Effects of cyanogenesis on morphology and estimated leaf flavonoid content in 51 white clover accessions
Jennifer Gabriel A B * , Nicole M. van Dam
A B * , Nicole M. van Dam  A B C and Henriette Uthe
A B C and Henriette Uthe  A B
A B
A German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, Leipzig 04103, Germany.
B Institute of Biodiversity, Friedrich Schiller University, Jena 07743, Germany.
C Leibniz Institute for Vegetable and Ornamental Crops (IGZ) e.V., Großbeeren 14979, Germany.
Crop & Pasture Science 74(5) 494-506 https://doi.org/10.1071/CP22140
Submitted: 19 April 2022 Accepted: 28 November 2022 Published: 30 January 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution 4.0 International License (CC BY)
Abstract
Context: Plant secondary metabolites are of increasing interest for agriculture due to their diverse beneficial ecological functions. The forage crop white clover (Trifolim repens L.) has been intensively studied for its heritable polymorphism in the production of hydrogen cyanide (HCN), a toxic defense phytochemical. In fodder production, white clover accessions are selected for biomass production, whereby HCN production is an unwanted trait.
Aim: Although white clover is a legume crop species of global importance, little is known about the linkage between cyanogenesis and growth traits, in particular in combination with resistance-related phytochemicals, such as flavonoids. We aimed to identify differences in biomass production, estimated leaf flavonoid content, and trait correlations in cyanogenic (HCN-producing) and acyanogenic (not HCN-producing) individuals and accessions of white clover.
Methods: We analysed 51 white clover accessions from a German germplasm collection for variability in selected traits: cyanogenesis as equivalent electrode potential, estimated leaf flavonoid content, root and shoot production, leaf area, specific leaf area, and number of leaves produced.
Key results: Most accessions considered as cyanogenic were heterogeneous for HCN production. Chemical–morphological trait correlations differed between cyanogenic and acyanogenic plants. Acyanogenic individuals and accessions produced more and larger leaves compared to cyanogenic ones. Within cyanogenic accessions, the higher the HCN level of a plant, the fewer but larger leaves were produced.
Conclusions: Our results highlight the variation in HCN production within the selected accessions, which calls for a consistent approach for cyanogenesis-based categorisation.
Implication: This study demonstrates the potential of combining phytochemical traits with biomass production in white clover when selecting material in a breeding program.
Keywords: cyanogenesis, forage legume, genotypic diversity, leaf flavonoids, legume chemistry, morphological traits, plant breeding, Trifolium repens, white clover germplasm.
Introduction
Cyanogenesis, or the ability to produce hydrogen cyanide (HCN), is a common trait in certain plant families. Most plants emit low levels of toxic HCN as a by-product of ethylene biosynthesis. Over 3000 higher plant species, including many important food plants, release HCN via cyanogenesis (Conn 1981). In cyanogenic plants, cell rupture enables the reaction of cyanogenic glycosides (CNglcs) with the corresponding enzymes (β-glucosidase, α-hydroxynitrile lyase) (Conn 1981; Seigler 1998; Gleadow and Møller 2014). The released HCN is toxic, which explains the major biological function as two-component defence against herbivores, primarily arthropod generalists (Nahrstedt 1985; Hughes et al. 1992; Ballhorn et al. 2010a). The effectiveness of reducing herbivore damage thereby depends on multiple factors, such as the concentration of the cyanogenic precursors, the amount of tissue damaged (Provenza et al. 1992; Gleadow and Woodrow 2002) and, as described by Ballhorn et al. (2005), the cyanogenic capacity (HCNc), a measurement of the amount of hydrogen cyanide released per minute. In addition, the production of CNglcs is affected by abiotic stresses, notably extreme temperatures, soil salinity, nitrogen supply and drought (Daday 1965; Vickery et al. 1987; Ballhorn and Elias 2014). However, cyanogenesis comes along with both advantages and disadvantages in the context of plant breeding for agricultural application. It has been shown that low levels of HCN production, leading to increased plant fitness, outweigh the negative effects of its toxicity. Cyanogenic plants showed higher yields, increased drought tolerance and were more resistant to pests and herbivores (Daday 1965; Foulds and Grime 1972; Ennos 1981). On the other hand, a direct trade-off between cyanogenesis and defence against fungal pathogens was shown in rubber tree and lima bean (Lieberei 1988; Ballhorn et al. 2010b). Cyanogenic white clover (Trifolium repens L., Fabaceae) plants perform even better under moderate persistent drought stress compared to those that are not able to produce HCN (acyanogenic morph) (Kooyers et al. 2014). This may be one aspect explaining why many cyanogenic crops, such as manioc, sorghum, and white clover are still cultivated for human consumption and animal forage production (Jones 1998).
On the other hand, high levels of CNglcs are detrimental since the consumption of cyanogenic plants entails risks for both human and livestock by inhibiting cellular respiration (Cheeke 1995; Leavesley et al. 2008; Hamel 2011). Among cyanogenic plant species, white clover is one of the most widely grown perennial pasture legumes of the temperate zones. Due to its high nutritive value, ability to fix nitrogen, and long seasonal growth, white clover is used as a pasture legume and forage crop, mainly for sheep and cattle (Frame and Newbould 1986; Caradus et al. 1996). Because of the ability to adapt to a wide range of climatic conditions, it is used in farming systems all over the world (Frame and Newbould 1986). Nevertheless, its competitiveness and growth is reduced under drought stress (Thomas 1984). Its beneficial properties, especially in crop rotation systems, makes it one of the major pasture legumes in New Zealand, Great Britain (UK), Ireland, the US, and Denmark (Mather et al. 1995). Its economic status in these countries explains the large number of accessions in regional seed collections and the continued research effort put into breeding programs to improve resilience and yield (Annicchiarico 2003; Widdup and Barrett 2011; Jahufer et al. 2012; Egan et al. 2021). White clover accessions have been therefore well characterised for agriculturally relevant traits (Caradus 1986; Caradus and Woodfield 1997; Aasmo Finne et al. 2000; Oliveira et al. 2013). The published checklists of varieties by Caradus and Woodfield (1997) compiles characteristics such as tolerance and susceptibility to pests, growth traits and HCN production of numerous accessions used for breeding (Caradus et al. 1989; Caradus and Woodfield 1997).
The two-gene system for cyanogenesis in white clover is one of the best-studied polymorphisms in plants (Coop 1940; Corkill 1942; Hughes 1991; Hayden and Parker 2002). Cyanogenic plants need to possess at least one dominant allele for substrate and enzyme (Olsen et al. 2007). Assessment in white clover plants has been commonly performed using a colorimetric test for either a simple 0/1 categorisation or a combination of colorimetric and spectrophotometric methods for quantitative analyses of HCN (Egan et al. 1998; Bradbury 2009). Although widely accepted and advantageous in its usage, the colorimetric test suffers from limitations such as the comparison among studies with regard to the differentiation of the cyanogenic level. When considering the intraspecific variation, plant breeding practices require reliable quantitative measurements that allow for consistent assessment of the level of cyanogenesis. Among other techniques, this could be achieved by using a cyanide (CN) ion-selective electrode (ISE) (Gillingham et al. 1969). These ion-selective (IS) measurements to assess cyanogenesis are based on measuring the number of effective cyanide ions (ionic activity) as proportional output potential.
