Growth characteristics, chlorophyll content and nutrients uptake in Tetragonia decumbens Mill. cultivated under different fertigation regimes in hydroponics
Milile Nkcukankcuka A , Muhali O. Jimoh A , Gerhardus Griesel A and Charles P. Laubscher A BA Department of Horticultural Sciences, Faculty of Applied Sciences, Cape Peninsula University of Technology, PO Box 1906, Bellville 7535, Symphony Way, City of Cape Town, South Africa.
B Corresponding author. Email: laubscherc@cput.ac.za
Crop and Pasture Science - https://doi.org/10.1071/CP20511
Submitted: 24 December 2020 Accepted: 17 February 2021 Published online: 22 April 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
This study was designed to investigate the growth characteristics, chlorophyll content and nutritional properties of Tetragonia decumbens, an indigenous plant species of the South African Western Cape. One hundred and eight cuttings generated from a mother plant obtained from a garden were cultivated in hydroponic systems over 7 weeks. The hydroponic experiment comprised three nutrient solutions of graded concentrations (0.2, 0.1, and 0.05% w/v) of Nutrifeed fertiliser prepared in the water whereas the control had no nutrients. Soilless treatments were made of 100% silica sand medium heaped in plastic pots near the brim. Each hydroponic system was run for 2 h/week at the pH and electrical conductivities (EC) of 4.5, 5.5, 6.5, and 3.38, 2.34, 1.10 dS/m respectively, whereas frequencies of irrigation were set at 2 L/h for 15 min at an intermittent break of 2 h between 0900 and 1700 hours. For the first time, this study reported macronutrients and micronutrients present in dune spinach. At P < 0.05, post-harvest results showed that fertigation did not affect the macronutrients, however, micronutrients were significantly affected. Therefore, a well-drained soilless media (100% silica) with a high nutrient application (0.2% w/v), low electrical conductivities, and moderate pH levels had better results in vegetative growth and nutritional composition compared with other treatments.
Keywords: Aizoaceae, diet diversity, dune spinach, food security, hydroponics, nutrient application, photosynthesis, soilless cultivation, Tetragonia decumbens.
Introduction
In literature, the population of known plant species in the world has been estimated to be ~374 000. This figure is constituted by 78 854 lower plants and 308 312 higher plant species (Christenhusz and Byng 2016). Out of this number, not less than 28 187 are used for medicinal purposes, 28 000 as ornamental plants, and 7000 as crops (Khoshbakht and Hammer 2008; Allkin 2017). A careful look into this number suggests that despite the rising demand for food caused by the increasing human population, less attention is given to wild plant species that are potentially edible. Although genetically modified plants can play an important role in significantly improving human survival, the growth of new plants through the introduction of wild edible plant species becomes imperative to provide a significant amount of energy needed for wellbeing (Chivenge et al. 2015).
Despite the opportunities inherent in the cultivation and consumption of wild plant species, ~1.02 billion people worldwide are undernourished (Gonzalez 2010). Although it has been widely reported that hundreds of millions of indigenous people, especially in developing worlds, regularly collect plant resources to fulfil their daily needs, wild edible plants can also serve as a supplement to non-indigenous people and can provide an alternative source of rich nutrients (Konsam et al. 2016). Thus, wild edible vegetables reduce the vulnerability of local communities to food insecurity and have great potential for evolving new plants through domestication by enlarging the pool of genetic resources for breeding and selection (Uprety et al. 2012).
Among the underutilised edible wild vegetables in the world is dune spinach, Tetragonia decumbens. This species is a spreading shrub that is characterised by sessile, glabrous, dark green, and succulent leaves (Forrester 2004). The species is native to the shores of Southern Africa and in Western Australia where it was first collected in 1932 near Cottesloe, a seaside suburb of Perth (Heyligers 1999). According to Tembo-Phiri (2019), the importance of T. decumbens as an edible plant species has been underestimated. It has never been cultivated but has been piloted for possible commercial cultivation at a community garden in Khayelitsha, Cape Town, South Africa, along with other potential winter rainfall crops. There is the need to develop a cultivation protocol for this edible plant and profile its macronutrient and micronutrient characteristics to ensure its sustainable use.
More importantly, the latest frontier in agricultural research is to maximise crop yield and quality and to keep production costs down. To accomplish sustainable agricultural systems, the environmental impact of each field industry must be considered. Optimised agricultural production practices involve optimising water supply and applying nutrients to maximise efficiency and minimise waste (Fernández and Hoeft 2009). Moreover, increasing demand for land for other purposes aside from agricultural needs has reduced the size of plots allocated to farming. This has seen many existing farmlands hitherto converted to schools, factories, sports centres, roads, and housing (Reed and Kleynhans 2009; Francis et al. 2012; Pham et al. 2015).
Therefore, it is imperative to embrace soilless cultivation where the land requirement is minimal, enabling maximum control over pH, electrical conductivity, and other environmental conditions. Hydroponic cultivation allows easy manipulation of nutrient supply to maximise yield. It tolerates recirculation of used water and prevents infestation of pests and diseases (Faber et al. 2020). The type of hydroponic system chosen for use is important and is based on the condition of the plant and its valuable properties desired. However, hydroponic systems need to be improved to encourage the growth of valuable plant traits (Lefever et al. 2017). Cultivating T. decumbens in hydroponics is therefore in tune with contemporary realities of the need to complement existing agricultural practices in terms of productivity and in developing an optimal growth protocol for the species since there is a dearth of information on its propagation, spacing, harvest times, and other cultivation methodologies for optimal yield (Araya et al. 2020). This study is the first research providing information about mineral analysis of T. decumbens and its hydroponic cultivation under different fertigation regimes. Since there is a lack of information on the nutritional characteristics of the plant in existing literature, it is therefore expected that data from this study will serve as a template for future researchers, households, farmers, and industrialists who may want to exploit the plant for diet diversity, food supplementation, and as pharmaceutical precursors.
