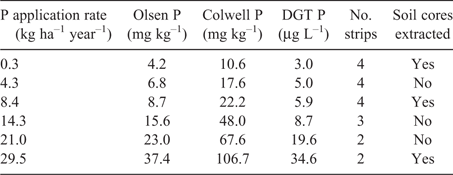
The source of soil for the glasshouse experiments, and the site of the field experiment, was the Hamilton LTPE (37°50′29″S 142°05′22″E, elevation 200 m). The site was established in 1977 when an area that had previously received no fertiliser was sown to perennial ryegrass, phalaris and subterranean clover, and treatments were applied with a design of six P application rates by three stocking pressures without replication (Cayley et al. 1999). From 1979 until spring 2012, the P application rates had averaged between 0.3 and 29 kg P ha–1 year–1 and Olsen P (Olsen et al. 1954) had ranged from 4 to 37 mg kg–1 (Table 1). Comparable values for the Colwell P test (Colwell 1963) ranged from 10 to 107 mg kg–1 and those for the diffuse gradient thin-films (DGT) test (Mason et al. 2010) ranged from 3 to 35 µg L–1. The soil is a Brown Chromosol (Isbell 1996) derived from basalt, consisting of a fine sandy clay loam topsoil 0.2 m deep overlying a heavy clay subsoil (Imhof and Rampant 2001). The topsoil (0–0.1 m) had a pH of 4.6 in calcium chloride, organic carbon of 4.6% (Rayment and Lyons 2011; Methods 4B3 and 6A1 respectively) and a phosphate buffering index (Burkitt et al. 2002) of 212. Previous studies had shown that there was no effect of P application on soil organic carbon and only small differences in pH, but that at the highest P rate total nitrogen was 20% higher than at the nil P rate (0.38% vs 0.45% N; Robertson and Nash 2008; Barlow et al. 2018).
|
Soil cores
Areas of the LTPE that had received either 0.3, 8 or 28 kg P ha–1 year–1 over the previous 36 years were mown in September 2012 and treated with glyphosate to control existing vegetation. In October 2012, polyvinyl chloride (PVC) rings 0.24 m in diameter and 0.25 m in length were pushed into the ground with an excavator and cores were extracted with minimal disturbance to the internal soil. The cores had a soil depth of 0.20 m and an average weight with soil at field capacity of 17 kg. The cores were placed on saucers to hold the soil in place.
Plants
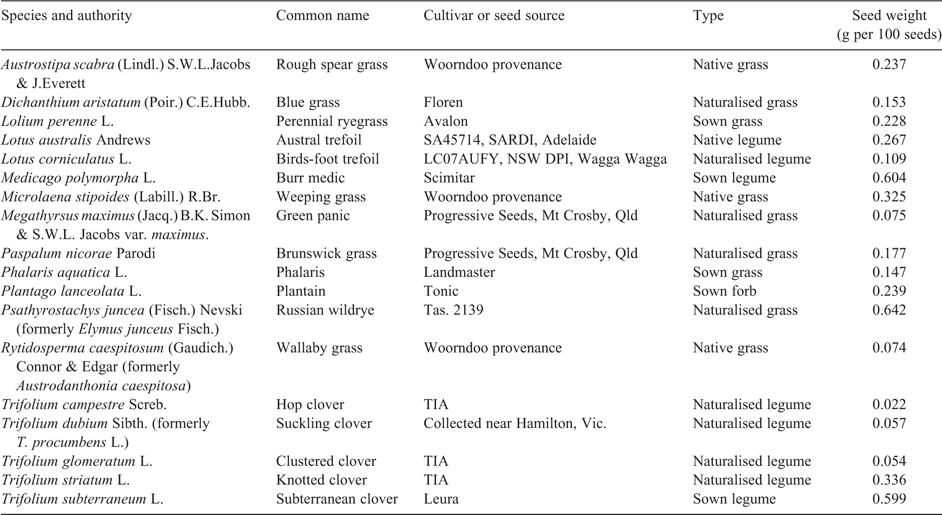
Eighteen plant species were selected for assessment, most of which are commonly found in pastures grazed by sheep (Table 2). Additional selection criteria included the availability of seed and its viability. In cases where a species is both naturalised and sown, the seed of a cultivar was chosen because of seed availability. The naturalised clovers had small seeds, with the smallest (T. campestre) less than 4% the size of subterranean clover. Because of this large difference in seed size, plants were grown until a sward formed before taking measurements. A subset of 10 species was sown or transplanted into a field experiment in May 2013, which formed Experiment 1. Species that normally grow in summer were sown into soil cores in January 2013 and formed Experiment 2, whereas species that normally grow in winter and spring, such as the annual legumes, were sown in May 2013 and formed Experiment 3. Legumes were inoculated with rhizobia supplied as an aqueous suspension; a mixture of Groups B and C was used for the Trifolium species, and SU343 was used for L. corniculatus. Sample legume plants dug up later in the experiment confirmed that all species had nodulated successfully.
Experiment 1: field microplots
A field experiment was established with 10 species planted on weed matting on the LTPE representing a range of fertility levels, but with an emphasis on the lower end of the fertility range (Table 1). In the months before establishment, 19 strips (10 m × 4 m) were selected for evenness and a botanical composition typical of the grazing plot. These areas were mown in October 2012 and treated with glyphosate to minimise regrowth. At most fertility levels there was one strip for each grazing plot, but at lower fertility levels some grazing plots had two strips. Superphosphate was applied at rates between 0 and 14 kg P ha–1 in March 2013, and potash supplying 50 kg K ha–1. White weed matting was selected rather than the normal black colour to minimise its effects on soil temperature, and each experimental area was fenced to exclude grazing. After installation, microplots were defined by cutting 64 holes, 30 mm in diameter, in a grid pattern within a 0.8-m × 0.8-m area into the weed matting. There was a gap of at least 0.5 m between microplots. Prior to planting, 10 soil cores (0–0.1 m) were taken from each microplot and analysed for Olsen P. This was 2 months after P application. Plugs of the native grasses that had been raised in a low-P potting mix at a commercial native plant nursery were transplanted into these holes in the weed matting in May 2013. All other species were sown into the holes as seed at a seeding rate that averaged 1.5–2 kg ha–1 across each microplot. Slug damage was noted in seedlings in late June, which was controlled with a bait containing metaldehyde. After transplanting, the native grasses treatments received 30 kg N ha–1 as aqueous sulfate of ammonia, and a further 30 kg N ha–1 was applied to all microplots using the same method after the first harvest. Plant material was harvested by cutting to a height of 30 mm on 16 September, 25 October and 2 December 2013, the last of which was at least 2 weeks before pasture in the grazed plots senesced. At the September harvest, biomass harvested from the species established by plugs averaged fivefold more than those established from seed, and these data are not presented because of a large effect of establishment method on responses to P. By the October harvest, the average yield from plugs was only 12% greater than from seed. Data are presented from the combined October and December harvests because this was the most responsive period, and differences between swards established from plugs and seeds were minimal. DM from the outer row within each microplot was excluded from measurements of DM to minimise edge effects. Where slug damage had resulted in a low density of the target species, the affected portions of the microplot were also excluded from measurements. At the October harvest, weed species (Hypochaeris radicata L., Anthoxanthum odoratum L., Cerastium glomeratum Thuill., Holcus lanatus L.) found within the adjacent grazed plot were harvested for analysis of mineral and nutritive value. Rainfall between May and November was 559 mm, which was 14% above the long-term average. Soil temperatures at 0.1 m averaged 14°C in the growth period until the October harvest, and 16°C from then until the final harvest.