Besides avoiding unwanted phytochemicals, breeders also target for beneficial biochemical properties. Particularly, breeders favour accessions with tolerance to abiotic stressors. The production of flavonoids, for example, enhances resistance to drought, one major cause of crop losses (Nakabayashi et al. 2014; Li et al. 2021). Furthermore, flavonoids serve as bioactive compounds mediating consumer-specific interactions with herbivores or pathogens (Mierziak et al. 2014; Whitehead et al. 2021). In addition, they play a key role as stimulants and chemoattractants in establishing and maintaining root colonisation by growth-promoting symbioses with mycorrhizal fungi and rhizobia (Siqueira et al. 1991; Larose et al. 2002; Eckardt 2006). These agriculturally relevant symbioses make flavonoids an additional target for breeding approaches (D’Amelia et al. 2018). However, it is as yet unknown if and how flavonoid content trades off with HCN production in white clover. Furthermore, when multiple traits together make up the quality of a breeding line, assessing their correlations is indispensable to breeders in order to avoid unwanted positive and negative correlations (Yan and Wallace 1995; Yan and Frégeau-Reid 2008; Chen and Lübberstedt 2010). Whether correlations among growth traits and secondary metabolite production follow similar trajectories in cyanogenic and acyanogenic white clover accessions/individuals is as yet unknown.
Due to their agronomic value, many white clover accessions have been deposited in germplasm collections worldwide. Several studies analysed the trait and genetic diversity of accessions from collections; notably from countries with extensive white clover farming e.g. New Zealand, Great Britain (UK), Ireland, the US, and Denmark (Mather et al. 1995; Zhang et al. 2010; Wu et al. 2021). Equally detailed information on growth and chemical traits of white clover cultivars in European gene bank collections is scarce. The characterisation of these collections is important for the identification of genetic sources for white clover breeding. In this study, we screened 51 white clover accessions from a German gene bank of the Leibnitz Institute of Plant Genetics and Crop Plant Research (IPK) for two chemical traits, cyanogenesis and total leaf flavonoid content, combined with growth traits. The aim was to identify positive and negative trait correlations among these traits. For this, we examined accessions used in agriculture for their cyanogenic status using a quantitative ion-selective electrode, and determined traits related to biomass production. Our work addressed the following questions: (1) Do cyanogenic and acyanogenic accessions differ in morphological traits, and which of those are the main drivers of variation in biomass production? (2) Do morphological traits and estimated leaf flavonoid production differ between cyanogenic and acyanogenic individuals? (3) Do traits correlate within cyanogenic and acyanogenic subsets and do these correlations differ between the two groups? We were thereby able to assess which agro-morphological traits best explain biomass production and whether growth and flavonoid production differ consistently among cyanogenic and acyanogenic plants.
Materials and methods
Plant material and cultivation
Seeds of 51 high agronomic performance white clover accessions were obtained from the Leibnitz Institute of Plant Genetics and Crop Plant Research (IPK), Seeland, Gatersleben, Germany. Seeds were surface sterilised by soaking them in 10% H2O2 for 10 min. Per accession, 15–30 seeds were germinated in a climate chamber (21°C, 50% relative humidity; 16:8 h photoperiod) on glass beads with water for 14 days. To ensure a comparable level of development among the replicates of each accession, we selected five seedlings with the first true leaf unfolded. Each replicate was planted in pots filled with a sand/soil mixture (1:1) and grown for 63 days in the greenhouse at 25°C (16 h day) and 21°C (8 h night) at ambient relative humidity. All pots were randomised and fertilised with half-strength Hoagland solution weekly (Hoagland and Arnon 1938).
Morphological traits
A total of three leaf traits (total number of leaves, leaf area, specific leaf area) and six biomass traits were measured and analysed to evaluate correlations between growth and chemical traits in cyanogenic and acyanogenic plants. At the end of the growth phase, at 9 weeks after transplanting the seedlings to the pots, the total number of leaves (TNL) was determined. Six fully unfolded trifoliate leaves from three ramets of two stolons per plant were harvested. Leaves were placed on a white paper and scanned immediately. Leaf areas (LAs) were determined using Adobe Photoshop CS6 and ImageJ (ver. 1.53, http://imagej.nih.gov/ij0). After freeze drying (−80°C, 800 Pa, 72 h) leaves were weighed and the specific leaf area (SLA) was calculated as average mm2 leaf area per mg dry weight. After assessing root fresh weight and shoot fresh weight, the plant materials were oven-dried (60°C, 72 h) to determine root dry weight (RDW) and shoot dry weight (SDW). Finally, the root:shoot ratios for dry biomass (RSD) were calculated.
Sample preparation
For chemical analyses, including the measurement of cyanogenic levels (CNG) and estimated total leaf flavonoid content (TFC), 5–7 additional fully unfolded trifoliate leaves were collected from each plant one day before the final harvesting. With regard to the leaf sampling procedure, the effect of leaf age on HCN production was tested in a pilot study. Cyanogenesis level along stolons was measured using a Cyanide Selective Electrode as also used in the main study (see section Determination of cyanogenic level). Seven leaves along stolons were sampled and measured for HCN production. Although there was only weak evidence of differences between the youngest and oldest leaves along stolons (P = 0.078, Table 1), we took this tendency into account when sampling leaves for the main experiment. The youngest fully enfolded leaves of one or two ramets of three randomly chosen stolons were selected. Freeze dried leaves (−80°C, 0.008 bar, 72 h) were ground two times for 10 min at 30 Hz using a ball mill (Mixer Mill MM 200, RETSCH GmbH, Haan, Germany).

|
Reagents and chemicals
Trichloromethane, potassium dihydrogen phosphate, sodium hydroxide, quercetin dihydrate, and aluminum chloride hexahydrate were obtained from Carl Roth GmbH and Co. KG (Karlsruhe, Germany). Copper ethyl acetoacetate and 4,4′-methylenebis(N,N-dimethylaniline) were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Methanol was purchased by VWR International GmbH (Darmstadt, Germany). Water for the preparation of reagents and extracts was purified by an ultrapure purification system (Thermo Scientific Barnstead GenPure, Fisher Scientific GmbH, Schwerte, Germany).