Materials and methods
Greenhouse experiment
This investigation was conducted over 7 weeks in a greenhouse facility of the Cape Peninsula University of Technology, Bellville Campus (GPS coordinates –33°55ʹ45.53S, 18°38ʹ31.16E). The environment within the greenhouse is controlled by the installed structure and technology that keeps the relative humidity of the facility between 40 and 87%, and a temperature range of 23–27°C.
Plant preparation
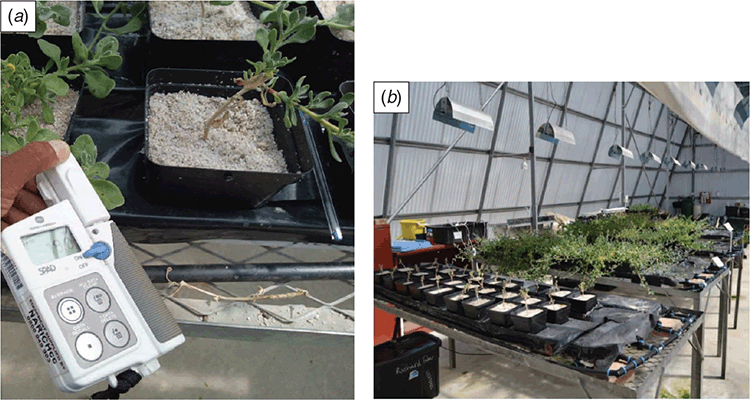
One hundred uniform cuttings of T. decumbens were made with a length of ~3 cm using the stock material and placed in cutting square trays containing washed and sterilised coarse river sand. The cuttings were dipped into a Seradix No. 1 rooting hormone containing 0.1% indolebutyric acid (IBA). The cutting trays were then placed in the main greenhouse on the Bellville campus of the Cape Peninsula, University of Technology, on heated propagation beds. Plants were later placed under 40% shade cloth for 1 week to harden off before being planted out into the experimental site. The plants were placed onto galvanised steel tables covered in black plastic sheeting with 10 replicates of each treatment (Fig. 1a, b).

|
Hydroponic experiment
The hydroponic structure was built from white plastic gutters (3 m long) purchased from Builders Warehouse in Cape Town. A hack saw was used to cut the gutters into lengths of 1.36 m. A stop end was put on each end of the gutter to prevent water from pouring over, and a silicone plug was applied to keep the two parts firm. The gutters were then placed on steel tables (2.5 × 1 m) that was used on a flat surface. The silicone was left to dry up for two days before the experiment started. On the second day, the dry silicone and the gutters were mounted onto the tables using cable tyres to prevent the gutters from tilting over. The gutters were covered with black plastic polyethylene sheets to avoid algae build-up and nine holes were cut for placing (12.5 cm) plastic pots. The plastic pots (Sanscape, Parow Industria, Cape Town) were lined with a shade net to prevent Consol® silica sand from leaching into the system. A 20 mL black LDPE irrigation pipe obtained from WM Spilhaus, Bellville, City of Cape Town, South Africa, was used for recirculating the nutrient solution from the reservoir into the Nutrient Film Technique (NFT) system. Beneath each table were reservoir tank (70 L) with a submersible pond master mk2 pump, model 2600 purchased from NESCO Engineering Pty Ltd, Bellville. The reservoirs were filled with water and left to run for two days. After two days the plastic pots with planted T. decumbens were placed on each hole of the gutter and left for three days to run only with water (Fig. 2a, b). Nutrifeed fertiliser supplied by Starke Ayres, Cape Town, containing the following ingredients: 65 g/kg nitrogen, 27 g/kg phosphorus, 130 g/kg potassium, 70 mg/kg calcium, 20 mg/kg copper, 1500 mg/kg iron, 10 mg/kg molybdenum, 22 mg/kg magnesium, 240 mg/kg manganese, 75 mg/kg sulfur, 240 mg/kg boron and mg/kg zinc was applied. There were three treatments, which were as follows: (Treatment 1) 140 g/70 L, (Treatment 2) 75 g/70 L and (Treatment 3) 35 g/70 L of the nutrient solution was controlled at a rate of 2 L/h. The pH and EC were maintained at 4.5, 5.5, 6.5, and 3.38, 2.34, 1.10 dS/m respectively. The nutrient solutions were refreshed every 2 weeks to minimise the build-up of salts in the growing media. As the water drained out of the pots, it drained back into the reservoirs and was reused. The experiment was arranged in a randomised complete block design to reduce variability due to absorbed sunrays within replicates (Singh et al. 2019; Faber et al. 2020).
Experimental treatments (preparation and application)
Soilless treatments were made of 100% silica sand medium. All silica sand used was thoroughly rinsed with tap water until water poured through the sand ran clear. Graded concentrations of nutrient solutions were prepared as 0.2, 0.1, and 0.05% w/v of Nutrifeed fertiliser mixed with water, and the control, which had no nutrients. Fertigation regimes (mid-frequent) were achieved by installing identical pumps to all hydroponic systems. The amount of aqueous nutrient solution delivered to each growing bed was controlled by adjusting all output valves.
Data collection
Roots and shoot growth
Root and shoot lengths were measured before transplanting into the hydroponic systems and again at the end of the experiment. Measurements were recorded in millimetres using a standard ruler.
Dry weight
Plants were placed in brown paper bags post-harvest and dried at 30–31°C for 48 h in a forced convection oven (LABTECH™ model LDO 150F, Daihan Labtech India Pty Ltd 3269 Ranjit Nagar, New Dehli, 110008) until there was no further weight loss. Plants were then weighed using an electronic balance model PS750/C/2 laboratory balance (Radwag®, Poland) with 0.001 g readability and recorded. The difference between the wet and dry weights correlates with the amount of water held within the plants’ tissues (Butcher 2016).