Experiment 2: summer core
This was a randomised block experiment consisting of 10 species × 3 P levels (0, 8 and 29 kg P ha–1 year–1) × 4 replicates grown in the glasshouse. Plants were sown by seed in January 2013. Initial shoot growth was trimmed after 28 and 63 days to produce a more even sward across species, after which harvests were undertaken 86, 115 and 147 days after sowing by cutting to 40 mm above ground level. Soil samples (0–0.1 m) were taken from each core in March 2013, and comprised three separate cores 10 mm in diameter. Basal nutrients were supplied as aqueous KNO3 (50 kg N ha–1, 140 kg K ha–1), (NH4)2SO4 (25 kg N ha–1, 28 kg S ha–1) and the micronutrient component of Hoagland solution (Hoagland and Arnon 1950) supplying 1.9 kg B ha–1, 0.2 kg Cu ha–1, 2.0 kg Mn ha–1, 0.2 kg Mo ha–1, 0.2 kg Zn ha–1, 0.1 kg Co ha–1 and an additional 1.4 kg S ha–1 as boric acid, copper sulfate, manganese sulfate, molybdic acid, zinc sulfate and cobalt chloride respectively. Non-target species were removed by hand weeding at the seedling stage. Soil cores were watered daily with rainwater up to a point where water drained into the saucer at the bottom of the core. Free water in the saucer was normally absorbed by the soil after watering. Cores were regularly rotated within their blocks. The glasshouse had natural lighting, evaporative cooling and gas heating. The natural lighting regime replicated conditions under which summer-growing species germinate in response to summer rainfall and grow into a declining daylength. Over the growth period reported here, the glasshouse had a mean daytime air temperature of 24°C, a mean night-time minimum of 13°C and a mean soil temperature of 18.5°C.
Experiment 3: winter core
This comprised a randomised block experiment consisting of 12 species × 3 P levels (0, 8 and 29 kg P ha–1 year–1) × 4 replicates grown in the glasshouse. The cores had the same size and collection strategy as Experiment 2, and were stored in the glasshouse adjacent to Experiment 2 until planting. Most treatments were sown from seed in May 2013, but the native grasses were transplanted into the cores 26 days after the initial sowing. Initial growth was trimmed 61 days after sowing to produce a more even sward across species, after which harvests were undertaken 82, 103, 124, 145 and 174 days after sowing by cutting to 40 mm above ground level. Only data from harvests on Days 124, 145 and 174 are presented because this was the most P-responsive period. Soil samples (0–0.1 m) were taken from each core in July 2013, 4 months after the equivalent samples were taken from Experiment 2. Basal nutrients were applied as in Experiment 1, except a second application of sulfate of ammonia was applied 142 days after sowing. Over the harvests reported here, the mean daytime air temperature was 26°C and mean night-time minimum was 10°C. Soil temperatures were logged in one soil core, and averaged 16°C between the first and second harvests and 18°C between the fourth and fifth harvests, with an overall daily range from 7°C to 27°C. Although the temperature regime was similar to that of Experiment 2, it replicated the seasonality of annual plants in the field where germination occurs in April or May, after which the plants are exposed to increasing daylength from late June until the final harvest.
Experiment 4: field botanical composition
To quantify how soil P fertility affects which plants predominate in a competitive situation under grazing, a series of transects was assessed for botanical composition and Olsen P across a plot boundary on the LTPE. This was intended to complement botanical assessments of the LTPE at the grazing plot level that have already been published (e.g. Cayley et al. 1999). Two series of transects were assessed across the fence line between a low- and high-fertility plot, which had received an average of 0.3 and 29.4 kg P ha–1 year–1 respectively since 1979. Transects were 9 m in length and ran parallel to the fence line at a spacing of either 0.5 or 1.0 m. The plots were stocked at 11 and 19 wethers ha–1 respectively. Each transect was assessed for botanical composition using the dry weight rank method (‘t Mannetje and Haydock 1963) and herbage mass visually on 10 × 0.1-m2 quadrats, which was calibrated to cut quadrats. Soil samples (0–0.1 m) were collected along each transect, and visible dung collected from 10 × 0.1-m2 quadrats. Assessments were undertaken on 8–9 October 2015, which coincided with the early flowering stage of T. subterraneum, sweet vernal (Anthoxanthum odoratum) and onion grass (Romulea rosea (L.) Eckl.).
Laboratory analysis
Plant samples from all experiments were dried at 60°C and ground for analysis of P concentration by acid digestion followed by inductively coupled plasma analysis (McQuaker et al. 1979; AOAC 1990). Samples from the October harvest of Experiment 1 were analysed by near-infrared reflectance for crude protein, digestible organic matter, acid detergent fibre and neutral detergent fibre using calibrations previously derived for mixed pasture as described by Smith and Flinn (1991). Soil samples were dried at 40°C. Soil samples from individual cores and microplots were analysed for available P by the method of Olsen et al. (1954), whereas composite samples of each fertility level in Experiments 1 were also analysed for Colwell P and DGT P (Colwell 1963; Mason et al. 2010).
Statistical analysis
Critical Olsen P levels were calculated using the approach of Dyson and Conyers (2013):
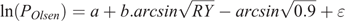
where POlsen (mg kg–1) is the soil Olsen P value of the microplot, a and b are coefficients fitted for each species by linear regression, RY is the relative yield, calculated as (microplot yield)/(maximal yield), and ϵ is experimental error. In a second stage of the process, points that exceed twice the initial critical value are omitted. Maximal yield was calculated for each species as the mean yield at highest fertility in each experiment (29 kg P ha–1 year–1). There is unlikely to be further response to additional P above this level because in subsequent field experiments with wheat (Triticum aestivum L.) and canola (Brassica napus L.) at the same site there were no significant responses to additional P at this fertility level (McCaskill et al. 2019), and these are among the most P-responsive species (Moody and Bolland 1999). Because (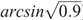 ) was subtracted from each value before the regression analysis, the critical Olsen P for 90% of maximal herbage yield could be calculated as (Critical P) = exp(a), and its 70% confidence range as exp(a ± SE), where SE is the standard error of a. Differences were considered significant (P < 0.05) where confidence ranges exp(a ± 2.SE) did not overlap. Regressions were conducted using linear regression with species as groups in Genstat 18 (VSN International, Hemel Hempstead, UK), with weightings for the area harvested to account for slug damage in some microplots. The same approach was adopted for the soil core experiments, as well as for P uptake as a measure of the P-foraging capacity of the species. Dyson and Conyers (2013) noted that their method calculated more conservative (i.e. lower) estimates of critical soil test values than a Mitscherlich function.