Determination of cyanogenic level
Hydrogen cyanide levels were measured with a Cyanide Solid State Half-Cell Ion Selective Electrode (CN-ISE), Cat. No. 9406BN combined with a double junction reference electrode, Cat. No. 900200 (Fisher Scientific, Schwerte, Germany). Per sample, 20 mg of dried and finely ground leaf material were extracted in 1 mL 0.2 M KH2PO4. The solution was incubated at 37°C for 30 min and the reaction stopped by adding 200 μL 10 M NaOH. After shaking (1 min, 30 Hz) and centrifuging for 10 min at 10.000 rpm (11.180g) at room temperature, the supernatant was taken up with a pipette and combined with 19.8 mL Millipore water in a new tube. The HCN measurement was conducted under stirring at room temperature by placing the electrode in the extract. Relative HCN release was expressed as electrode potential (mV).
Before testing the accessions, the electrode read-out was aligned with the result of a sensitive colorimetric test, a modified Feigl-Anger HCN assay (Feigl and Anger 1966; Olsen et al. 2007). Filter paper was soaked in a solution of 5 mg 4,4′-methylenebis(N,N-dimethylaniline) and 5 mg copper ethyl acetoacetate in 2 mL trichloromethane. After drying, the test paper was stored until further use at 4°C. For a subset of genotypes, 4–5 medium-sized leaves were harvested, transferred into two 2 mL tubes, and immediately stored for at least 30 min at −80°C. One tube with leaf samples was subsequently defrosted for 10 min at 37°C and the thawed leaf material was crushed with a pestle directly in the tube. Freshly prepared test filter paper was placed on top of the open tube and the lid was closed to fix the paper. The tubes with the test paper were incubated for another 90 min at 37°C. Samples for which the paper turned blue were classified as cyanogenic. The resulting data was combined with CN-ISE measurements to determine at which electrode potential a colour reaction was detected. Based on this combination, samples from the main experiment with a value below −58.5 mV were considered as cyanogenic. Accessions were categorised as cyanogenic when at least one out of five replicates per accession tested positive for HCN production. On the basis of our results and the range of the measured electrode potentials (EP), we categorised the selected accessions within our study in low (EP ≥ −58.5 mV), medium (EP < −80.0 mV), and high (EP < −100.0 mV) cyanogenic.
Determination of the estimated total flavonoid content
To estimate total flavonoid content (TFC) in white clover leaves, we used the aluminum chloride colorimetric method (Chang et al. 2002). Quercetin was used to prepare a standard calibration curve. For this, we prepared a stock solution of 5.595 mg quercetin dihydrate dissolved in 50 mL 80% methanol. Serial dilutions of 5 μg and 10–100 μg (in steps of 10 μg) were prepared. Of each solution, 0.5 mL was mixed 1:1 with a solution of 2% AlCl3 in 1.5 mL tubes. Per sample, 10 mg dried ground leaf material was extracted in 80% methanol, sonicated for 45 min at 40°C and centrifuged for 10 min at 2555g (room temperature). The supernatant was stored at −20°C until measurement. A solution of 0.5 mL 2% AlCl3 was combined with the extract. Both the standard dilutions and the extracts were incubated for 60 min before measuring the absorbance against a blank at 420 nm on a UV-Vis spectrophotometer using 1 mL cuvettes (Jasco V-620, Pfungstadt, Germany). Based on the readings of the quercetin dilution series, we calculated TFC from the calibration curve (Y = 0.0325x + 0.32, R2 = 0.9256) as mg quercetin equivalent (QE) per g of dried leaf material.
Statistical analysis
All statistical analyses were performed using R v4.0.2 (R Core Team 2020) using the packages ‘Rmisc’ (Hope 2013), ‘ggplot’ (Wickham et al. 2016), ‘car’ (Fox and Weisberg 2019), ‘multcomp’ (Hothorn et al. 2008), ‘corrplot’ (Wei and Simko 2017), ‘ggbiplot’ (Vu 2011), and ‘nlme’ (Pinheiro et al. 2018). To identify significant intercorrelations (P < 0.05) we calculated Pearson correlation coefficients for all measured traits. Considering the potential impact of cyanogenesis on these correlations, the analysis was conducted for cyanogenic and acyanogenic individuals separately. For distance measures between the accessions, we conducted a principal component analysis (PCA) on the standardised means of all morphological traits. To identify the impact of each morphological trait and TFC on the variability we calculated the mean of the coefficients of variance (CV; significant differences at a level of P < 0.05; Supplementary Table S1) among all cyanogenic and acyanogenic accessions. We tested further differences between cyanogenic and acyanogenic individuals and accessions for each measured trait. Shapiro–Wilk and Levene’s test were performed to test for normality of residuals and homogeneity of variances. After ensuring that the requirements for parametric analyses were met, analyses of variances (ANOVAs) were run and pairwise analyses based on multiple comparisons of means (Tukey’s HSD) were performed. In case of non-parametric data, we used Dunn multiple comparisons with P-values adjusted with the Benjamin Hochberg method after a Kruskal–Wallis test. Least significant differences were identified at a level of P < 0.05.
Results
Cyanogenesis on the individual and the accession level
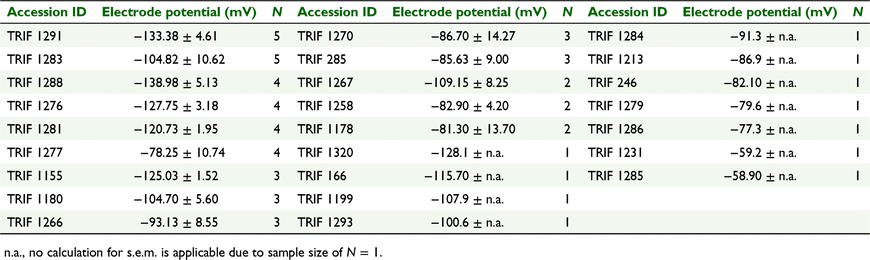
In order to classify the accessions according to their HCN production, we examined how many of the tested individuals produced HCN per accession and to what extent (Table 2). HCN production was tested for each replicate (single individual). Over all tested accessions (n = 51), 28 were homogenous with regard to cyanogenesis, since all replicates per accession were either positive or negative for HCN production. Of these, 26 were acyanogenic and only two were fully cyanogenic (TRIF 1291, TRIF 1283). The remaining 23 were heterogenous as we identified cyanogenic and acyanogenic replicates within these accessions. In total, we identified 199 acyanogenic and 56 cyanogenic individuals.
|
Growth traits and estimated total flavonoid content among and within cyanogenic and acyanogenic accessions
The measured growth variables were analysed for acyanogenic and cyanogenic accessions and individuals (Table 3). On the level of accessions, specific leaf area was higher in accessions classified as acyanogenic (PSLA = 0.019). Estimated total flavonoid content of leaves was higher in cyanogenic plants on both the individual (P = 0.025) and the accession level (P = 0.0002).