Measurement of chlorophyll content of leaves
A single-photon avalanche diode (SPAD-502) counter purchased from Konica Minolta (Chiyoda City, Tokyo, Japan) was used to measure chlorophyll content every two weeks. This device measures red light transmission at 650 nm (the threshold frequency for chlorophyll to absorb light) and infrared light transmission at 940 nm (zero absorption frequency). The gadget (Fig. 2a) uses these two transmission values to estimate a SPAD value, which indicates the chlorophyll content in µmol per m2 of leaf area. SPAD data was taken from two fully expanded leaves selected from each plant and the numbers were averaged by the SPAD-502 counter to provide a final number (Dunn et al. 2018; Singh et al. 2019).
Nutrient analysis
After harvesting, replicate samples of T. decumbens obtained from the four treatments were dried in an oven and pulverised using an electric blender before being taken to BemLab Pty Ltd located at Gant’s Sentrum, 16 Van Der Berg Cres, Strand, Cape Town, 7140, South Africa, for proximate analysis. BemLab is a standard analytical laboratory certified by the South African National Accreditation System (SANAS), the only National Accreditation Body that grants official recognition to Laboratories, Certification Bodies, Inspection Bodies, Proficiency Testing Scheme Providers and Good Laboratory Practice (GLP) test facilities to carry out specific tasks. At BemLab, nutritional characteristics of the plant samples vis-à-vis macronutrients (N, K, P, Ca, Mg, and Na) and micronutrients (Cu, Zn, Mn, Fe, and B) were determined with the use of an inductively coupled plasma-optical emission spectrometer.
Statistical analysis
A one-way analysis of variance (ANOVA) was used to determine variability in growth parameters. The significant differences between treatment means at P ≤ 0.05 were compared using Fisher’s least significant difference (l.s.d.). All calculations were done on STATISTICA ver. 10, and MINITAB 17 statistical software was used to compute l.s.d.
Results
Number of shoots in T. decumbens
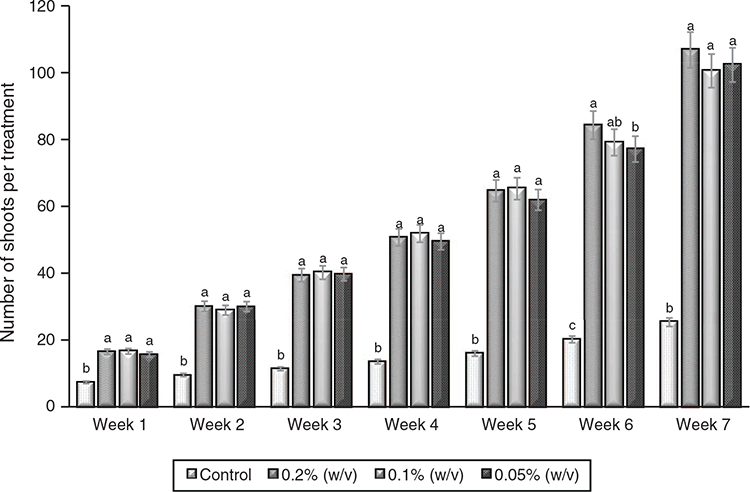
As presented in Fig. 3, the results showed that there was no significant difference in the number of shoots for treatments 1, 2, and 3 during the 7 weeks of the experiment except during week 6 when significant variation occurred between the three treatments and control. At week 6, the number of shoots recorded was in this order; 0.2 > 0.1 > 0.05% (w/v) treatments. However, the control differed significantly from other treatments that were investigated.
Fresh and dry weight of T. decumbens
The results showed variability in the fresh and dry weight for all treatments investigated during the 7 weeks of the experiment most especially in the fresh shoot and root weights where significant differences were recorded in the mean weight values at P < 0.05 (Fig. 4). The highest fresh weight was recorded in the shoots germinated under 0.2% (w/v) treatment condition whereas the least was obtained in the control. Likewise, the same trend was observed in the root samples germinated under the control and treatments conditions. However, equivalent weight was recorded for the dry shoots obtained from 0.1 and 0.05% (w/v) treatments whereas no significant difference was observed in the dry root weight of the samples cultivated under the control and treatment three conditions.
Chlorophyll content
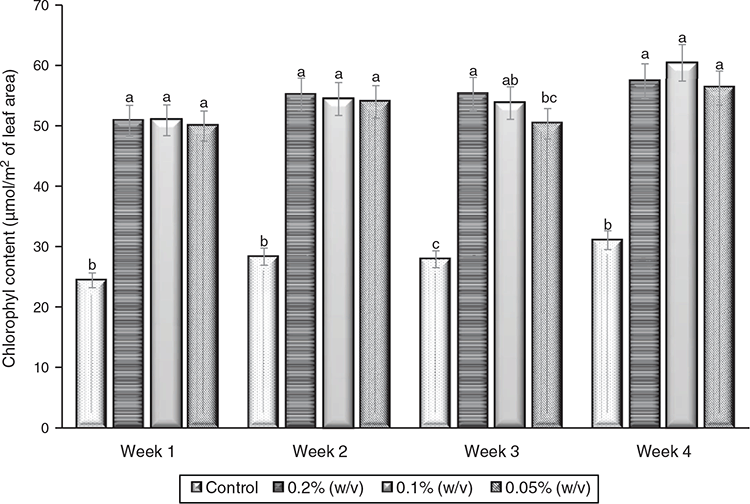
Findings from this research showed that fertigation regimes had no significant effect on the chlorophyll content of the leaves measured from week 1 to week 4, except in week 3 when slight significant variability occurred. At week 3, the highest chlorophyll was recorded in 0.2% (w/v) treatment whereas 0.1 and 0.05% (w/v) treatments had equivalent chlorophyll content (Fig. 5). Similarly, the control differs significantly from other treatments as low chlorophyll content was recorded at P ≤ 0.05.