) was subtracted from each value before the regression analysis, the critical Olsen P for 90% of maximal herbage yield could be calculated as (Critical P) = exp(a), and its 70% confidence range as exp(a ± SE), where SE is the standard error of a. Differences were considered significant (P < 0.05) where confidence ranges exp(a ± 2.SE) did not overlap. Regressions were conducted using linear regression with species as groups in Genstat 18 (VSN International, Hemel Hempstead, UK), with weightings for the area harvested to account for slug damage in some microplots. The same approach was adopted for the soil core experiments, as well as for P uptake as a measure of the P-foraging capacity of the species. Dyson and Conyers (2013) noted that their method calculated more conservative (i.e. lower) estimates of critical soil test values than a Mitscherlich function.
To calculate internal P concentrations at the critical soil test value, tissue P concentration was regressed against ln(POlsen) to linearise the relationship, so linear regression with groups could be used. The same method was used to test the relationship between Olsen P and DM yield, P concentration and nutritive value. These relationships were used to calculate DM yield and plant P concentration at an Olsen P of 8 mg kg–1, as representative of the lowest fertility level at which the sown species persist.
Multiple linear regression was used to test the significance of associations between pasture components (e.g. percentage T. subterraneum within the sward) in Experiment 4 and soil fertility or standing biomass. These calculations were conducted using the means of each of the 26 transects.
Results
Nearly all species tested showed significant responses to Olsen P, but there were also large differences in maximal yield among species (Fig. 1; Table 3). Critical Olsen P was lowest for the native grasses Austrostipa scabra (Lindl.) S.W.L.Jacobs & J.Everett and Rytidosperma caespitosum, whereas critical values for M. stipoides and most of the naturalised and sown species were significantly higher. Contrary to expectations, in Experiment 3 T. subterraneum had one of the lowest critical Olsen P values, whereas the native legume and the naturalised legumes had among the highest. However, this low critical value under glasshouse conditions did not translate to the field (Experiment 1), where T. subterraneum recorded among the highest critical values. The critical Olsen P for P uptake produced similar species rankings to those for DM, but there was greater statistical precision in separating species. The summer-growing species in Experiment 2 had much lower critical values than the winter-growing species, and there were few significant differences between species. Across all the response experiments, species with a low critical Olsen soil P value also had low internal P concentrations at this Olsen level; the exceptions were L. corniculatus and Paspalum nicorae Parodi, which had among the highest internal P concentrations. In all three experiments there was a high correlation (r = 0.79–0.99) between the yield under low-P conditions (predicted for an Olsen P of 8 mg kg–1) and yield under high-P conditions (based on data in Table 3). In the field (Experiment 1), the species producing the highest cumulative shoot DM at an Olsen P of 8 mg kg–1 was R. ceaspitosum, followed by L. perenne and T. subterraneum. In the winter cores experiment (Experiment 3), the highest-yielding species was T. subterraneum, which produced 45% higher more shoot DM than the next highest-ranking species, L. perenne.
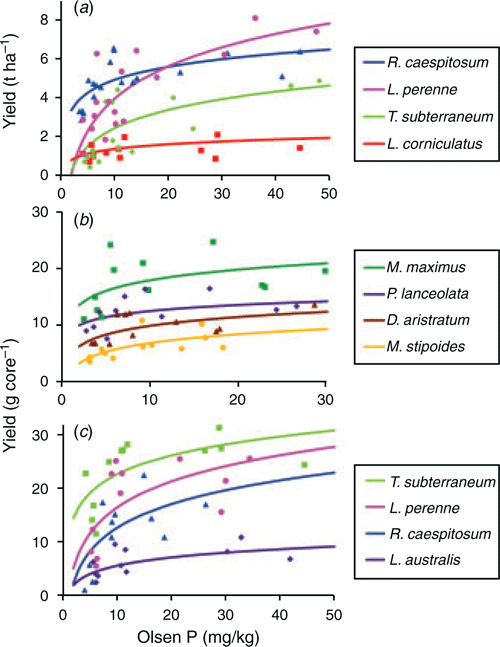
|
All species responded to higher soil fertility with higher internal P concentrations (Table 4). At both low and high levels of soil P fertility, the lowest internal shoot P concentrations were recorded in the native and improved grasses, followed by T. subterraneum, whereas the other legumes and volunteer species had significantly higher shoot P concentrations.
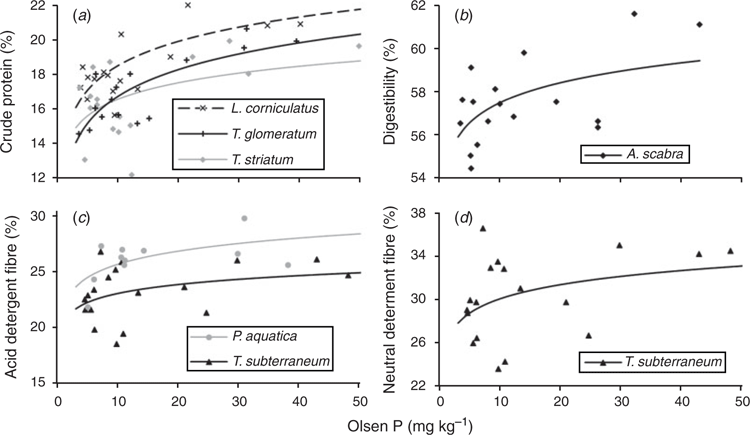
In most species there was little effect of increasing soil fertility on nutritive value (Table 5). Crude protein was increased in three species (L. corniculatus, T. glomeratum and T. striatum L.), and in vitro organic matter digestibility was increased in one species (A. scabra). Acid detergent fibre was increased in two species (P. aquatica and T. subterraneum), and neutral detergent fibre was increased in one species (T. subterraneum; Fig. 2). Organic matter digestibility was lowest for the native grasses A. scabra and R. caespitosum, but there were few significant differences among the sown grasses and legumes (Tables 4, 5).