|
Comparing acyanogenic and cyanogenic individuals, we found differences in the measured growth traits and estimated total flavonoid content in leaves. The number of leaves was higher in acyanogenic plants (PTNL = 0.033).
Trait variation on accession level
On the basis of the previously described results we were able to determine the trait variation for the acyanogenic and cyanogenic accessions. We found low variability in leaf area (LA), specific leaf area (SLA), estimated total flavonoid content (TFC) of leaves, and total number of leaves (TNL), as shown by the coefficient of variance (CV; CV < 25%, Fig. 1). Growth parameters, notably root weight (RDW), explained most variability (CV > 50%). LA, SLA, TFC and TNL thereby differed from the growth traits each (P = 0.000). Also, among growth traits CVs differed between SDW and both RDW and RSD (P = 0.000), as well as between RDW and RSD (P = 0.008). There was no evidence for an effect of cyanogenesis (Table S1). The first two principal component axes explained 69.6% of the variation in the nine measured morphological traits (Fig. 2). PCA axis 1 explained 43.4% of this variation and was positively associated with SDW, RDW, RSD and TNL, whereas axis 2 (26.2%) was positively associated with SLA and LA. Mean values of all measured traits for each accession are provided in Table S2.
Trait correlation on the individual level
Since most of the cyanogenic accessions turned out to be heterogenous for HCN production (see above), we could only perform trait correlations on the individual level. All measured variables were analysed for correlations between biomass traits, leaf traits, and chemical traits for cyanogenic and acyanogenic plants separately. For acyanogenic plants (n = 199), we found positive and negative correlations between the measured leaf traits and biomass data (Fig. 3a). Leaf area (LA) correlated positively with shoot dry weight (SDW; PSDW = 0.025). Specific leaf area (SLA) showed a negative correlation to root dry weight (RDW), and dry root/shoot ratio (PRDW = 0.020, PRSD = 0.039). The total number of leaves per plant (TNL) correlated strongly and positively with all biomass traits (PSDW, PRDW and PRSD = 0.000). For the estimated total leaf flavonoid content (TFC) we could detect a positive correlation to SDW, SLA, and TNL (PSDW = 0.003, PSLA = 0.004 and PTNL = 0.053).
Contrary to the results for acyanogenic plants, we found no evidence for correlations between either LA or SLA with the selected biomass traits (Fig. 3b). As in acyanogenic plants, TNL correlated mostly strongly with all biomass traits (PSDW and PRDW = 0.000, PRSD = 0.001). Cyanogenic level (CNG) correlated positively with LA (P = 0.000) and negatively with TNL (P = 0.024). Furthermore, there was no correlation between the two measured molecular parameters, TFC and CNG (P = 0.857).
Discussion
In the study presented here, we examined 51 agriculturally relevant white clover accessions from Central Europe for HCN production levels. The selected accessions were variable for cyanogenesis; most accessions contained both cyanogenic and acyanogenic individuals. We found that overall growth traits did not differ between cyanogenic and acyanogenic accessions. On the individual level the number of leaves produced was lower and estimated leaf flavonoid content higher in cyanogenic compared to acyanogenic plants. We were also able to identify positive and negative trait correlations within the cyanogenic and acyanogenic subgroups. Additionally, these trait correlations differed between the two groups. The strength of the differences and direction of correlations found are discussed below with regard to agricultural usage of white clover accessions.
Accessions are mostly heterogenous for cyanogenesis
We tested all individuals of the selected accessions for their hydrogen cyanide (HCN) production. Most of the accessions that could be classified as cyanogenic were heterogenous for the production of HCN. This result is in line with previous studies (Caradus and Woodfield 1997). Among all tested accessions, 78% of the individual plants were acyanogenic. It was hypothesised that cyanogenesis is linked with altitude assuming a selection for the acyanogenic morph in regions with low winter temperature (Daday 1954; de Araújo 1976; Till-Bottraud et al. 1988). The low frequency of cyanogenic accessions could reflect this trait’s status as mostly undesirable in breeding practice e.g. when using white clover as forage or green manure (Bjarnholt et al. 2008).
Differences of qualitative and quantitative HCN production of accessions among studies
Over the decades of white clover breeding, HCN production has always been taken into account when characterising accessions. These classifications are partly inconsistent, both between previously published data and in comparison with those presented here. Caradus (1986) and Caradus and Woodfield (1997) collected data from different studies including qualitative HCN production (cyanogenic/acyanogenic), the level (low, moderate, high), and the frequency (percentage) of cyanogenic plants. As a measure of the incidence of cyanogenic activity, we report in the present work the number of positive replicates, expressed as a percentage. In contrast, previous studies reported an average of the intensity of cyanogenesis across individual samples. Nevertheless, the comparison reveals a comparable rating for most of the cyanogenic accessions; indicating a similar relationship in the classification despite different levels of analysis (Table 4). An apparent limitation of the method described here is its unsuitability for determining the exact cyanotype. The classification as acyanogenic does not consider the presence of cyanogenic glycosides in the absence of the corresponding enzyme. The use of a linamarase enzyme assay would have completed the results. Nevertheless, this limitation should not crucially affect the outcome of the study.

|
Four accessions have been previously stated as acyanogenic in at least one study while we identified one or more cyanogenic replicates. For eight accessions no positive result for HCN production could be detected in any of the replicates whereby those were described as cyanogenic (Table 4).
Due to the variable number of replicates between studies, data on the frequency of cyanogenic plants can only illustrate the rarity of fully cyanogenic accessions. A replicate number of n = 5 seems to be insufficient to identify cyanogenic individuals in accessions in which the ability for cyanogenesis is a rare trait. Nevertheless, there is no clear indication how frequently cyanogenesis has to occur within an accession for being classified as cyanogenic. The same applies when categorising into low, medium or high cyanogenic accessions; which is additionally limited by the usage of different measurement methods. Crush and Caradus (1995) already stated that differences in the method can cause quantitative discrepancies. Nevertheless, they conceded similarities in the ranking of accessions within studies.
Chemical–morphological trait correlations are affected by the cyanogenic status
We evaluated the importance of agro-morphological trait correlation with phytochemical properties in white clover on the level of the cyanogenic status of individuals. Correlations between leaf and biomass traits showed a few similarities among the acyanogenic and cyanogenic subsets. In both cyanogenic and acyanogenic plants, trait variability was mainly driven by root biomass. The total number of leaves per plant (TNL) was positively linked to biomass production regardless of the cyanogenic status, which corresponds to previous findings (Hawkins 1959; Caradus et al. 1989). Interestingly, leaf area (LA) and shoot biomass showed a positive correlation only for acyanogenic plants. Similarly, SLA correlated positively and significantly with LA in acyanogenic plants only, which means that leaf thickness increases with leaf size. There is contrasting information on the linkage between these two leaf traits from previous studies in other plant species (Cunningham et al. 1999; Fonseca et al. 2000). Our results indicate that LA and SLA are decoupled in cyanogenic but not in acyanogenic plants in white clover. This observation suggests that cyanogenic plants produce thicker leaves than acyanogenic ones, which might result from different growth strategies possibly related to adaptation to abiotic conditions. Furthermore, cyanogenesis was significantly linked with leaf traits. We found that individuals producing higher levels of HCN produced fewer leaves but with a greater leaf area. Possibly, the negative correlation between HCN level and leaf number reflects a cost of being highly cyanogenic. Indeed, previous experiments found that acyanogenic plants perform better in mixed and pure stands (Kakes 1989; Noitsakis and Jacquard 1992). Leaf size may compensate for having fewer leaves to some extent. Since the leaves of cyanogenic plants are better defended, they might need to compensate for herbivory resistance less with leaf number.