Effects of fertigation regimes on macronutrients uptake
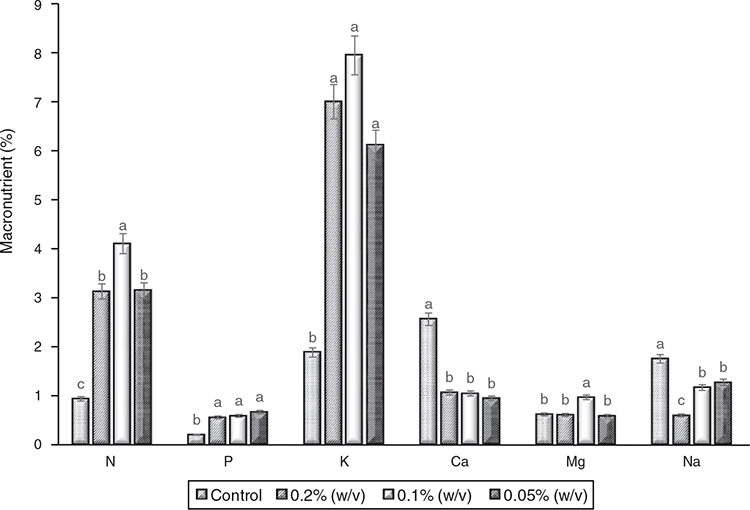
Results from this study showed that at moderate fertiliser application, N composition increased although it was expected to see higher readings of N with an increased nutrient application. These results were obtained in the control and the treatments (0.2 > 0.1% >0.05%, w/v). The highest N composition was recorded in 0.1% (w/v) treatment whereas the 0.2 and 0.05% (w/v) treatments had an equivalent composition of nitrogen at P ≤ 0.05. Of all the treatments, the control had the lowest N concentration (Fig. 6). Fertiliser application did not have significant effects on the percentage phosphorus content of the analysed samples regardless of treatments. This is evident in the fact that an equivalent yield of phosphorus was recorded in all the treatments except in the control sample with a significantly lower composition of phosphorus (P ≤ 0.05) compared with other treatments. Variability was observed in the percentage K content of the control sample; however, there was no significant difference between 0.2, 0.1, and 0.05% (w/v) at P ≤ 0.05. At P ≤ 0.05, results showed that fertigation had no significant effect on the percentage calcium of T. decumbens cultivated in hydroponics under different fertigation regimes as the control had a higher calcium content than other treatments (0.2, 0.1, and 0.05% w/v) where equivalent calcium yield was recorded. The % magnesium content was highest in 0.1% (w/v) treatment, however other treatments had equivalent Mg with the control. The highest sodium composition was recorded in T. decumbens harvested from the control treatments suggesting that fertilisation did not affect % Na content. At P ≤ 0.05, treatment with 0.2% (w/v) had the lowest Na content whereas 0.1% and 0.05% (w/v) had equivalent Na composition.
Effects of fertigation regimes on micronutrients uptake
Results showed fertigation had a significant effect on Mn uptake and availability. However, an equivalent yield of Mn was recorded in all treatments including the control (Fig. 7). However, fertigation had no significant effect on the iron composition of T. decumbens. Moreover, a higher yield in Fe was recorded in the control than other treatments. At P ≤ 0.05, an equal yield of Fe was recorded in 0.2, 0.1, and 0.05% (w/v) treatments. Although Cu yield was low, variability occurred in the Cu composition of the tested plant samples. The control and 0.2% (w/v) treatment had equal and the highest Cu content whereas 0.05% (w/v) had the least. A slightly higher Cu composition was recorded in 0.1% (w/v) treatment compared with plants grown under 0.05% (w/v) treatment. There was significant variability in the Zn composition of the samples. Although an equivalent Zn yield was recorded in the control, 0.2 and 0.1% (w/v) samples; the Zn composition of the sample from 0.05% (w/v) was significantly high compare with the control, 0.2 and 0.1% (w/v). In all treatments, however, fertigation had no significant effect on % Zn composition. Likewise, a significant difference was observed in the B component of the tested species indicating that fertigation had a significant effect on % B composition. At P ≤ 0.05, T2 had the highest B yield whereas the least was recorded in the control whereas 0.2 and 0.05% (w/v) had equivalent B composition that is slightly higher than the control but lower than 0.1% (w/v).
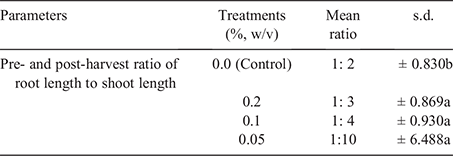
Pre-planting and post-harvest ratio of root to shoot
As presented in Table 1, there was no significant difference between treatments at P ≤ 0.05. However, the control treatment differed from other treatments.
|
Discussion
The growth rate of a plant depends primarily on the supply of nutrients to the roots and the temperature around the plant among other factors. Each species has a specific temperature range represented by minimum and maximum values. These values differ in different varieties, either grains, vegetables, or fruits (Hatfield and Prueger 2015; Jimoh et al. 2020a). Treatments applied in this investigation had a significant effect on the vegetative root and shoot growth of T decumbens. Results also suggest that a well-drained medium with a high nutrient application rate yielded better results in terms of vegetative growth. This agrees with Dorais (2007) and Blair et al. (1994) who reported that gas diffusion, soil activity, and fertility are optimised in a well-drained medium, giving rise to higher yield. Generally, it was noticed that the plant responded better to the high nutrient application compared with low or medium nutrient applications. These findings support that of Fallovo et al. (2009) who reported that the application of fertiliser is one of the most practical and effective ways of controlling and improving the yield and nutritional quality of crops for human consumption. This opportunity was availed by the fact that hydroponic systems allow easy manipulation of nutrient supply to maximise yield in a limited space (Treftz and Omaye 2016). Results obtained from this research also agree with previous studies that a suitable pH and EC adjustment in hydroponics regarding plant type improved overall plant growth (Wortman 2015; Singh and Bruce 2016). According to Sardare and Admane (2013), the medium pH changes constantly as the plant grows in a hydroponic system; thus, pH control is a necessity. In this study, the pH and electrical conductivity (EC) were maintained at 4.5, 5.5, 6.5, and 3.38, 2.34, 1.10 dS/m (S) respectively. The choice of pH range may be said to be responsible for the optimal yield in T. decumbens since a pH range of 5.5–6.5 has been reported to be optimal for the availability of nutrients from most nutrient solutions for most species although some species may differ significantly (Singh and Bruce 2016).