A summary of DM production and in vitro DM digestibility for each species predicted for an Olsen P of 8 mg kg–1 shows no overall relationship (Fig. 3). Dividing the range into notional high versus low DM production and high versus low digestibility, most of the species tested are in the quadrant for low growth and high digestibility. Within this quadrant, T. subterraneum had the highest DM production, whereas L. corniculatus had the lowest DM production but the highest digestibility.
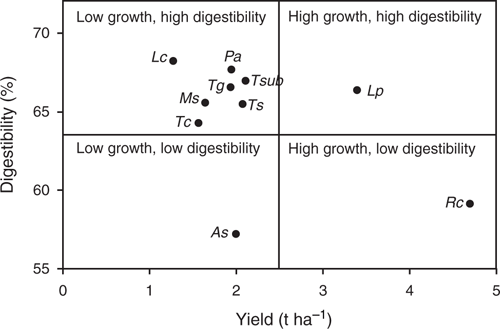
|
The field transect study of Experiment 4 showed that on the low-fertility plot T. subterraneum comprised 10–38% of the sward, despite Olsen P being in the range 3.4–5.2 mg kg–1 (Fig. 4). This occurred within 2 m of the fence, where biomass was lower than further into the plot and the mass of dung marginally higher, both of which are indicative of high grazing pressure as sheep in the low fertility plot seek the company of sheep in the adjacent high-fertility plot. Dung in the low-P plot had a much lower P concentration than in the high-P plot (0.40% vs 1.25% P), suggesting that dung transfer is unlikely to be a major mechanism to move P into the strip within 2 m of the fence of the low-P plot. The percentage of T. subterraneum within a transect was negatively related to biomass (P < 0.05) but not significantly related to Olsen P (P = 0.32). The total percentage of a suite of species normally associated with low fertility (A. odoratum, R. rosea, Vulpia spp., H. radicata, H. lanatus) was negatively related to both Olsen P and biomass (both P < 0.01). In contrast, the percentage of capeweed (Arctotheca calendular (L.) Levyns) was positively related to Olsen P, but negatively related to biomass (both P < 0.01). There were no significant relationships between the percentage of sown grasses (P. aquatica and L. perenne) and either Olsen P or biomass.
Discussion
Of the legumes tested here, only L. corniculatus had a significantly lower critical external P requirement than subterranean clover. The naturalised clovers T. campestre, T. glomeratum, and T. dubium had critical external P requirements similar to subterranean clover but a lower growth potential. This concurs with Maxwell et al. (2013), who found that under both low- and high-P conditions the yield of T. subterraneum exceeded that of T. glomeratum, T. dubium and T. striatum. The native grasses A. scabra and R. caespitosum had the lowest critical values in the field experiment, but were of low nutrient value as livestock feed. The other native grass, M. stipoides, was of high nutrient value, but its critical soil test value was similar to the sown species. Root architecture studies have related the low external P requirement of L. corniculatus with a root hair length that is nearly twice that of T. subterraneum, and a root hair cylinder volume threefold greater (Yang et al. 2017). However, off-setting its high P foraging capacity, the present study found L. corniculatus had three agronomic weaknesses. First, it is a relatively poor producer of DM, both under high- and low-P conditions. Second, it had among the highest internal P concentrations at its critical soil test value, indicating that it has relatively poor internal use of P. Ideally, a candidate legume for pastures of low P status would combine a low external requirement with a low internal requirement (provided animal dietary P requirements are met). Third, its combination of high digestibility and low fibre put it at risk of being grazed out through selective grazing in a mixed pasture.
Contrary to expectations, subterranean clover had among the highest DM production under low-P conditions. In the field at low fertility (Olsen P 8 mg kg–1), the DM production of T. subterraneum was only exceeded by two grasses, whereas in the glasshouse at an Olsen P of 4 mg kg–1 it produced more DM than any other species tested. This was under conditions where interspecific competition was eliminated by hand weeding. However, in a mixed pasture on the LTPE that was grazed, T. subterraneum typically contributed less than 7% of biomass at this level of soil fertility (Cayley et al. 1999; M. R. McCaskill, unpubl. data). However, the field transect study showed that T. subterraneum could comprise 10–38% of biomass at an Olsen P of 4 mg kg–1 provided the competition from taller species was eliminated by heavy grazing. A similar finding was made by Hill et al. (2005), namely that abundance of T. subterraneum was favoured by high grazing pressure, whereas taller species were favoured by low grazing pressure. Hill et al. (2005) also found that a low external P requirement was only one characteristic related to species abundance under infertile conditions and that other characteristics, such as deep rootedness or the ability to fix atmospheric nitrogen, were also associated with abundance under such conditions. Other features noted in the present study that favour T. subterraneum in infertile conditions include a large seed size, low internal P requirements and a low-growing form that enables a portion of its biomass to evade grazing. Nevertheless, beyond the heavily grazed strip of the transect study, the low-P plots were dominated by taller-growing species, most of which are of lower digestibility and likely to be selected against by grazing livestock. These species include onion grass (R. rosea), along with smaller amounts of sweet vernal (A. odoratum), wallaby grass (Rhytidosperma pilosa, R. laevis and R. caespitosum), dandelions (H. radicata) and silvergrass (Vulpia spp.; Saul et al. 1999). Sweet vernal and dandelions evade grazing by a prostrate growth habit that avoids prehension, with only a fibrous stem and inflorescence emerging above biting height. Onion grass, wallaby grass and silvergrass are low in digestibility and high in fibre (Table 5; Saul et al. 1999). Grazing preference studies have shown that these characteristics are associated with low relative grazing preference (Maxwell et al. 2015). Therefore, the grazing-avoidance strategies place greater grazing pressure on the small amount of T. subterraneum in the pasture that is above grazing height, and the plant must take up more P to regrow, which ultimately leads to a low seed set at the end of the growing season. During summer and autumn, sheep graze little of the unpalatable dominant species, leaving a residue of low-quality dried pasture in which there are few pockets of bare soil where clover can germinate at the autumn break. This feedback loop is driven by relative palatability rather than the relative external P requirement of each species.