SLA, as a characteristic trait used to estimate biomass production, increases with increasing latitude, which can be related to differences in efficiency in the use of light energy (Dolph and Dilcher 1980; Gong and Gao 2019). Along a latitudinal and altitudinal gradient, HCN production is lower with more abundant acyanogenic populations in colder regions and in higher altitude (Daday 1954; de Araújo 1976). Our results support these findings on the accession level since SLA was higher in acyanogenic plants.
Estimated total leaf flavonoid content was higher in cyanogenic accessions and individual plants (Table 3). A distinct trade-off between cyanogenesis and phenolic compounds was previously described for the legume species Phaseolus lunatus (Ballhorn et al. 2010b). However, estimated total flavonoid levels significantly increased with specific leaf area and the number of leaves in acyanogenic plants whereas the direction was inverse in cyanogenic plants. In cyanogenic plants estimated flavonoid production seems to come at the expense of leaf area per dry matter. A trade-off effect between flavonoid content and biomass production has been described for white clover. Low levels of quercetin correlated with increased plant growth (Hofmann and Jahufer 2011). The cyanogenic accessions we analysed may have originated from populations occurring in stressed habitats since flavonoids are involved in stress resistances. At the same time cyanogenic plants produced less biomass supporting the described negative trade-off. Since we measured TFC representatively as quercetin equivalent, it is not possible to determine which flavonoids are affected in their synthesis with reduced biomass accumulation in cyanogenic plants. A targeted HPLC (high-performance liquid chromatography) measurement would have allowed a precise determination of leaf flavonoid content. Nevertheless, our results underline the previously suggested potential of flavonoid content as a selection target in white clover breeding.
Cyanide ion-selective electrode measurements for identifying cyanogenic plants
For our analyses we used cyanide ion-selective electrode measurements. The reliability of electrode measurements for forage crops had been proven by combined photometric determination with alkaline picrate, as this method was considered sensitive, specific and reproducible (Gillingham et al. 1969). In contrast to the colorimetric methods, the IS measurement is less complex and avoids the use of instable or toxic colour-forming reagents. Nevertheless, electrochemical methods have limitations. Measurements are not specific due to interferences from other substances in the plant tissue (Blaedal et al. 1971). We found that it is a valuable alternative to conventional methods when assessing intraspecific variance in HCN production, which allowed us to make quantitative trait correlations. After proper calibration, we found this method to be fast and straightforward, which made it suitable for large numbers of samples with almost similar reproducibility to colorimetric methods (Gillingham et al. 1969). By measuring leaves at different positions along stolons, we found weak evidence that there is variation for HCN even within one individual (Table 1). Potentialintraindividual variation should definitely be considered in future assessments, for example this can be achieved by consistent sampling procedures such as harvesting leaves from different stolons and the same position, as presented in this study. Considering that HCN genotypes may be more resilient under drought, breeders may want to keep this trait at a level that is low enough for using white clover as a forage crop. Based on our experiences, we recommend this fast and easy method as a semi-quantitative way of identifying low cyanogenic genotypes that isrelevant for crop breeding due to its accuracy and easy handling.
The study presented here supported the detailed categorisation of registered white clover accessions into cyanogenic and acyanogenic types, and documented the heterogeneity therein. We based our classification on an easy-to-use method and integrated it with a multi-trait approach, combining biomass production and relevant phytochemical properties. We thereby resolved some discrepancies among previous studies in the categorisation of cyanogenic accessions. Our results also identified agriculturally relevant trait correlations in cyanogenic and acyanogenic white clover plants over all accessions. This provides valuable information for breeders aiming to combine biomass productivity with optimised levels of cyanogenesis and flavonoids.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
All authors acknowledge the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig funded by the German Research Foundation (DFG-FZT 118, 202548816). The study was funded by a doctoral scholarship (project no. 20018/566) for JG by the Deutsche Bundesstiftung Umwelt (DBU).
References
Aasmo Finne M, Rognli OA, Schjelderup I (2000) Genetic variation in a Norwegian germplasm collection of white clover (Trifolium repens L.). Euphytica 112, 45–56.| Genetic variation in a Norwegian germplasm collection of white clover (Trifolium repens L.).Crossref | GoogleScholarGoogle Scholar |
Annicchiarico P (2003) Breeding white clover for increased ability to compete with associated grasses. The Journal of Agricultural Science 140, 255–266.
| Breeding white clover for increased ability to compete with associated grasses.Crossref | GoogleScholarGoogle Scholar |
Ballhorn DJ, Elias JD (2014) Salinity-mediated cyanogenesis in white clover (Trifolium repens) affects trophic interactions. Annals of Botany 114, 357–366.
| Salinity-mediated cyanogenesis in white clover (Trifolium repens) affects trophic interactions.Crossref | GoogleScholarGoogle Scholar |
Ballhorn DJ, Lieberei R, Ganzhorn JU (2005) Plant cyanogenesis of Phaseolus lunatus and its relevance for herbivore-plant interaction: the importance of quantitative data. Journal of Chemical Ecology 31, 1445–1473.
| Plant cyanogenesis of Phaseolus lunatus and its relevance for herbivore-plant interaction: the importance of quantitative data.Crossref | GoogleScholarGoogle Scholar |
Ballhorn DJ, Kautz S, Lieberei R (2010a) Comparing responses of generalist and specialist herbivores to various cyanogenic plant features. Entomologia Experimentalis et Applicata 134, 245–259.
| Comparing responses of generalist and specialist herbivores to various cyanogenic plant features.Crossref | GoogleScholarGoogle Scholar |
Ballhorn DJ, Pietrowski A, Lieberei R (2010b) Direct trade-off between cyanogenesis and resistance to a fungal pathogen in lima bean (Phaseolus lunatus L.). Journal of Ecology 98, 226–236.
| Direct trade-off between cyanogenesis and resistance to a fungal pathogen in lima bean (Phaseolus lunatus L.).Crossref | GoogleScholarGoogle Scholar |
Bjarnholt N, Laegdsmand M, Hansen HCB, Jacobsen OH, Møller BL (2008) Leaching of cyanogenic glucosides and cyanide from white clover green manure. Chemosphere 72, 897–904.