Chlorophyll is used by plants for light-trapping and energy transduction during the anabolic process of photosynthesis. Until recently, when the fifth type of chlorophyll was added, only four variants of chlorophyll were known. According to (Chen et al. 2010), the fifth chlorophyll otherwise called chlorophyll ‘f’ (C55H70O6N4Mg) is red shifted, suggesting it can absorb light from the infrared region. Chlorophyll metabolism is critical because intracellular chloroplast-nucleus interaction depends on it (Tanaka and Tanaka 2006). Likewise, chlorophyll has been a parameter used to measure the wellbeing of a plant. In this study, the different fertigation regimes applied showed no significant effect on estimated chlorophyll between treatments; however, the control varied significantly from other treatments. This implies that the application of nutrients affected chlorophyll content. This agrees with Nemadodzi et al. (2017) who reported earlier that the rate of nitrogen and phosphorus in fertilisers affected chlorophyll content in Spinacia oleracea. Also, these results align with Cetin et al. (2015) who reported no variability in chlorophyll content and the rate of photosynthesis in cotton after it was subjected to different fertigation treatments. Similarly, significant variability was observed in the chlorophyll content of Zea mays and Sorghum bicolour cultivated in soils amended with organic and inorganic fertilisers (Amujoyegbe et al. 2007). Although outside the scope of this research, a combination of an air-pump, high-frequency H2O2 application, and vortex oxygenation was reported to have yielded the highest mean value of chlorophyll in Pelargonium tomentosum (Chen et al. 2010; Butcher et al. 2017), suggesting that oxygenation may drive photosynthesis through increased chlorophyll production. Findings from this research, therefore, corroborate what has been reported in earlier literature; that nutrient application increases chlorophyll content.
The relationship between N and chlorophyll biosynthesis cannot be overemphasised as the former forms an important precursor to the latter. That is why N status in the leaves of many crops can be simply estimated with a chlorophyll meter that ordinarily measures chlorophyll content (von Wettstein et al. 1995; Zhang et al. 2020). In N deficient plants, there is a lack of green colour in the leaves, a decrease in leaf surface area, and a reduction in photosynthesis (Hawkesford 2014) Thus, this finding being the first of its kind to report N yield in T. decumbens proves to be important in the establishment of cultivation practices in hydroponics in the future. Phosphorus is stored as phytates in plants. It is required for cell reparation, growth, and maintenance. It is an integral component of ATP (ATP), ribonucleic acid (RNA), and DNA (DNA) (Lewu and Mavengahama 2010; Adegbaju et al. 2019). Compared with other indigenous vegetables, the higher phosphorus per cent that was recorded in this study for T. decumbens (0.66%), is slightly higher than earlier values recorded for Spinacia oleracea (0.0437%), Chenopodium album (0.37%), and Solanum nigrum (0.239%) respectively reported by Ndlovu and Afolayan (2008) and Afolayan and Jimoh (2009). However, the phosphorus content of T. decumbens is comparable to that of Amaranthus spp., Rumex crispus, and Celosia argentea reported respectively by Soriano-García et al. (2018), Idris et al. (2019), and Adegbaju et al. (2019).
Also, the per cent potassium composition of T. decumbens obtained in this experiment was slightly lower than that of S. oleracea and Amaranthus caudatus respectively reported by Tang et al. (2019) and Jimoh et al. (2020b). According to White and Karley (2010), potassium is the most abundant inorganic cation in plants that are needed for the activation of many enzymes that facilitate signalling and nutrient sensing. In animals, it is a vital macronutrient needed for efficient blood circulation, transmission of nerve impulses, muscular contraction, and ionic balance (Stein 2010; Jimoh et al. 2020b).
Compared with earlier reports for notable vegetables, the calcium content of T. decumbens was higher than that of spinach (0.423%), S. nigrum (0.308%), and amaranth (0.159%) respectively reported by Tang et al. (2019), Afolayan and Jimoh (2009) and Soriano-García et al. (2018). Given the fact that 1200 mg of Ca is recommended in the human diet daily (Jimoh et al. 2018; Salami and Afolayan 2021), a moderate serving of T. decumbens is sufficient to meet this dietary need.
The concentration of magnesium and sodium obtained in this study is low compared with popular vegetables. This may have been influenced by a higher K+ concentration that has been variously reported to work antagonistically against Na+, Ca2+, and Mg2+ uptake (Voogt 2002; Xie et al. 2020). Magnesium is the central atom in the chlorophyll molecule. It is a cationic element whose availability aids transport mechanism and is crucial to the functionality of key photosynthetic processes in plants, as well as nerve impulses transmission, oxidative metabolism, cellular respiration, regulation of internal temperature, energy generation, and bone formation in animals (Shaul 2002; Unuofin et al. 2017). Additionally, results of moderate micronutrient concentration in T. decumbens are a pointer to the fact that the species is a good candidate for phytoremediation. However, these results are comparable with various experimental results reported by Soriano-García et al. (2018), (Idris et al. 2019) and (Jimoh et al. 2018) for other leafy vegetables like spinach, R. crispus, and amaranths, although Lion and Olowoyo (2013) had cautioned against health risk associated with over-consumption of vegetables containing these toxic metals.