There are several potential paths out of this cycle. First, a full pasture renovation involving herbicide treatment, followed by 1 or 2 years of cropping and resowing with T. subterraneum and a perennial grass, controls the unpalatable species. However, industry experience indicates that unless the maintenance P exceeds a threshold the pasture first loses its perennial grass and then the clover as it becomes invaded by unpalatable species. On the LTPE this threshold occurs at an Olsen P of 7 mg kg–1. Second, sufficient P can be applied to shift the balance towards desirable species without undertaking a full pasture renovation. This tends to encourage annual grasses and legumes and should only be contemplated when there is sufficient density of native or sown perennial grasses to ensure the pasture has stability (Garden et al. 2000). A third potential approach is to use legumes of lower relative palatability. In the high country of New Zealand, Maxwell et al. (2010) reported that, on commercial paddocks with low P inputs, there was greater vegetative cover from naturalised T. striatum, T. dubium and T. glomeratum than from the sown Trifolium repens and T. subterraneum, despite the latter having been resown every few years. An accompanying study of sheep preference ranked their relative palatability as T. repens > T. subterraneum > T. striatum > T. glomeratum > T. dubium (Maxwell et al. 2015). Of these species, the density of T. striatum was negatively associated with P fertility, whereas that of T. repens was positively associated with P fertility (Maxwell et al. 2010). It was concluded that the lower palatability of the naturalised clovers during seed set increased their persistence by allowing these species to set more seed. Selection of legume species or cultivars for reduced palatability could be a promising approach to reduce the threshold fertility at which the unpalatable non-legumes invade. A fourth potential approach is to alter the grazing management to reduce the ability of the livestock to be selective (e.g. Badgery et al. 2017; Cox et al. 2017; Gregorini et al. 2017). However, there are limits to this approach because it only affects preferential grazing by domesticated livestock; in the present study, livestock were excluded but there was grazing by slugs, which preferred legume seedlings over native grasses.
Among legumes we studied, T. subterraneum was the first to achieve canopy closure in both the field and winter core experiments. It had larger seeds than the naturalised clovers T. campestre, T. dubium and T. glomeratum, which enabled it to establish quickly to capture light and plant-available P ahead of small-seeded species at the start of the growing season. Similar findings were reported by Maxwell et al. (2013), who attributed the higher DM production of T. subterraneum over the naturalised clovers to its larger seeds. Although a small-seeded species has a dispersal advantage where seed spread is through wind or animals, and can set a large number of seeds from the same biomass, a large-seeded species has an advantage where the dispersal is through sowing equipment. Therefore, as a large-seeded species, T. subterraneum has advantages over smaller-seeded species at the start of the growing season, particularly under conditions of low fertility.
In our test regime, plant growth from the various species was compared across soils with a wide range of P fertility and soil fertility, described by a soil test taken from the microplot or core. This produced a wider scatter of data points than experiments where different amounts of P were added to a low-P soil (e.g. Hill et al. 2005; Maxwell et al. 2013; Haling et al. 2016a, 2016b, 2018). One reason for this is that in our approach soil fertility is described by a soil test value around which is an experimental error component, whereas the experimental error in adding P to a low-P soil is so low it can be ignored in statistical analysis. To accommodate the error component around the soil test value, critical soil test values were calculated using a statistical technique developed for soil test interpretation in field crops (Dyson and Conyers 2013). Rankings of critical external P requirements were consistent with Hill et al. (2005), where P. aquatica, M. stipoides and T. subterraneum were in a group with high external P requirements and Rytidosperma richardsonii (Cashmore) Connor & Edgar in a low group. A similar study by Sandral et al. (2019) also found that rankings in the field matched those of controlled conditions. There is no evidence to date that our field studies produced a different or more valid ranking. Its advantages are that plants are tested with a combination of soil chemistry, mycorrhizae and root diseases that plants face under field conditions, but it comes at the cost of increased variability.
Each species was represented by only a single cultivar or ecotype. However, considerable intraspecific variation has been found in response to P in species such as T. subterraneum (Haling et al. 2018). The cultivar representing T. subterraneum in our study (cv. Leura) was ranked third out of 30 cultivars for shoot dry mass under low-P conditions (Haling et al. 2018). Our study showed a much larger difference in shoot DM production between low and high P in T. dubium, T. glomeratum and T. striatum than from collections of these species from the New Zealand high country (Maxwell et al. 2013). In this case, within-species genetic differences in response are unlikely to be the main contributor, because our lowest Olsen P was much lower than that in the study of Maxwell et al. (2013; 4 vs 11 mg kg–1).
The forage samples collected in mid-spring barely meet the allowances for lactating or rapidly growing sheep recommended by Freer et al. (2007). A lactating ewe requires at least 0.30% P in the diet, whereas a weaner sheep weighing 30 kg and growing at 200 g day–1 requires at least 0.20% P. Higher weaner growth rates of 350 g day–1 have been reported (Thompson et al. 2010), which, by extrapolation, would require at least 0.25% P in the diet. None of the forages from the low-fertility pasture (Olsen P 8 mg kg–1 in Table 4) met the requirement for lactating ewes, whereas under high-fertility conditions (Olsen P 20 mg kg–1) only half the species supplied sufficient P in the forage. Most notably, even under high-fertility conditions the commonly sown pasture species were below the 0.30% P requirement of lactating ewes, including phalaris (0.21% P), perennial ryegrass (0.24% P) and subterranean clover (0.27% P). Because the forages are barely meeting the dietary P needs of lactating or rapidly growing sheep even under high-P conditions, there is little scope to improve P efficiency by selecting plants with high internal P efficiency, and the focus of selection instead needs to be on plants with roots that are more effective at acquiring P.
Although the present study showed large variation in protein, digestibility and fibre between species, there were few significant effects of soil fertility within a species. This contrasts with the findings of Saul et al. (1999), who collected samples from the same site and time of year and used almost identical methods of chemical analysis. Increases in legume crude protein with P fertility have been reported by others (e.g. Robson et al. 1981; Saul et al. 1999), but in the present study the only significant differences were found in L. corniculatus, T. glomeratum and T. striatum. The present study found a significant increase in digestibility with soil fertility in A. scabra, which was the species with the lowest overall digestibility. In P. aquatica and T. subterraneum there was a significant increase in fibre with fertility. The latter effect would normally be regarded as a decrease in nutritive value, but there were no associated effects on digestibility. This effect may have been caused by a greater amount of stem tissue required to support the high yields under high-P conditions.
Critical Olsen P values were inconsistent across experiments, as illustrated by M. stipoides being much lower in the summer core experiment than in the field experiment (Olsen P 9.5 vs 19.2 mg kg–1). The two values are not directly comparable because of differences in mineralisation after the initial soil samples were collected and because the core experiments only partially simulated sward conditions. The cores for both Experiments 2 and 3 were collected at the time of peak growth in spring, which would have depleted the pools of available P. Soil samples for Experiment 2 were collected in February after 3 months of mineralisation, followed by warm soil temperatures while the experimental plants were growing. In contrast, the field experiment had a further 3 months of mineralisation and a superphosphate application that would have transferred more P into pools detected by the Olsen P test, but was then followed by an extended period of low soil temperatures that would have limited mineralisation. Furthermore, plants in the core experiments could access light from a greater area than the core. In a glasshouse pot study where access to light was constrained to the core area by cylindrical reflective sleeves to more accurately simulate the mutual shading that occurs in swards, aboveground and below-ground growth were decreased relative to unconstrained pots, and P uptake was enhanced by increased production of rhizosphere carboxylates (Jeffery et al. 2017). Because of these limitations, critical values are not directly portable across experiments undertaken at different times of the year.