| Leaching of cyanogenic glucosides and cyanide from white clover green manure.Crossref | GoogleScholarGoogle Scholar |
Blaedal WJ, Easty DB, Anderson L, Farrell TR (1971) Potentiometric determination of cyanide with an ion selective electrode. Application to cyanogenic glycosides in Sudan grasses. Analytical Chemistry 43, 890–894.
| Potentiometric determination of cyanide with an ion selective electrode. Application to cyanogenic glycosides in Sudan grasses.Crossref | GoogleScholarGoogle Scholar |
Bradbury JH (2009) Development of a sensitive picrate method to determine total cyanide and acetone cyanohydrin contents of gari from cassava. Food Chemistry 113, 1329–1333.
| Development of a sensitive picrate method to determine total cyanide and acetone cyanohydrin contents of gari from cassava.Crossref | GoogleScholarGoogle Scholar |
Caradus JR (1986) World checklist of white clover varieties. New Zealand Journal of Experimental Agriculture 14, 119–164.
| World checklist of white clover varieties.Crossref | GoogleScholarGoogle Scholar |
Caradus JR, Woodfield DR (1997) Review: World checklist of white clover varieties II. New Zealand Journal of Agricultural Research 40, 115–206.
| Review: World checklist of white clover varieties II.Crossref | GoogleScholarGoogle Scholar |
Caradus JR, MacKay AC, Woodfield DR, van den Bosch J, Wewala S (1989) Classification of a world collection of white clover cultivars. Euphytica 42, 183–196.
| Classification of a world collection of white clover cultivars.Crossref | GoogleScholarGoogle Scholar |
Caradus JR, Hay RJM, Woodfield DR (1996) The positioning of white clover cultivars in New Zealand. Agronomy Society of New Zealand Special Publication 11/Grassland Research and Practice Series 6, 45–50.
| The positioning of white clover cultivars in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Chang C-C, Yang M-H, Wen H-M, Chern J-C (2002) Estimation of total flavonoid content in propolis by two complementary colometric methods. Journal of Food and Drug Analysis 10, 3
| Estimation of total flavonoid content in propolis by two complementary colometric methods.Crossref | GoogleScholarGoogle Scholar |
Cheeke PR (1995) Endogenous toxins and mycotoxins in forage grasses and their effects on livestock. Journal of Animal Science 73, 909–918.
| Endogenous toxins and mycotoxins in forage grasses and their effects on livestock.Crossref | GoogleScholarGoogle Scholar |
Chen Y, Lübberstedt T (2010) Molecular basis of trait correlations. Trends in Plant Science 15, 454–461.
| Molecular basis of trait correlations.Crossref | GoogleScholarGoogle Scholar |
Conn EE (1981) Cyanogenic glycosides. In ‘The biochemistry of plants. A comprehensive treatise, Vol. 7’. Secondary Plant Products. (Ed. EE Conn) pp. 479–499. (Academic Press: New York)
Coop IE (1940) Cyanogenesis in white clover (Trifolium repens L.). III. A study of linamarase, the enzyme which hydrolyses lotaustralin. New Zealand Journal of Science and Technology 22, 71B–83B.
Corkill L (1942) Cyanogenesis in white clover (Trifolium repens L.). V. The inheritance of cyanogenesis. New Zealand Journal of Science and Technology 23, 178–193.
Crush JR, Caradus JR (1995) Cyanogenesis potential and iodine concentration in white clover (Trifolium repens L.) cultivars. New Zealand Journal of Agricultural Research 38, 309–316.
| Cyanogenesis potential and iodine concentration in white clover (Trifolium repens L.) cultivars.Crossref | GoogleScholarGoogle Scholar |
Cunningham SA, Summerhayes B, Westoby M (1999) Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecological Monographs 69, 569–588.
| Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients.Crossref | GoogleScholarGoogle Scholar |
Daday H (1954) Gene frequencies in wild populations of Trifolium repens. Heredity 8, 61–78.
| Gene frequencies in wild populations of Trifolium repens.Crossref | GoogleScholarGoogle Scholar |
Daday H (1965) Gene frequencies in wild populations of Trifolium repens L. IV Mechanisms of natural selection. Heredity 20, 355–365.
| Gene frequencies in wild populations of Trifolium repens L. IV Mechanisms of natural selection.Crossref | GoogleScholarGoogle Scholar |
de Araújo AM (1976) The relationship between altitude and cyanogenesis in white clover (Trifolium repens, L.). Heredity 37, 291–293.
| The relationship between altitude and cyanogenesis in white clover (Trifolium repens, L.).Crossref | GoogleScholarGoogle Scholar |
Dolph GE, Dilcher DL (1980) Variation in leaf size with respect to climate in Costa Rica. Biotropica 12, 91–99.
| Variation in leaf size with respect to climate in Costa Rica.Crossref | GoogleScholarGoogle Scholar |
D’Amelia V, Aversano R, Chiaiese P, Carputo D (2018) The antioxidant properties of plant flavonoids: their exploitation by molecular plant breeding. Phytochemistry Reviews 17, 611–625.
| The antioxidant properties of plant flavonoids: their exploitation by molecular plant breeding.Crossref | GoogleScholarGoogle Scholar |
Eckardt NA (2006) The role of flavonoids in root nodule development and auxin transport in Medicago truncatula. The Plant Cell 18, 1539–1540.
| The role of flavonoids in root nodule development and auxin transport in Medicago truncatula.Crossref | GoogleScholarGoogle Scholar |
Egan SV, Yeoh HH, Bradbury JH (1998) Simple picrate paper kit for determination of the cyanogenic potential of cassava flour. Journal of the Science of Food and Agriculture 76, 39–48.
| Simple picrate paper kit for determination of the cyanogenic potential of cassava flour.Crossref | GoogleScholarGoogle Scholar |
Egan LM, Hofmann RW, Ghamkhar K, Hoyos-Villegas V (2021) Prospects for Trifolium improvement through germplasm characterisation and pre-breeding in New Zealand and beyond. Frontiers in Plant Science 12, 653191
| Prospects for Trifolium improvement through germplasm characterisation and pre-breeding in New Zealand and beyond.Crossref | GoogleScholarGoogle Scholar |
Ennos RA (1981) Detection of selection in populations of white clover (Trifolium repens L.). Biological Journal of the Linnean Society 15, 75–82.
| Detection of selection in populations of white clover (Trifolium repens L.).Crossref | GoogleScholarGoogle Scholar |
Feigl F, Anger V (1966) Replacement of benzidine by copper ethylacetoacetate and tetra base as spot-test reagent for hydrogen cyanide and cyanogen. Analyst 91, 282–284.