Conclusion and recommendations
Findings from this study suggest that a nutrient application is needed to enhance the quality of edible plants grown in hydroponics. Moreover, a well-drained, yet less water-holding capacity soilless media in conjunction with a pH range of 5.5–6.5 will likely yield the best results in terms of vegetative growth. Also, this study revealed no variability in chlorophyll content between treatments under different fertigation regimes except for the control, in which the chlorophyll content varied significantly at P < 0.05. Findings also corroborate what has been previously reported in the literature: nutrient application increases chlorophyll content. Marginal variability observed in the effects of fertigation regimes on the nutritional characteristics of the species is an indication that the plant requires a minimal nutrient application, which makes it more economical to cultivate. Apart from being the maiden research reporting macronutrient and micronutrient compositions of T. decumbens, the revelation that the plant has phytonutrients that compare with or higher than most popular leafy vegetables presents the species to plant enthusiasts, marginal income households, researchers, and food processing industries as an alternative source of plant-based nutrients. To achieve diet diversity, reliable nutrient allowance, and food security, it is therefore recommended that the plant should be cultivated widely for increased human consumption.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
Authors acknowledge the financial assistance of the National Research Foundation towards this research.
References
Adegbaju OD, Otunola GA, Afolayan AJ (2019) Proximate, mineral, vitamin and anti-nutrient content of Celosia argentea at three stages of maturity. South African Journal of Botany 124, 372–379.| Proximate, mineral, vitamin and anti-nutrient content of Celosia argentea at three stages of maturity.Crossref | GoogleScholarGoogle Scholar |
Afolayan AJ, Jimoh FO (2009) Nutritional quality of some wild leafy vegetables in South Africa. International Journal of Food Sciences and Nutrition 60, 424–431.
| Nutritional quality of some wild leafy vegetables in South Africa.Crossref | GoogleScholarGoogle Scholar | 19037794PubMed |
Allkin B (2017) ‘Useful Plants – Medicines: State of the World’s Plants 2017.’ (Royal Botanic Gardens: Kew, United Kingdom)
Amujoyegbe BJ, Opabode JT, Olayinka A (2007) Effect of organic and inorganic fertilizer on yield and chlorophyll content of maize (Zea mays L.) and sorghum (Sorghum bicolor (L.) Moench). African Journal of Biotechnology 6, 1869–1873.
| Effect of organic and inorganic fertilizer on yield and chlorophyll content of maize (Zea mays L.) and sorghum (Sorghum bicolor (L.) Moench).Crossref | GoogleScholarGoogle Scholar |
Araya H, Amoo S, Laurie R, Seetseng K, Ibraimo N, Masemola C, Mamadi N, Mndzebele B, Ramusandiwa T, Lebese J (2020) Indigenous / Traditional African Leafy Vegetables. Agricultural Research Council, South Africa. https://www.arc.agric.za/arc-vopi/Pages/Crop Science/Indigenous-Crops.aspx.
Blair G, Conteh A, Lefroy R (1994) Fate of organic matter and nutrients in upland agricultural systems. In ‘Australian Centre for International Agricultural Research’. pp. 41–49. (ACIAR: Canberra, ACT)
Butcher JD (2016) ‘A comparative study of oxygenation techniques in the hydroponic.’ (Cape Peninsula University of Technology: Cape Town, South Africa)
Butcher JD, Laubscher CP, Coetzee JC (2017) A study of oxygenation techniques and the chlorophyll responses of Pelargonium tomentosum grown in deep water culture hydroponics. HortScience 52, 952–957.
| A study of oxygenation techniques and the chlorophyll responses of Pelargonium tomentosum grown in deep water culture hydroponics.Crossref | GoogleScholarGoogle Scholar |
Cetin O, Uzen N, Temiz MG (2015) Effect of N-fertigation frequency on the lint yield, chlorophyll, and photosynthesis rate of cotton. Journal of Agricultural Science and Technology 17, 909–920.
Chen M, Schliep M, Willows RD, Cai Z-L, Neilan BA, Scheer H (2010) A red-shifted chlorophyll. Science 329, 1318–1319.
| A red-shifted chlorophyll.Crossref | GoogleScholarGoogle Scholar | 20724585PubMed |
Chivenge P, Mabhaudhi T, Modi AT, Mafongoya P (2015) The potential role of neglected and underutilised crop species as future crops under water-scarce conditions in Sub-Saharan Africa. International Journal of Environmental Research and Public Health 12, 5685–5711.
| The potential role of neglected and underutilised crop species as future crops under water-scarce conditions in Sub-Saharan Africa.Crossref | GoogleScholarGoogle Scholar | 26016431PubMed |
Christenhusz MMJ, Byng JW (2016) The number of known plants species in the world and its annual increase. Phytotaxa 261, 201–217.
| The number of known plants species in the world and its annual increase.Crossref | GoogleScholarGoogle Scholar |
Dorais M (2007) Organic production of vegetables: state of the art and challenges. Canadian Journal of Plant Science 87, 1055–1066.
| Organic production of vegetables: state of the art and challenges.Crossref | GoogleScholarGoogle Scholar |
Dunn BL, Singh H, Goad C (2018) Relationship between chlorophyll meter readings and nitrogen in poinsettia leaves. Journal of Plant Nutrition 41, 1566–1575.
| Relationship between chlorophyll meter readings and nitrogen in poinsettia leaves.Crossref | GoogleScholarGoogle Scholar |
Faber RJ, Laubscher CP, Rautenbach F, Jimoh MO (2020) Variabilities in alkaloid concentration of Sceletium tortuosum (L.) N.E. Br in response to different soilless growing media and fertigation regimes in hydroponics. Heliyon 6, e05479
| Variabilities in alkaloid concentration of Sceletium tortuosum (L.) N.E. Br in response to different soilless growing media and fertigation regimes in hydroponics.Crossref | GoogleScholarGoogle Scholar | 33235938PubMed |
Fallovo C, Rouphael Y, Rea E, Battistelli A, Colla G (2009) Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. Journal of the Science of Food and Agriculture 89, 1682–1689.
| Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture.Crossref | GoogleScholarGoogle Scholar |
Fernández FG, Hoeft RG (2009) Managing soil pH and crop nutrients. In ‘Illinois agronomy handbook’. pp. 91–112. (Crop Science Extension & Outreach, University of Illinois: Urbana IL, USA)
Forrester J (2004) Tetragonia decumbens. Plantz Africa. South African National Biodiversity Institute. 1–3. Available at: http://opus.sanbi.org/bitstream/20.500.12143/3865/1/Tetragoniadecumbens_PlantzAfrica.pdf
Francis CA, Hansen TE, Fox AA, Hesje PJ, Nelson HE, Lawseth AE, English A (2012) Farmland conversion to non-agricultural uses in the US and Canada: Current impacts and concerns for the future. International Journal of Agricultural Sustainability 10, 8–24.
| Farmland conversion to non-agricultural uses in the US and Canada: Current impacts and concerns for the future.Crossref | GoogleScholarGoogle Scholar |
Gonzalez CG (2010) The global food crisis: law, policy, and the elusive quest for justice. Yale Human Rights and Development Law Journal 13, 463–479.
Hatfield JL, Prueger JH (2015) Temperature extremes: effect on plant growth and development. Weather and Climate Extremes 10, 4–10.
| Temperature extremes: effect on plant growth and development.Crossref | GoogleScholarGoogle Scholar |
Hawkesford MJ (2014) Reducing the reliance on nitrogen fertilizer for wheat production. Journal of Cereal Science 59, 276–283.
| Reducing the reliance on nitrogen fertilizer for wheat production.Crossref | GoogleScholarGoogle Scholar | 24882935PubMed |
Heyligers P (1999) The occurrence and history of Tetragonia decumbens (Aizoaceae) in New South Wales. Telopea 8, 371–373.
| The occurrence and history of Tetragonia decumbens (Aizoaceae) in New South Wales.Crossref | GoogleScholarGoogle Scholar |
Idris OA, Wintola OA, Afolayan AJ (2019) Comparison of the proximate composition, vitamins analysis of the essential oil of the root and leaf of Rumex crispus L. Plants 51, 1–15.
Jimoh MO, Afolayan AJ, Lewu FB (2018) Suitability of Amaranthus species for alleviating human dietary deficiencies. South African Journal of Botany 115, 65–73.
| Suitability of Amaranthus species for alleviating human dietary deficiencies.Crossref | GoogleScholarGoogle Scholar |
Jimoh MO, Afolayan AJ, Lewu FB (2020a) Heavy metal uptake and growth characteristics of Amaranthus caudatus L. under five different soils in a controlled environment. Notulae Botanicae Horti Agrobotanici 48, 417–425.
| Heavy metal uptake and growth characteristics of Amaranthus caudatus L. under five different soils in a controlled environment.Crossref | GoogleScholarGoogle Scholar |
Jimoh MO, Afolayan AJ, Lewu FB (2020b) Nutrients and antinutrient constituents of Amaranthus caudatus L. cultivated on different soils. Saudi Journal of Biological Sciences 27, 3570–3580.
| Nutrients and antinutrient constituents of Amaranthus caudatus L. cultivated on different soils.Crossref | GoogleScholarGoogle Scholar | 33304168PubMed |
Khoshbakht K, Hammer K (2008) How many plant species are cultivated? Genetic Resources and Crop Evolution 55, 925–928.
| How many plant species are cultivated?Crossref | GoogleScholarGoogle Scholar |
Konsam S, Thongam B, Handique AK (2016) Assessment of wild leafy vegetables traditionally consumed by the ethnic communities of Manipur, northeast India. Journal of Ethnobiology and Ethnomedicine 12, 1–15.
Lefever K, Laubscher CP, Ndakidemi PA, Nchu F (2017) Effects of pH and phosphorus concentrations on the chlorophyll responses of Salvia chamelaeagnea (Lamiaceae) grown in hydroponics. In ‘Chlorophyll’. (Ed. E Jacob-Lopes) pp. 79–92. (IntechOpen)
Lewu FB, Mavengahama S (2010) Wild vegetables in northern KwaZulu Natal, South Africa: Current status of production and research needs. Scientific Research and Essays 5, 3044–3048.
Lion GN, Olowoyo JO (2013) Population health risk due to dietary intake of toxic heavy metals from Spinacia oleracea harvested from soils collected in and around Tshwane, South Africa. South African Journal of Botany 88, 178–182.
| Population health risk due to dietary intake of toxic heavy metals from Spinacia oleracea harvested from soils collected in and around Tshwane, South Africa.Crossref | GoogleScholarGoogle Scholar |
Ndlovu J, Afolayan A (2008) Nutritional analysis of the South African wild vegetable Corchorus olitorius. Asian Journal of Plant Sciences 7, 615–618.
| Nutritional analysis of the South African wild vegetable Corchorus olitorius.Crossref | GoogleScholarGoogle Scholar |
Nemadodzi LE, Araya H, Nkomo M, Ngezimana W, Mudau NF (2017) Nitrogen, phosphorus, and potassium effects on the physiology and biomass yield of baby spinach (Spinacia oleracea L.). Journal of Plant Nutrition 40, 2033–2044.
| Nitrogen, phosphorus, and potassium effects on the physiology and biomass yield of baby spinach (Spinacia oleracea L.).Crossref | GoogleScholarGoogle Scholar |
Pham VC, Pham TTH, Tong THA, Nguyen TTH, Pham NH (2015) The conversion of agricultural land in the peri-urban areas of Hanoi (Vietnam): patterns in space and time. Journal of Land Use Science 10, 224–242.
| The conversion of agricultural land in the peri-urban areas of Hanoi (Vietnam): patterns in space and time.Crossref | GoogleScholarGoogle Scholar |
Reed LL, Kleynhans TE (2009) Agricultural land purchases for alternative uses – evidence from two farming areas in the Western Cape province, South Africa. Agrekon 48, 332–351.