Conclusions
We compared the DM response of 18 pasture species to varying concentrations of Olsen soil P in three experiments using a soil where previous applications of applied P had sufficient time to equilibrate into the chemical forms (inorganic and organic) and stratification layers in the soil profile that would be typically expected under commercial paddock conditions. There was no overall trend for either the native grasses or naturalised legumes to have lower external P requirements than each other or the sown species. Instead, in each experiment there was a high correlation between DM production under low-soil P conditions with those under high-P conditions. In a field experiment in which the native grasses were planted from plugs raised in a nursery, the highest production at both low and high P was from the native grass R. caespitosum, whereas the highest-producing legume was T. subterraneum. Despite the high production of T. subterraneum under low-P conditions in both the field and glasshouse, this species forms a relatively low proportion of the pasture sward under the same P conditions in the field. This was attributed to its relatively high nutritive value, which leads to it being preferentially grazed, and favours species that are either less palatable or less accessible to grazing livestock. In low-P environments there is a much stronger selection pressure for low relative palatability than for P efficiency. It is concluded that to maintain desirable species in these temperate low-input pastures, sufficient P needs to be applied to maintain fertility above a threshold at which the less-palatable species begin to invade.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Australian Wool Innovation Ltd and Agriculture Victoria Research (and its predecessors) for funding this study through the project ‘Maximising Pasture Production Under Low P Inputs’ (Project WP601). The authors thank Bronwyn Clark and Caroline MacDonald for technical work on the project, and CSBP for undertaking the Olsen P, Colwell P soil tests and total P in plant analysis. The authors also thank the Agriculture Victoria Research laboratory in Horsham for the nutritive value analyses, and Sean Mason for undertaking the DGT soil tests. Graeme Sandral of the New South Wales Department of Primary Industries supplied the seed of L. corniculatus, Eric Hall of the University of Tasmania supplied the seed of T. striatum, T. campestre and T. glomeratum, David Franklin supplied seedlings of A. scabra, M. stipoides and R. caespitosum and Steve Hughes of the South Australian Research and Development Institute (SARDI), Adelaide supplied the seed of L. australis. The authors thank John Howieson of the Centre for Rhizobium Studies, Murdoch University for advice on rhizobial strains for the legumes, and Michael Russelle of the US Department of Agriculture for advice in planning the experiments.
References
AOAC (1990) Metals in plants: Inductively coupled plasma spectroscopic method 985.01. In ‘Official methods of analysis’. 15th edn. Vol. 1. (Ed. K Helrich) p. 42. (AOAC: Rockville, MD, USA)Badgery WB, Millar GD, Michalk DL, Cranney P, Broadfoot K (2017) The intensity of grazing management influences lamb production from native grassland. Animal Production Science 57, 1837–1848.
| The intensity of grazing management influences lamb production from native grassland.Crossref | GoogleScholarGoogle Scholar |
Barlow KM, McCaskill MR, Partington DL (2018) Trends in soil pH in a long-term phosphorus and stocking rate trial. In ‘National Soil Science Conference’. Canberra, ACT, 18–23 November 2018. (Eds N Hulugalle, T Biswas, R Greene, P Bacon) pp. 390–391. (Soil Science Society of Australia: Bridgewater, S. Aust.) https://www.soilscienceaustralia.org.au/national-conferences/ (accessed 11 September 2019)
Burkitt LL, Moody PW, Gourley CJP, Hannah MC (2002) A simple buffering index for Australian soils. Australian Journal of Soil Research 40, 497–513.
| A simple buffering index for Australian soils.Crossref | GoogleScholarGoogle Scholar |
Cayley JWD, Kearney GA, Saul GR, Lescun CL (1999) The long-term influence of superphosphate and stocking rate on the production of spring-lambing Merino sheep in the high rainfall zone of southern Australia. Australian Journal of Agricultural Research 50, 1179–1190.
| The long-term influence of superphosphate and stocking rate on the production of spring-lambing Merino sheep in the high rainfall zone of southern Australia.Crossref | GoogleScholarGoogle Scholar |
Coates DB, Kerridge PC, Miller CP, Winter WH (1990) Phosphorus and beef production in northern Australia. 7. The effect of phosphorus on the composition, yield and quality of legume-based pasture and their relation to animal production. Tropical Grasslands 24, 209–220.
Colwell JD (1963) The estimation of the phosphorus requirements of wheat in southern New South Wales by soil analysis. Australian Journal of Experimental Agriculture and Animal Husbandry 3, 190–197.
| The estimation of the phosphorus requirements of wheat in southern New South Wales by soil analysis.Crossref | GoogleScholarGoogle Scholar |
Cook SJ, Lazenby A, Blair GJ (1978) Pasture degradation. 1. Effect on total and seasonal pasture production. Australian Journal of Agricultural Research 29, 9–18.
| Pasture degradation. 1. Effect on total and seasonal pasture production.Crossref | GoogleScholarGoogle Scholar |
Cox F, Badgery W, Kemp D, Krebs G (2017) Seasonal diet selection by ewes grazing within contrasting grazing systems. Animal Production Science 57, 1824–1836.
| Seasonal diet selection by ewes grazing within contrasting grazing systems.Crossref | GoogleScholarGoogle Scholar |
Dyson CB, Conyers MK (2013) Methodology for online biometric analysis of soil test–crop response datasets. Crop & Pasture Science 64, 435–441.
| Methodology for online biometric analysis of soil test–crop response datasets.Crossref | GoogleScholarGoogle Scholar |
Fortune JA, Cocks PS, Macfarlane CK, Smith FP (1995) Distribution and abundance of annual legume seeds in the wheatbelt of Western Australia. Australian Journal of Experimental Agriculture 35, 189–197.
| Distribution and abundance of annual legume seeds in the wheatbelt of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Freer M, Dove H, Nolan JV (2007) ‘Nutrient requirements of domesticated ruminants.’ (CSIRO Publishing: Melbourne, Vic.)
Garden DL, Lodge GM, Friend DA, Dowling PM, Orchard BA (2000) Effects of grazing management on botanical composition of native grass-based pastures in temperate south-east Australia. Australian Journal of Experimental Agriculture 40, 225–245.
| Effects of grazing management on botanical composition of native grass-based pastures in temperate south-east Australia.Crossref | GoogleScholarGoogle Scholar |
Gourley CJP, Allan DL, Russelle MP (1993) Difference in response to available phosphorus among white clover cultivars. Agronomy Journal 85, 296–301.
| Difference in response to available phosphorus among white clover cultivars.Crossref | GoogleScholarGoogle Scholar |
Gregorini P, Villalba JJ, Chilibroste P, Provenza FD (2017) Grazing management: setting the table, designing the menu and influencing the diner. Animal Production Science 57, 1248–1268.
| Grazing management: setting the table, designing the menu and influencing the diner.Crossref | GoogleScholarGoogle Scholar |
Griffith Davies J, Scott AE, Fraser KM (1934) Natural pastures: their response to superphosphate. Bulletin 83, CSIRO, Melbourne, Vic., Australia.