| Replacement of benzidine by copper ethylacetoacetate and tetra base as spot-test reagent for hydrogen cyanide and cyanogen.Crossref | GoogleScholarGoogle Scholar |
Fonseca CR, Overton JM, Collins B, Westoby M (2000) Shifts in trait-combinations along rainfall and phosphorus gradients. Journal of Ecology 88, 964–977.
| Shifts in trait-combinations along rainfall and phosphorus gradients.Crossref | GoogleScholarGoogle Scholar |
Foulds W, Grime JP (1972) The response of cyanogenic and acyanogenic phenotypes of Trifolium repens to soil moisture supply. Heredity 28, 181–187.
| The response of cyanogenic and acyanogenic phenotypes of Trifolium repens to soil moisture supply.Crossref | GoogleScholarGoogle Scholar |
Fox J, Weisberg S (2019) An R companion to applied regression, Third Edition. Thousand Oaks CA. Available at https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Frame J, Newbould P (1986) Agronomy of white clover. Advances in Agronomy 40, 1–88.
| Agronomy of white clover.Crossref | GoogleScholarGoogle Scholar |
Gillingham JT, Shirer MM, Page NR (1969) Evaluation of the orion cyanide electrode for estimating the cyanide content of forage samples. Agronomy Journal 61, 717–718.
| Evaluation of the orion cyanide electrode for estimating the cyanide content of forage samples.Crossref | GoogleScholarGoogle Scholar |
Gleadow RM, Møller BL (2014) Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity. Annual Review of Plant Biology 65, 155–185.
| Cyanogenic glycosides: synthesis, physiology, and phenotypic plasticity.Crossref | GoogleScholarGoogle Scholar |
Gleadow RM, Woodrow IE (2002) Constraints on effectiveness of cyanogenic glycosides in herbivore defense. Journal of Chemical Ecology 28, 1301–1313.
| Constraints on effectiveness of cyanogenic glycosides in herbivore defense.Crossref | GoogleScholarGoogle Scholar |
Gong H, Gao J (2019) Soil and climatic drivers of plant SLA (specific leaf area). Global Ecology and Conservation 20, e00696
| Soil and climatic drivers of plant SLA (specific leaf area).Crossref | GoogleScholarGoogle Scholar |
Hamel J (2011) A Review of acute cyanide poisoning with a treatment update. Critical Care Nurse 31, 72–82.
| A Review of acute cyanide poisoning with a treatment update.Crossref | GoogleScholarGoogle Scholar |
Hawkins RP (1959) Botanical characters for the classification and identification of varieties of white clover. National Institute of Agricultural Botany Journal 8, 675–682.
Hayden KJ, Parker IM (2002) Plasticity in cyanogenesis of Trifolium repens L.: inducibility, fitness costs and variable expression. Evolutionary Ecology Research 4, 155–168.
Hoagland DR, Arnon DI (1938) The water culture method for growing plants without soil. California Agricultural Experiment Station Publications C347, 36–39.
Hofmann RW, Jahufer MZZ (2011) Tradeoff between biomass and flavonoid accumulation in white clover reflects contrasting plant strategies. PLoS ONE 6, e18949
| Tradeoff between biomass and flavonoid accumulation in white clover reflects contrasting plant strategies.Crossref | GoogleScholarGoogle Scholar |
Hope RM (2022) Rmisc: Ryan Miscellaneous. R package version 1.5.1. Available at https://CRAN.R-project.org/package=Rmisc
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical Journal 50, 346–363.
Hughes MA (1991) The cyanogenic polymorphism in Trifolium repens L. (white clover). Heredity 66, 105–115.
| The cyanogenic polymorphism in Trifolium repens L. (white clover).Crossref | GoogleScholarGoogle Scholar |
Hughes MA, Brown K, Pancoro A, Murray BS, Oxtoby E, Hughes J (1992) A molecular and biochemical analysis of the structure of the cyanogenic β-glucosidase (linamarase) from cassava (Manihot esculenta Cranz). Archives of Biochemistry and Biophysics 295, 273–279.
| A molecular and biochemical analysis of the structure of the cyanogenic β-glucosidase (linamarase) from cassava (Manihot esculenta Cranz).Crossref | GoogleScholarGoogle Scholar |
Jahufer MZZ, Ford JL, Widdup KH, Harris C, Cousins G, Ayres JF, Lane LA, Hofmann RW, Ballizany WL, Mercer CF, Crush JR, Williams WM, Woodfield DR, Barrett BA (2012) Improving white clover for Australasia. Crop & Pasture Science 63, 739–745.
| Improving white clover for Australasia.Crossref | GoogleScholarGoogle Scholar |
Jones DA (1998) Why are so many food plants cyanogenic? Phytochemistry 47, 155–162.
| Why are so many food plants cyanogenic?Crossref | GoogleScholarGoogle Scholar |
Kakes P (1989) An analysis of the costs and benefits of the cyanogenic system in Trifolium repens L. Theoretical and Applied Genetics 77, 111–118.
| An analysis of the costs and benefits of the cyanogenic system in Trifolium repens L.Crossref | GoogleScholarGoogle Scholar |
Kooyers NJ, Gage LR, Al-Lozi A, Olsen KM (2014) Aridity shapes cyanogenesis cline evolution in white clover (Trifolium repens L.). Molecular Ecology 23, 1053–1070.
| Aridity shapes cyanogenesis cline evolution in white clover (Trifolium repens L.).Crossref | GoogleScholarGoogle Scholar |
Larose G, Chênevert R, Moutoglis P, Gagne S, Piché Y, Vierheilig H (2002) Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. Journal of Plant Physiology 159, 1329–1339.
| Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus.Crossref | GoogleScholarGoogle Scholar |
Leavesley HB, Li L, Prabhakaran K, Borowitz JL, Isom GE (2008) Interaction of cyanide and nitric oxide with cytochrome c oxidase: implications for acute cyanide toxicity. Toxicological Sciences 101, 101–111.
| Interaction of cyanide and nitric oxide with cytochrome c oxidase: implications for acute cyanide toxicity.Crossref | GoogleScholarGoogle Scholar |
Li B, Fan R, Sun G, Sun T, Fan Y, Bai S, Guo S, Huang S, Liu J, Zhang H, Wang P, Zhu X, Song C-P (2021) Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species. Plant and Soil 461, 389–405.
| Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species.Crossref | GoogleScholarGoogle Scholar |
Lieberei R (1988) Relationship of cyanogenic capacity (HCN-c) of the rubber tree Hevea brasiliensis to susceptibility to Microcyclus ulei, the agent causing South American leaf blight. Journal of Phytopathology 122, 54–67.
| Relationship of cyanogenic capacity (HCN-c) of the rubber tree Hevea brasiliensis to susceptibility to Microcyclus ulei, the agent causing South American leaf blight.Crossref | GoogleScholarGoogle Scholar |
Mather RDJ, Melhuish DT, Herlihy M (1995) Trends in the global marketing of white clover cultivars. Agronomy Society of New Zealand 6, 7–14.