| Agricultural land purchases for alternative uses – evidence from two farming areas in the Western Cape province, South Africa.Crossref | GoogleScholarGoogle Scholar |
Salami SO, Afolayan AJ (2021) Evaluation of nutritional and elemental compositions of green and red cultivars of roselle: Hibiscus sabdariffa L. Scientific Reports 11, 1030–3.
| Evaluation of nutritional and elemental compositions of green and red cultivars of roselle: Hibiscus sabdariffa L.Crossref | GoogleScholarGoogle Scholar | 33441870PubMed |
Sardare MD, Admane SV (2013) A review on plant without soil – hydroponics. International Journal of Research in Engineering and Technology 02, 299–304.
| A review on plant without soil – hydroponics.Crossref | GoogleScholarGoogle Scholar |
Shaul O (2002) Magnesium transport and function in plants: the tip of the iceberg. Biometals 15, 307–321.
| Magnesium transport and function in plants: the tip of the iceberg.Crossref | GoogleScholarGoogle Scholar |
Singh H, Bruce D (2016) ‘Electrical conductivity and pH guide for hydroponics.’ (Oklahoma State University: Stillwater, OK, USA)
Singh H, Dunn B, Payton M, Brandenberger L (2019) Fertilizer and cultivar selection of lettuce, basil, and Swiss Chard for hydroponic production. HortTechnology 29, 50–56.
| Fertilizer and cultivar selection of lettuce, basil, and Swiss Chard for hydroponic production.Crossref | GoogleScholarGoogle Scholar |
Soriano-García M, Ilnamiqui Arias-Olguín I, Pablo Carrillo Montes J, Genaro Rosas Ramírez D, Silvestre Mendoza Figueroa J, Flores-Valverde E, Rita Valladares-Rodríguez M (2018) Nutritional functional value and therapeutic utilization of Amaranth. Journal of Analytical & Pharmaceutical Research 7, 596–600.
| Nutritional functional value and therapeutic utilization of Amaranth.Crossref | GoogleScholarGoogle Scholar |
Stein AJ (2010) Global impacts of human mineral malnutrition. Plant and Soil 335, 133–154.
| Global impacts of human mineral malnutrition.Crossref | GoogleScholarGoogle Scholar |
Tanaka A, Tanaka R (2006) Chlorophyll metabolism. Current Opinion in Plant Biology 9, 248–255.
| Chlorophyll metabolism.Crossref | GoogleScholarGoogle Scholar | 16603411PubMed |
Tang L, Hamid Y, Sahito ZA, Gurajala HK, He Z, Feng Y, Yang X (2019) Evaluation of variation in essential nutrients and hazardous materials in spinach (Spinacia oleracea L.) genotypes grown on contaminated soil for human consumption. Journal of Food Composition and Analysis 79, 95–106.
| Evaluation of variation in essential nutrients and hazardous materials in spinach (Spinacia oleracea L.) genotypes grown on contaminated soil for human consumption.Crossref | GoogleScholarGoogle Scholar |
Tembo-Phiri C (2019) ‘Edible fynbos plants: a soil types and irrigation regime investigation on Tetragonia decumbens and Mesembryanthemum crystallinum.’ (Stellenbosch University: South Africa)
Treftz C, Omaye ST (2016) Hydroponics: potential for augmenting sustainable food production in non-arable regions. Nutrition & Food Science 46, 672–684.
| Hydroponics: potential for augmenting sustainable food production in non-arable regions.Crossref | GoogleScholarGoogle Scholar |
Unuofin JO, Otunola GA, Afolayan AJ (2017) Nutritional evaluation of Kedrostis africana (L.) Cogn: an edible wild plant of South Africa. Asian Pacific Journal of Tropical Biomedicine 7, 443–449.
| Nutritional evaluation of Kedrostis africana (L.) Cogn: an edible wild plant of South Africa.Crossref | GoogleScholarGoogle Scholar |
Uprety Y, Poudel RC, Shrestha KK, Rajbhandary S, Tiwari NN, Shrestha UB, Asselin H (2012) Diversity of use and local knowledge of wild edible plant resources in Nepal. Journal of Ethnobiology and Ethnomedicine 8,
| Diversity of use and local knowledge of wild edible plant resources in Nepal.Crossref | GoogleScholarGoogle Scholar | 22546349PubMed |
von Wettstein D, Gough S, Kannangara CG (1995) Chlorophyll biosynthesis. The Plant Cell 7, 1039–1057.
| Chlorophyll biosynthesis.Crossref | GoogleScholarGoogle Scholar | 12242396PubMed |
Voogt W (2002) Potassium management of vegetables under intensive growth conditions. In ‘Proceedings of the Colloquium of the International Potash Institute’. (Eds NS Pasricha, SK Bansal) pp. 347–362. (International Potash Institute: Bern, Switzerland)
White PJ, Karley AJ (2010) Potassium. In ‘Cell biology of metals and nutrients. Plant Cell Monograph’. pp. 199–224. (Springer: Berlin, Heidelberg)
Wortman SE (2015) Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Scientia Horticulturae 194, 34–42.
| Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system.Crossref | GoogleScholarGoogle Scholar |
Xie K, Cakmak I, Wang S, Zhang F, Guo S (2020) Synergistic and antagonistic interactions between potassium and magnesium in higher plants. The Crop Journal.
| Synergistic and antagonistic interactions between potassium and magnesium in higher plants.Crossref | GoogleScholarGoogle Scholar | in press
Zhang C, Huang Y, Xiao Z, Yang H, Hao Q, Yuan S, Chen H, Chen L, Chen S, Zhou X, Huang W (2020) A GATA transcription factor from soybean (Glycine max) regulates chlorophyll biosynthesis and suppresses growth in the transgenic Arabidopsis thaliana. Plants 9, 1036
| A GATA transcription factor from soybean (Glycine max) regulates chlorophyll biosynthesis and suppresses growth in the transgenic Arabidopsis thaliana.Crossref | GoogleScholarGoogle Scholar |