Haling RE, Yang Z, Shadwell N, Culvenor RA, Stefanksi A, Ryan MH, Sandral GA, Kidd DR, Lambers H, Simpson RJ (2016a) Growth and root dry matter allocation by pasture legumes and a grass with contrasting external phosphorus requirements. Plant and Soil 407, 67–79.
| Growth and root dry matter allocation by pasture legumes and a grass with contrasting external phosphorus requirements.Crossref | GoogleScholarGoogle Scholar |
Haling RE, Yang Z, Shadwell N, Culvenor RA, Stefanski A, Ryan MH, Sandral GA, Kidd DR, Lambers H, Simpson RJ (2016b) Root morphological traits that determine phosphorus-acquisition efficiency and critical external phosphorus requirement in pasture species. Functional Plant Biology 43, 815–826.
| Root morphological traits that determine phosphorus-acquisition efficiency and critical external phosphorus requirement in pasture species.Crossref | GoogleScholarGoogle Scholar |
Haling RE, Brown LK, Stefanski A, Kidd DR, Ryan MH, Sandral GA, George TS, Lambers H, Simpson RJ (2018) Differences in nutrient foraging among Trifolium subterraneum cultivars deliver improved P-acquisition efficiency. Plant and Soil 424, 539–554.
| Differences in nutrient foraging among Trifolium subterraneum cultivars deliver improved P-acquisition efficiency.Crossref | GoogleScholarGoogle Scholar |
Hill JO, Simpson RJ, Wood JT, Moore AD, Chapman DF (2005) The phosphorus and nitrogen requirements of temperate pasture species and their influence on grassland botanical composition. Australian Journal of Agricultural Research 56, 1027–1039.
| The phosphorus and nitrogen requirements of temperate pasture species and their influence on grassland botanical composition.Crossref | GoogleScholarGoogle Scholar |
Hill JO, Simpson RJ, Moore AD, Chapman DF (2006) Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant and Soil 286, 7–19.
| Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition.Crossref | GoogleScholarGoogle Scholar |
Hill JO, Simpson RJ, Ryan MH, Chapman DF (2010) Root hair morphology and mycorrhyzal colonisation of pasture species in response to phosphorus and nitrogen nutrition. Crop & Pasture Science 61, 122–131.
| Root hair morphology and mycorrhyzal colonisation of pasture species in response to phosphorus and nitrogen nutrition.Crossref | GoogleScholarGoogle Scholar |
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular 347, College of Agriculture, University of California, Berkeley, CA, USA.
Imhof M, Rampant P (2001) PVI6 soil description. Available at: http://p00011/dpi/vro/glenregn.nsf/pages/glenelg_soil_rises_pvi6 (accessed 11 September 2019).
Isbell RF (1996) ‘The Australian soil classification.’ (CSIRO Publishing: Melbourne, Vic.)
Jeffery RP, Simpson RJ, Lambers H, Kidd DR, Ryan MH (2017) Plants in constrained canopy micro-swards compensate for decreased root biomass and soil exploration with increased amounts of rhizosphere carboxylates. Functional Plant Biology 44, 552–562.
| Plants in constrained canopy micro-swards compensate for decreased root biomass and soil exploration with increased amounts of rhizosphere carboxylates.Crossref | GoogleScholarGoogle Scholar |
Kemp DR, King WMcG, Gilmour AR, Lodge GM, Murphy SR, Quigley PE, Sanford P, Andrew MH (2003) SGS biodiversity theme: impact of plant biodiversity on the productivity and stability of grazing systems across southern Australia. Australian Journal of Experimental Agriculture 43, 961–975.
| SGS biodiversity theme: impact of plant biodiversity on the productivity and stability of grazing systems across southern Australia.Crossref | GoogleScholarGoogle Scholar |
Mason S, McNeill A, McLaughlan MJ, Zhang H (2010) Prediction of wheat response to an application of phosphorus under field conditions using diffuse gradients in thin-films. Plant and Soil 337, 243–258.
| Prediction of wheat response to an application of phosphorus under field conditions using diffuse gradients in thin-films.Crossref | GoogleScholarGoogle Scholar |
Maxwell TMR, Moir JL, Edwards GR (2010) Influence of environmental factors on the abundance of naturalised annual clovers in the South Island hill and high country. Proceedings New Zealand Grassland Association 72, 165–170.
Maxwell TMR, Moir JL, Edwards GR (2013) Phosphorus response and efficiency of four adventive annual clovers grown in a New Zealand high country soil under glasshouse conditions. New Zealand Journal of Agricultural Research 56, 203–214.
| Phosphorus response and efficiency of four adventive annual clovers grown in a New Zealand high country soil under glasshouse conditions.Crossref | GoogleScholarGoogle Scholar |
Maxwell TMR, Moir JL, Edwards GR (2015) Grazing preference of Merino sheep for naturalised annual clover species relative to commonly sown clover species. Grass and Forage Science
| Grazing preference of Merino sheep for naturalised annual clover species relative to commonly sown clover species.Crossref | GoogleScholarGoogle Scholar |
McCaskill MR, Cayley JWD (2000) Soil audit of a long-term phosphate experiment in south-western Victoria: total P, S, N and major cations. Australian Journal of Agricultural Research 51, 737–748.
| Soil audit of a long-term phosphate experiment in south-western Victoria: total P, S, N and major cations.Crossref | GoogleScholarGoogle Scholar |
McCaskill M, Riffkin P, Pearce A, Christy B, Norton R, Speirs A, Clough A, Midwood J, Partington D (2019) Critical Colwell P values for wheat and canola in the high rainfall zone. In ‘Proceedings 19th Australian Agronomy Conference’. Wagga Wagga, NSW, 25–29 August 2019. (Ed. J Pratley) Available at: http://agronomyaustraliaproceedings.org (accessed 11 Sep 2019)
McQuaker NR, Brown DF, Kluckner PD (1979) Digestion of environmental materials for analysis by ICP-AES. Analytical Chemistry 51, 1082.
Moody PW, Bolland MDA (1999) Phosphorus. In ‘Soil analysis – an interpretation manual’. (Eds KI Peverill, LA Sparrow, DJ Reuter) pp. 187–220. (CSIRO Publishing: Melbourne, Vic.)
Moore RM (1970) South-eastern temperate woodlands and grasslands. In ‘Australian grasslands’. (Ed. RM Moore) pp. 169–190. (Australian National University Press: Canberra, ACT)
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Circular No. 939, US Department of Agriculture, Washington, DC.