| Trends in the global marketing of white clover cultivars.Crossref | GoogleScholarGoogle Scholar |
Mierziak J, Kostyn K, Kulma A (2014) Flavonoids as important molecules of plant interactions with the environment. Molecules 19, 16240–16265.
| Flavonoids as important molecules of plant interactions with the environment.Crossref | GoogleScholarGoogle Scholar |
Nahrstedt A (1985) Cyanogenic compounds as protecting agents for organisms. Plant Systematics and Evolution 150, 35–47.
| Cyanogenic compounds as protecting agents for organisms.Crossref | GoogleScholarGoogle Scholar |
Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, Michael AJ, Tohge T, Yamazaki M, Saito K (2014) Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. The Plant Journal 77, 367–379.
| Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids.Crossref | GoogleScholarGoogle Scholar |
Noitsakis B, Jacquard P (1992) Competition between cyanogenic and acyanogenic morphs of Trifolium repens. Theoretical and Applied Genetics 83, 443–450.
| Competition between cyanogenic and acyanogenic morphs of Trifolium repens.Crossref | GoogleScholarGoogle Scholar |
Oliveira JA, López JE, Palencia P (2013) Agromorphological characterization, cyanogenesis and productivity of accessions of White Clover (Trifolium repens L.) collected in Northern Spain. Czech Journal of Genetics and Plant Breeding 49, 24–35.
| Agromorphological characterization, cyanogenesis and productivity of accessions of White Clover (Trifolium repens L.) collected in Northern Spain.Crossref | GoogleScholarGoogle Scholar |
Olsen KM, Sutherland BL, Small LL (2007) Molecular evolution of the Li/li chemical defence polymorphism in white clover (Trifolium repens L.). Molecular Ecology 16, 4180–4193.
| Molecular evolution of the Li/li chemical defence polymorphism in white clover (Trifolium repens L.).Crossref | GoogleScholarGoogle Scholar |
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2018) nlme: Linear and nonlinear mixed effects models. R Package Version 3.1-137. http://CRAN.R-project.org/package=nlme
Provenza FD, Pfister JA, Cheney CD (1992) Mechanisms of learning in diet selection with reference to phytotoxicosis in herbivores. Journal of Range Management 45, 36–45.
| Mechanisms of learning in diet selection with reference to phytotoxicosis in herbivores.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/
Seigler DS (1998) Cyanogenic glycosides and cyanolipids. In ‘Plant secondary metabolism’. (Ed. DS Seigler) pp. 273–299. (Springer: Boston) https://doi.org/10.1007/978-1-4615-4913-0_16
Siqueira JO, Nair MG, Hammerschmidt R, Safir GR, Putnam AR (1991) Significance of phenolic compounds in plant-soil-microbial systems. Critical Reviews in Plant Sciences 10, 63–121.
| Significance of phenolic compounds in plant-soil-microbial systems.Crossref | GoogleScholarGoogle Scholar |
Stochmal A, Oleszek W (1997) Changes of cyanogenic glucosides in white clover (Trifolium repens L.) during the growing season. Journal of Agricultural and Food Chemistry 45, 4333–4336.
| Changes of cyanogenic glucosides in white clover (Trifolium repens L.) during the growing season.Crossref | GoogleScholarGoogle Scholar |
Thomas H (1984) Effects of drought on growth and competitive ability of perennial ryegrass and white clover. Journal of Applied Ecology 21, 591–602.
| Effects of drought on growth and competitive ability of perennial ryegrass and white clover.Crossref | GoogleScholarGoogle Scholar |
Till-Bottraud I, Kakes P, Dommee B (1988) Variable phenotypes and stable distribution of the cyanotypes of Trifolium repens L. in southern France. Acta Oecologica 9, 393–404.
Vickery PJ, Wheeler JL, Mulcahy C (1987) Factors affecting the hydrogen cyanide potential of white clover (Trifolium repens L.). Australian Journal of Agricultural Research 38, 1053–1059.
| Factors affecting the hydrogen cyanide potential of white clover (Trifolium repens L.).Crossref | GoogleScholarGoogle Scholar |
Viette M, Tettamanti C, Saucy F (2000) Preference for acyanogenic white clover (Trifolium Repens) in the vole Arvicola Terrestris. II. Generalization and further investigations. Journal of Chemical Ecology 26, 101–122.
| Preference for acyanogenic white clover (Trifolium Repens) in the vole Arvicola Terrestris. II. Generalization and further investigations.Crossref | GoogleScholarGoogle Scholar |
Vu VQ (2011) ggbiplot: A ggplot2 based biplot. R Package version 0.55.
Wei T, Simko V (2017) R package ‘corrplot’: Visualization of a correlation matrix. (Version 0.84), Available at https://github.com/taiyun/corrplot
Whitehead SR, Bass E, Corrigan A, Kessler A, Poveda K (2021) Interaction diversity explains the maintenance of phytochemical diversity. Ecology Letters 24, 1205–1214.
| Interaction diversity explains the maintenance of phytochemical diversity.Crossref | GoogleScholarGoogle Scholar |
Wickham H, Chang W, Henry L, Pedersen TL, Takahashi K, Wilke K, Woo K, Yutani H, Dunnington D (2016) Ggplot2: Elegant graphics for data analysis. 2nd ed. Cham, Switzerland: Springer International Publishing; 2016. 260 p.
Widdup KH, Barrett BA (2011) Achieving persistence and productivity in white clover. NZGA: Research and Practice Series 15, 173–180.
| Achieving persistence and productivity in white clover.Crossref | GoogleScholarGoogle Scholar |
Wu F, Ma S, Zhou J, Han C, Hu R, Yang X, Nie G, Zhang X (2021) Genetic diversity and population structure analysis in a large collection of white clover (Trifolium repens L.) germplasm worldwide. PeerJ 9, e11325
| Genetic diversity and population structure analysis in a large collection of white clover (Trifolium repens L.) germplasm worldwide.Crossref | GoogleScholarGoogle Scholar |
Yan W, Frégeau-Reid J (2008) Breeding line selection based on multiple traits. Crop Science 48, 417–423.
| Breeding line selection based on multiple traits.Crossref | GoogleScholarGoogle Scholar |
Yan W, Wallace DH (1995) Breeding for negatively associated traits. In ‘Plant breeding reviews. Vol. 13’. (Ed. J Janick) pp. 141–177. (John Wiley & Sons, Inc.) https://doi.org/10.1002/9780470650059.ch4
Zhang X, Zhang Y, Yan R, Han J, Fuzeng-Hong F, Cao K (2010) Genetic variation of white clover (Trifolium repens L.) collections from China detected by morphological traits, RAPD and SSR. African Journal of Biotechnology 9, 3032–3041.