Rayment GE, Lyons DJ (2011) ‘Soil chemical methods – Australasia.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Richardson AE, Hocking PJ, Simpson RJ, George TS (2009) Plant mechanisms to optimise access to soil phosphorus. Crop & Pasture Science 60, 124–143.
| Plant mechanisms to optimise access to soil phosphorus.Crossref | GoogleScholarGoogle Scholar |
Richardson A, Lynch J, Ryan P, Delhaize E, Smith FA, Smith S, Harvey P, Ryan M, Veneklaas E, Lambers H, Oberson A, Culvenor R, Simpson R (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant and Soil 349, 121–156.
| Plant and microbial strategies to improve the phosphorus efficiency of agriculture.Crossref | GoogleScholarGoogle Scholar |
Robertson FA, Nash DM (2008) Phosphorus and nitrogen in soil, plants, and overland flow from sheep-grazed pastures fertilized with different rates of superphosphate. Agriculture, Ecosystems & Environment 126, 195–208.
| Phosphorus and nitrogen in soil, plants, and overland flow from sheep-grazed pastures fertilized with different rates of superphosphate.Crossref | GoogleScholarGoogle Scholar |
Robson AD, O’Hara GW, Abbott LK (1981) Involvement of phosphorus in nitrogen fixation by subterranean clover (Trifolium subterraneum L.). Australian Journal of Plant Physiology 8, 427–436.
Sandral GA, Haling RE, Ryan MH, Price A, Pitt WM, Hildebrand SM, Fuller CG, Kidd DR, Stefanksi A, Lambers H, Simpson RJ (2018) Intrinsic capacity for nutrient foraging predicts critical external phosphorus requirement of 12 pasture legumes. Crop & Pasture Science 69, 174–182.
| Intrinsic capacity for nutrient foraging predicts critical external phosphorus requirement of 12 pasture legumes.Crossref | GoogleScholarGoogle Scholar |
Sandral GA, Price A, Hildebrand SM, Fuller CG, Haling RE, Stefanski A, Yang Z, Culvenor RA, Ryan MH, Kidd DR, Diffey S, Lambers H, Simpson RJ (2019) Field benchmarking of the critical external phosphorus requirements of pasture legumes for southern Australia. Crop & Pasture Science 70, in press
Sanford P, Cullen BR, Dowling PM, Chapman DF, Garden DL, Lodge GM, Andrew MH, Quigley PE, Murphy SR, King WMcG, Johnston WH, Kemp DR (2003) SFS pasture theme: effect of climate, soil factors and management on pasture production and stability across the high rainfall zone of southern Australia. Australian Journal of Experimental Agriculture 43, 945–959.
| SFS pasture theme: effect of climate, soil factors and management on pasture production and stability across the high rainfall zone of southern Australia.Crossref | GoogleScholarGoogle Scholar |
Saul GR, Kearney GA, Flinn PC, Lescun CL (1999) Effects of superphosphate fertiliser and stocking rate on the nutritive value of perennial ryegrass and subterranean clover herbage. Australian Journal of Agricultural Research 50, 537–545.
| Effects of superphosphate fertiliser and stocking rate on the nutritive value of perennial ryegrass and subterranean clover herbage.Crossref | GoogleScholarGoogle Scholar |
Schefe CR, Barlow KM, Robinson NJ, Crawford DM, McLaren TI, Smernik RJ, Croatto G, Walsh RD, Kitching M (2015) 100 Years of superphosphate addition to pasture in an acid soil – current nutrient status and future management. Soil Research 53, 662–676.
| 100 Years of superphosphate addition to pasture in an acid soil – current nutrient status and future management.Crossref | GoogleScholarGoogle Scholar |
Simpson RJ, Oberson A, Culvenor RA, Ryan MH, Veneklaas EJ, Lambers H, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Richardson AE (2011a) Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant and Soil 349, 89–120.
| Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems.Crossref | GoogleScholarGoogle Scholar |
Simpson RJ, Richardson AE, Riley IT, McKay AC, McKay SF, Ballard RA, Ophel-Keller K, Hartley D, O’Rourke TA, Li H, Sivasithamparam K, Ryan MH, Barbetti MJ (2011b) Damage to roots of Trifolium subterraneum L. (subterranean clover), failure of seedlings to establish and the presence of root pathogens during autumn–winter. Grass and Forage Science 66, 585–605.
| Damage to roots of Trifolium subterraneum L. (subterranean clover), failure of seedlings to establish and the presence of root pathogens during autumn–winter.Crossref | GoogleScholarGoogle Scholar |
Simpson RJ, Stefanski A, Marshall DJ, Moore AD, Richardson AE (2015) Management of soil phosphorus fertility determines the phosphorus budget of a temperate grazing system and is the key to improving phosphorus efficiency. Agriculture, Ecosystems & Environment 212, 263–277.
| Management of soil phosphorus fertility determines the phosphorus budget of a temperate grazing system and is the key to improving phosphorus efficiency.Crossref | GoogleScholarGoogle Scholar |
Smith KF, Flinn PC (1991) Monitoring the performance of a broad-based calibration for measuring the nutritive value of two independent populations of pasture using near infrared reflectance spectroscopy. Australian Journal of Agricultural Research 31, 205–210.
‘t Mannetje L, Haydock KP (1963) The dry-weight-rank method for the botanical analysis of pasture. Journal of the British Grassland Society 18, 268–275.
| The dry-weight-rank method for the botanical analysis of pasture.Crossref | GoogleScholarGoogle Scholar |
Thompson AN, Kennedy AJ, Holmes J, Kearney G (2010) Arrowleaf clover improves lamb growth rates in late spring and early summer compared with subterranean clover pastures in south-west Victoria. Animal Production Science 50, 807–816.
| Arrowleaf clover improves lamb growth rates in late spring and early summer compared with subterranean clover pastures in south-west Victoria.Crossref | GoogleScholarGoogle Scholar |
Weaver DM, Wong MTF (2011) Scope to improve phosphorus (P) management and balance efficiency of crop and pasture soils with contrasting P status and buffering indices. Plant and Soil 349, 37–54.
| Scope to improve phosphorus (P) management and balance efficiency of crop and pasture soils with contrasting P status and buffering indices.Crossref | GoogleScholarGoogle Scholar |
Yang Z, Culvenor RA, Haling RE, Stefanski A, Ryan MH, Sandral GA, Kidd DR, Lambers H, Simpson RJ (2017) Variation in root traits associated with nutrient foraging among temperate pasture legumes and grasses. Grass and Forage Science 72, 93–103.
| Variation in root traits associated with nutrient foraging among temperate pasture legumes and grasses.Crossref | GoogleScholarGoogle Scholar |